INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of diseases ranging from simple hepatic steatosis through steatohepatitis (NASH) to increasing fibrosis and eventual cirrhosis. NAFLD used to be considered the leading cause of chronic liver damage in Western countries. With the spread of a Westernised lifestyle, NAFLD is exhibiting an increasingly universal distribution[1,2]. NAFLD is strongly associated with obesity, type 2 diabetes (T2DM), dyslipidemia and cardiovascular disease[3]. Although the cornerstone of NAFLD therapy remains lifestyle intervention, many patients have difficulty maintaining lifestyle changes and achieving the treatment goals.
As novel anti-diabetic treatment options, glucagon-like peptide-1 (GLP-1)-based agents have been shown to improve the hepatic parameters in T2DM patients with NAFLD. GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient ingestion, and has various bioactivities including enhancing glucose-dependent insulin secretion from pancreatic β-cells, promoting β-cell survival, inhibiting glucagon secretion from α-cell, slowing gastric empty and controlling food intake. These effects help to maintain a euglycaemic level and are beneficial for weight control. However, under physiological conditions, the intact active GLP-1 is quickly degraded by dipeptidyl peptidase-4 (DPP-4) into the inactive GLP-1. Therefore, GLP-1 receptor (GLP-1R) agonists, which are resistant to DPP-4, or DPP-4 inhibitors, which protect the endogenous GLP-1 from degradation, were developed by pharmaceutical enterprises. These GLP-1-based therapies have been widely accepted in the treatment of T2DM in recent years. It has been proven that GLP-1-based therapies are beneficial for body weight control, improve insulin sensitivity and dyslipidemia, and prevent oxidative stress in patients with T2DM[4-7]. Apart from these well-documented effects that are helpful for the metabolic disorders in NAFLD, recent studies have shown that GLP-1 agents may also have direct effects on hepatocytes.
In this article, we review the data from both clinical and preclinical studies that investigate the relationship between GLP-1 and NAFLD. The possible mechanisms, especially the direct effects of GLP-1-based therapies on hepatocytes, are also discussed.
ASSOCIATION OF GLP-1 SIGNALING WITH NAFLD
Observations from bariatric surgery
Bariatric surgery is recommended as a treatment option for adults with morbid obesity or obese patients with T2DM. The benefits of weight loss and lowering of blood glucose have been well documented in patients undergoing the procedure. Additionally, clinical studies and systemic reviews have reported that the procedure also improves hepatic histology in most obese patients with NAFLD or NASH, reversing steatosis, inflammation and even fibrosis in the liver[8,9]. Although weight loss is one of the main factors associated with the improvement of liver histology in individuals with NAFLD, several other factors that are independent of weight loss may also contribute to this effect. For example, one important factor is an elevated serum level of GLP-1 resulting from the altered secretion pattern of this gut hormone after re-routing the flow of chyme, whereby food more quickly reaches the distal ileum and stimulates the secretion of GLP-1 from the L cells located there. Nevertheless, whether NAFLD improvement is associated with the elevation of GLP-1 level in patients undergoing bariatric surgery remains to be clarified.
Evidence from NAFLD patients and animal models
GLP-1 levels and DPP-4 expression in NAFLD: Bernsmeier et al[10] evaluated the plasma level of active GLP-1 in patients with NAFLD or NASH and healthy controls with a standardised oral glucose tolerance test. The glucose-induced GLP-1 secretion was dramatically decreased in patients with NAFLD or NASH compared to the controls, suggesting a deficiency of GLP-1 signalling in NAFLD. McDonald et al[11] found that the fasting GLP-1 level was not significantly different between the high fat diet (HFD)-treated rats and the control animals throughout the development of NAFLD, although there was an age-related decline. Unfortunately, the ELISA kit that this previous study used for measuring plasma GLP-1 level had a 100% cross-reaction of active GLP-1 and inactive GLP-1 and could not therefore identify the level of active GLP-1 from that of total GLP-1. On the other hand, DPP-4, which inactivates intact GLP-1, has been shown to be upregulated in the livers of patients with NAFLD. In a recent report, hepatic DPP-4 mRNA expression level in liver biopsy samples was significantly higher in patients with NAFLD compared to healthy subjects[12]. Moreover, serum DPP-4 activity and the intensity of DPP-4 expression in the human liver were correlated with markers of liver damage, histopathological grade and insulin resistance in patients with NAFLD[13,14]. These results suggest that excessive GLP-1 inactivation resulting from DPP-4 overactivity may have a role in the development of NAFLD.
Expression of GLP-1R in NAFLD: The GLP-1R has been detected in human liver biopsy samples, primary human hepatocytes and human hepatoma cell lines at both the mRNA and protein level[15,16], which raises the possibility that GLP-1 may exert a direct effect on hepatocytes via functional GLP-1R. Hepatic GLP-1R expression level in patients with NASH was significantly lower than that in healthy controls. A similar phenomenon was also found in animal models of NASH. In HFD-induced NASH rats, GLP-1R mRNA expression was significantly decreased in the liver after one month of HFD exposure, indicating an association of downregulated GLP-1R expression with NASH[15].
Based on the aforementioned human data and animal studies, defective GLP-1 signalling may play a role in the development and progression of NAFLD. GLP-1-based agents have been well accepted as safe and efficacious options in the treatment of T2DM, which shares similar pathophysiological mechanisms with NAFLD. Currently, increasing amount of data from both clinical and animal studies suggest that GLP-1-based agents are also beneficial for NAFLD.
BENEFICIAL EFFECTS OF GLP-1-BASED THERAPIES ON NAFLD
Suggestions from small clinical studies
In one case report, a 59-year-old male T2DM patient was treated with exenatide, a GLP-1R agonist, adding to metformin monotherapy. His liver fat content declined from 15.8% to 4.3% as measured by magnetic resonance spectroscopy (MRS) following a 44-wk treatment. This dramatic decrease in liver fat content was accompanied by a significant improvement of liver enzymes, in particular alanine transaminase (ALT), the most important marker of liver steatosis[17].
Cuthbertson et al[18] investigated the impact of GLP-1R agonist therapy on intrahepatic lipid in obese T2DM patients with hepatic steatosis in a prospective clinical study. Twenty-five patients were given 6 mo of treatment with GLP-1R agonist (exenatide in 19, and liraglutide in 6 subjects). After 6 mo of treatment with the GLP-1R agonists, the relative reduction from baseline in intrahepatic lipid, as quantified by MRS, was 42%. This reduction was significantly correlated with a relative reduction in haemoglobin A1c (HbA1c) but not with that in total body weight.
Thiazolidinediones (TZDs) are considered an effective option in the treatment of NAFLD[19]. In a small randomised controlled trial, Sathyanarayana et al[20] compared the effects of exenatide and pioglitazone combination therapy with pioglitazone alone on hepatic fat content in T2DM patients who were already on diet control and/or metformin therapy. The exenatide and pioglitazone combination therapy was associated with a significantly greater decrease in hepatic fat content as evaluated by MRS when compared to pioglitazone monotherapy, despite a lack of a significant change in body weight. Reduction in plasma levels of ALT and triglycerides (TG) was also significantly greater following the combination therapy with pioglitazone and exenatide. Moreover, the fasting plasma level of fibroblast growth factor-21, an independent predictor of NAFLD, was significantly decreased in the exenatide and pioglitazone combination therapy and was unchanged in the pioglitazone monotherapy[21]. These results indicate an additive effect of exenatide on the TZDs for improving hepatic parameters in T2DM patients with NAFLD.
Analogous to GLP-1R agonists, DPP-4 inhibitors also show potential benefit for NAFLD patients with T2DM. In a small clinical study, 30 NAFLD patients with T2DM were given sitagliptin, a DPP-4 inhibitor. After a 4-month treatment, not only glycaemic control but also serum ALT, aspartate aminotransferase (AST) and γ-glutamyl transpeptidase levels were significantly improved relative to baseline[22].
Evidence from prospective placebo-controlled trials
The Liraglutide Effect and Action in Diabetes (LEAD) program assessed the efficacy and safety of liraglutide therapy on liver parameters in comparison with placebo-controls in a meta-analysis performed using data from six 26-wk, phase-III, and randomised controlled trials in patients with T2DM. In this program, 2241 (50.8%) patients had an abnormal elevation of plasma ALT levels at baseline. Liraglutide dose-dependently reduced the ALT levels in these patients, with similar adverse effects between the liraglutide and control groups. Additionally, in a sub-study of LEAD-2 where hepatic steatosis was measured by computerised tomography scan, once-daily subcutaneous injection of liraglutide (1.8 mg) showed a trend towards improving hepatic steatosis compared with placebo (liver-to-spleen attenuation ratio: 0.10 vs 0.00; P = 0.07). However, this difference became smaller after adjusting for reduction in body weight and HbA1c[6].
Evidence from animal studies
Zhang et al[23] investigated the effect of liraglutide on hepatic lipid accumulation in hypoadiponectinemia ApoE-/- mice fed on HFD. Hepatic steatosis in this animal model was improved histologically, and hepatic TG content, total cholesterol (TC) content and total lipid content were all dramatically reduced by an 8-wk liraglutide treatment. Similar results were found in a high fat and high fructose diet-induced NAFLD model in mice[24].
In agreement with the beneficial effects found in patients with T2DM, exendin-4 (also known as exenatide) therapy led to a reduction in the net weight gain, serum glucose level and insulin resistance in obese mice compared to their counterparts. Moreover, the increased liver weight, elevated hepatic TG content and hepatic steatosis were greatly attenuated by exendin-4 treatment[21,25]. Administration of AC3174, an exendin-4 analogue, to ob/ob mice ameliorated hepatic steatosis and fibrosis histologically in high trans-fat or high lard-fat diet models. Interestingly, this positive effect on the liver endpoints was at least partly body weight-independent. AC3174 treatment significantly reduced liver mass (-14.2%), liver lipid content (-12.9%), and plasma level of ALT and TG, whereas a calorie-restricted, weight-matched group displayed only modest nonsignificant reductions in liver mass (-9%) and liver lipid content (-5.1%) relative to the controls. Moreover, the improvement by AC3174 in liver and lipid parameters were abolished in GLP-1R knockout mice, suggesting the benefits resulted from the exendin-4 analogue was dependent on GLP-1R[26].
Shirakawa et al[27] found that the DPP-4 inhibitor sitagliptin monotherapy improved the diet-induced hepatic steatosis histologically, and significantly decreased the grade of steatosis and hepatic TC contents in both wild-type and pancreatic β-cell-specific glucokinase haplo-insufficient (GCK+/−) diabetic mice. In a DPP-4-deficient rat model, the serum active GLP-1 level was chronically elevated. The DPP-4-deficient rats had markedly less hepatic fat and TG content and exported significantly less TG into the circulation than the wild-type animals. HFD-induced hepatic fat accumulation, blood lipid abnormality, and serum ALT and AST level elevation were much less in the DPP-4-deficient rats than those of the wild-type animals[28,29]. Similar results were also observed in a DPP-4-deficient mouse model[30].
Collectively, the results from both clinical data and animal studies suggest that GLP-1-based therapies may have beneficial effects in the treatment of NAFLD.
Safety issues of GLP-1-based therapies
On the other hand, the safety concerns of GLP-1-based therapies, especially the long-term safety, have been raised with their wide usage in the treatment of T2DM. Two major areas of interest are whether these therapies have an impact on pancreatitis and cancer. Elashoff et al[31] raised safety concerns in an analysis of case reports of pancreatitis, pancreatic cancer and other cancers in patients treated with exenatide or sitagliptin for T2DM. The data showed that the use of sitagliptin or exenatide increased the odds ratio for reported pancreatitis compared with 4 other anti-diabetic medications. Pancreatic cancer was more commonly reported among patients who took sitagliptin or exenatide compared with the other therapies. However, it is noteworthy that Elashoff’s study was based on the United States Food and Drug Administration database containing spontaneous reports of adverse events, where over-reporting biases were highly likely to exist. Moreover, T2DM itself is associated with increased risk of acute pancreatitis and solid tumours, including pancreatic cancer[32,33]. Retrospective case control studies do not associate pancreatitis with GLP-1R agonists or DPP-4 inhibitors[34,35]. In line with the observations, two large-scale endpoint trials have recently shown that the DPP-4 inhibitors saxagliptin and alogliptin do not increase the risk of acute and chronic pancreatitis[36,37]. It has been reported that GLP-1R activation promoted C-cell hyperplasia and medullary thyroid cancer in rodents[38]. However, C cells within the monkey and human thyroid gland exhibit lower levels of GLP-1R expression. Long-term clinical studies of sufficient size and duration to permit conclusions regarding the association of GLP-1-based therapies with cancer have not yet been completed[35].
POTENTIAL MECHANISMS OF GLP-1-BASED THERAPIES IN AMELIORATING NAFLD
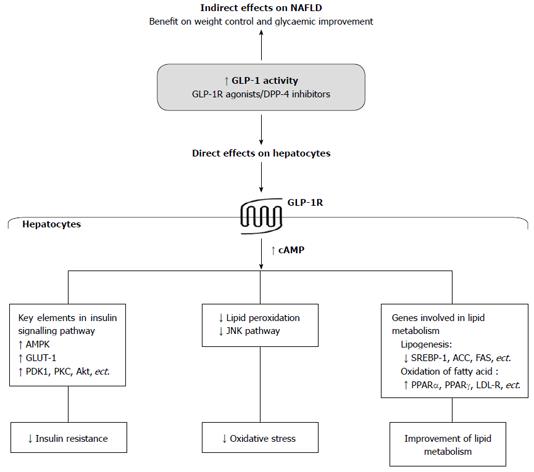
It has been well accepted that GLP-1-based therapies lead to weight control and glycaemic improvement, which are both important for the improvement of NAFLD. Moreover, recent reports have shown that GLP-1-based therapies also have direct effects on the liver (Figure 1).
Figure 1 Potential mechanistic roles of Glucagon-like peptide-1 and Glucagon-like peptide-1-based therapies in non-alcoholic fatty liver disease.
GLP-1: Glucagon-like peptide-1; GLP-1R: Glucagon-like peptide-1 receptor; DPP-4: Dipeptidyl peptidase-4; NAFLD: Non-alcoholic fatty liver disease; cAMP: Cyclic adenosine monophosphate; AMPK: AMP-activated protein kinase; PDK-1: Phosphoinositide-dependent kinase-1; PKC: Protein kinase C; Akt: Protein kinase B; GLUT-1: Glucose transporter-1; JNK: c-Jun N-terminal protein kinase; SREBP-1: Sterol regulatory element-binding protein 1; ACC: Acetyl-CoA carboxylase; FAS: Fatty acid synthase; PPAR: Peroxisome proliferator activator receptor; LDL-R: Low-density lipoprotein receptor.
Functional GLP-1R is expressed in hepatocytes
GLP-1R was detected in human liver biopsies at both mRNA and protein levels, although it was downregulated in patients with NASH[15]. Gupta et al[16] confirmed that GLP-1R was expressed in primary human hepatocytes and in two human hepatocellular carcinoma cell lines, HepG2 and HuH-7. Likewise, GLP-1R was also detectable in rodent hepatocytes[15,25]. Moreover, GLP-1R was present on the cell membrane of human hepatocytes and was internalised upon GLP-1 and exendin-4 stimulation[16]. Both GLP-1 and exendin-4 treatments resulted in a dramatic increase of cyclic adenosine monophosphate (cAMP) production in the hepatocytes. When the hepatocytes were pre-treated with the GLP-1R antagonist exendin (9-39), cAMP production that was induced by either GLP-1 or exendin-4 was significantly reduced to below basal levels[25]. These results indicate that functional GLP-1R is expressed in hepatocytes, and native GLP-1 or GLP-1R agonists may have a direct effect on hepatocytes via activation of GLP-1R.
Attenuation of insulin resistance in the liver by GLP-1-based therapies
Insulin resistance is an essential requirement and is believed to influence “the first hit” in the development of NAFLD. Recent studies have shown that this crucial process in the liver may be attenuated by GLP-1-based therapies.
In rodent NAFLD animal models and isolated primary rodent hepatocytes, either elevated endogenous GLP-1 level or liraglutide treatment was able to enhance activation of AMP-activated protein kinase (AMPK), a critical signal molecule involved in the regulation of hepatic insulin sensitivity[23,28]. GLP-1 overexpression increased insulin receptor substrate 1 (IRS-1) expression in the liver, promoted the hepatic activation of IRS-1 and protein kinase C (PKC) by insulin, and decreased hepatic glucose production (HGP) and hepatic fatty acid synthesis in the diabetic ob/ob mice[39]. In an HFD-induced insulin resistance model of ApoE-/- mice, hypoadiponectinemia induced by siRNA further led to a decrease in glucose infusion rate and insulin’s ability to suppress HGP, accompanied by a reduction in the expression of glucose transporter-1 and elevation in the expression of phosphoenolpyruvate carboxykinase, which is a key enzyme of HGP. Liraglutide treatment completely or partially restored the deterioration in insulin resistance and the alteration of regulatory factors involved in hepatic insulin sensitivity[40]. Another GLP-1R agonist exendin-4 stimulated the phosphorylation of other key elements of the insulin signalling pathway, including phosphoinositide-dependent kinase-1 (PDK-1), protein kinase B (also called Akt) and PKC, in human hepatocellular cell lines. GLP-1R knockdown in the cells abolished the effects of exendin-4 on PDK-1 and PKC[16]. Exendin-4 treatment increased protein kinase A (PKA) activity and Akt phosphorylation in the hepatocytes isolated from NASH rats[15], indicating that the GLP-1R agonist attenuates insulin resistance in the liver of NASH animals.
Suppression of oxidative stress in the liver by GLP-1-based therapies
Exendin-4-treated ob/ob mice exhibited a significant decrease in hepatic level of thiobarbituric reactive substance, an important assessment of lipid peroxidation, compared with saline-treated group[25]. As a key mechanism involved in hepatic oxidative stress, c-Jun N-terminal protein kinase signalling pathway was depressed by treatment with either exenatide[15] or liraglutide[23]. These findings indicate that GLP-1R agonists attenuate liver cell oxidative stress, one of the essential steps in the progression of NAFLD or NASH.
Interestingly, liver cells seem to be able to synthesise and secrete GLP-1 as intestinal L cells. Nobili et al[41] evaluated hepatic progenitor cells (HPCs) in liver biopsies from 30 paediatric NAFLD subjects. They found that the HPC compartment expanded in the paediatric NAFLD individuals relative to healthy controls. GLP-1 expression in the HPCs of the paediatric NAFLD was upregulated, and GLP-1 expression level was associated with the degree of steatosis and NAFLD activity score. Therefore, the authors hypothesised that HPC activation was involved in the response of the liver to oxidative stress in the paediatric NAFLD subjects. GLP-1 may play a protective role in this process by inducing the resistance of HPCs to oxidative stress, favouring their proliferation and differentiation.
Regulation of lipid metabolism-related gene expression in the liver by GLP-1-based therapies
GLP-1-treated hepatocytes presented a significant increase in cAMP production and an alteration of gene expression profile, including increased expression of both peroxisome proliferator activator receptor α (PPAR-α) and acetyl-CoA oxidase, as well as decreased expression of stearoyl-CoA desaturase 1, sterol regulatory element-binding protein 1 (SREBP-1) and acetyl-CoA carboxylase (ACC)[25]. In ob/ob mice, overexpression of GLP-1 reduced hepatic expression of fatty acid synthase (FAS)[39]. These findings suggest that GLP-1 impairs hepatocyte lipogenesis and enhances β-oxidation of fatty acids. Moreover, pre-treatment with exendin (9-39) abolished the effects of GLP-1 on the expression of these genes, suggesting that GLP-1R is required in this regulatory process[25].
Similarly, GLP-1R agonists are also effective in modulating the expression of these aforementioned genes. Liraglutide downregulated the expression of ACC and FAS in the liver of NAFLD mice[23] and restored hepatic transcripts of PPAR-α, low-density lipoprotein receptor and insulin-induced geng-2, which were downregulated by hypo-adiponectin in a NAFLD model[40]. Exenatide increased PPAR-γ expression and determined a PKA-dependent increase of PPAR-α activity in rats with NASH. The regulatory effect of exenatide on the expression of genes related to fatty acid β-oxidation was abolished by either PKA or AMPK inhibitors[15], indicating that the effects of the GLP-1R agonists on lipid metabolism are mediated via PKA and AMPK signalling pathway.
In mice fed on HFD, the DPP-4 inhibitor vildagliptin reduced hepatic expression of phosphomevalonate kinase and farnesyl diphosphate transferase 1, which were important for cholesterol synthesis[42]. In DPP-4-deficient mice, elevated GLP-1 level was associated with depressed SREBP-1 expression in hepatocytes[30].
Taken together, these findings indicate that GLP-1-based agents are able to regulate the expression of hepatic genes that are important for lipid metabolism.
Other effects of GLP-1-based therapies that may be beneficial for NAFLD
Liraglutide downregulated the expression of proinflammatory cytokines and transcription factors, including tumour necrosis factor α and nuclear factor κB, in the liver tissues of NAFLD[23]. In fat-loaded primary human hepatocytes, exendin-4 restored the change in endoplasmic reticulum stress markers, GPR78 and C/EBP homologous protein. Moreover, exendin-4 clearly promoted hepatocyte autophagy-associated events, as shown by enhanced production of both beclin-1 and LC3B-II detected by western blot and increased autophagic vacuoles visualised by transmission electron microscopy. As a result, the survival of hepatocytes in the fat load was promoted, and apoptosis was inhibited by exendin-4 treatment. Similar observations were made in mouse liver lysates after mice were fed on a high-fat, high-fructose diet and treated with liraglutide[24].
CONCLUSION
NAFLD has been reported to be associated with defective GLP-1 signalling. GLP-1-based therapies were effective in improving hepatic endpoints in NAFLD, such as reducing hepatic fat content, hepatic steatosis and plasma transaminase, and preventing fibrosis. Apart from the benefits in controlling body weight and blood glucose, GLP-1-based agents may directly exert an action on the liver through activation of functional GLP-1R in hepatocytes. The possible mechanisms include regulating the expression of genes associated with insulin resistance and lipid metabolism, and suppressing oxidative stress in the liver cells, thus preventing the development and progression of NAFLD. With these promising findings, large-scale randomised controlled trials are warranted to investigate the efficacy and safety of GLP-1-based therapies in the treatment of patients with NAFLD.