Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9055
Revised: February 7, 2014
Accepted: May 12, 2014
Published online: July 21, 2014
Processing time: 237 Days and 17.3 Hours
Over the last two decades, the rise in the prevalence rates of overweight and obesity explains the emergence of nonalcoholic fatty liver disease (NAFLD) as the leading cause of chronic liver disease worldwide. As described in adults, children and adolescents with fatty liver display insulin resistance, glucose intolerance, and dyslipidemia. Thus NAFLD has emerged as the hepatic component of the metabolic syndrome (MetS) and a strong cardiovascular risk factor even at a very early age. Several studies, including pediatric populations, have reported independent associations between NAFLD and markers of subclinical atherosclerosis including impaired flow-mediated vasodilation, increased carotid artery intima-media thickness, and arterial stiffness, after adjusting for cardiovascular risk factors and MetS. Also, it has been shown that NAFLD is associated with cardiac alterations, including abnormal left ventricular structure and impaired diastolic function. The duration of these subclinical abnormalities may be important, because treatment to reverse the process is most likely to be effective earlier in the disease. In the present review, we examine the current evidence on the association between NAFLD and atherosclerosis as well as between NAFLD and cardiac dysfunction in the pediatric population, and discuss briefly the possible biological mechanisms linking NAFLD and cardiovascular changes. We also address the approach to treatment for this increasingly prevalent disease, which is likely to have an important future global impact on the burden of ill health, to prevent not only end-stage liver disease but also cardiovascular disease.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is an important and emerging health problem in childhood. It is recognized as part of the metabolic syndrome and especially the necroinflammatory form is associated with a high risk for the development of functional and structural vascular changes as well as left ventricular dysfunction at an early age. In addition, there seems to be a complex bidirectional relationship between the progression to nonalcoholic steatohepatitis (NASH) and the development of insulin resistance and cardiovascular abnormalities. Early intervention during childhood to recognize NAFLD, as well as to prevent its progression to NASH, may be a crucial step in averting an unfavorable cardiac phenotype.
- Citation: Pacifico L, Chiesa C, Anania C, Merulis AD, Osborn JF, Romaggioli S, Gaudio E. Nonalcoholic fatty liver disease and the heart in children and adolescents. World J Gastroenterol 2014; 20(27): 9055-9071
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9055
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of fat-associated liver conditions that can result in end-stage liver disease and the need for liver transplantation[1]. Simple steatosis, or fatty liver, occurs early in NAFLD and may progress to nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis with increased risk of hepatocellular carcinoma[1]. Over the last two decades, the rise in the prevalence rates of overweight and obesity explains the emergence of NAFLD as the leading cause of chronic liver disease in pediatric populations worldwide[2,3]. The liver is one of the main ectopic sites where lipids may accumulate in obese subjects. Ectopic fat disposition occurs particularly when the energy storage capacity of the adipose tissue is exceeded, leading to increased net lipid flux to non-adipose organs, thereby causing lipotoxiciy and insulin resistance[4,5]. As described in adults, children and adolescents with fatty liver display insulin resistance, glucose intolerance, and dyslipidemia[6,7]. Thus NAFLD has emerged as the hepatic component of the metabolic syndrome (MetS)[8] and a strong cardiovascular risk factor even at a very early age[9,10].
Several studies (including pediatric populations) have reported independent associations between NAFLD and markers of subclinical atherosclerosis such as impaired flow-mediated vasodilation (FMD), increased carotid artery intima-media thickness (cIMT) and arterial stiffness, after adjusting for cardiovascular risk factors and MetS[9-14]. Also, it has been shown that NAFLD is associated with cardiac alterations, including myocardial insulin resistance[15], altered cardiac energy metabolism[16], abnormal left ventricular (LV) structure and impaired diastolic function[17,18]. The duration of these subclinical abnormalities may be important, because treatment to reverse the process is most likely to be effective earlier in the disease. In the present review, we examine the current evidence on the association between NAFLD and subclinical atherosclerosis as well as between NAFLD and cardiac dysfunction in the pediatric population, and discuss briefly the possible biological mechanisms linking NAFLD and cardiovascular changes. We also address the approach to treatment for this increasingly prevalent disease, which is likely to have an important future global impact on the burden of ill health, to prevent not only end-stage liver disease but also cardiovascular disease (CVD).
This is a clinical, narrative review and not a systematic review and meta-analysis. PubMed was extensively searched for articles using keywords and mesh terms: “nonalcoholic fatty liver disease”, “fatty liver”, “cardiovascular risk”, “atherosclerosis”, “endothelial dysfunction”, arterial stiffness”, “cardiac structure”, “cardiac dysfunction”, and “children”.
Pathologic studies have shown that atherosclerosis is an early process beginning in childhood, with fatty streaks observed in the aorta and the coronary and carotid arteries of children and adolescents[19,20]. Early assessment of the arterial damage is therefore important to prevent future vascular risk since subclinical atherosclerosis can be reversible if detected early and intervention is provided[10].
Several studies have focused on the relation between NAFLD and atherosclerosis in the pediatric population (Table 1)[9,14,21-36]. In the earliest study, involving 817 children (aged 2 to 19 years) who died of external causes (accident, homicide, suicide) from 1993 to 2003, Schwimmer et al[21] showed that the prevalence of atherosclerosis was increased by a factor of 2 among those with NAFLD. Fatty liver was present in 15% of the children. Mild atherosclerosis was present in 21% of the children, and moderate to severe atherosclerosis in 2%. Atherosclerosis was significantly more common in children with fatty liver than those without. Body mass index (BMI) was not independently associated with the presence of atherosclerosis, but fatty liver status and BMI did interact significantly (P < 0.01). Consequently, for obese subjects, the odds of having atherosclerosis was more than 6 times higher in children with fatty liver than those without[21]. Despite this, there are few data regarding the possible association between liver histopathologic changes and atherogenic risk in children.
| Ref. | Study population and sample size | Diagnosis | Outcome | Main results |
| Schwimmer et al[21] | Children (n = 817) who died of external causes from 1993 to 2003; 15% with NAFLD | Autoptic liver biopsy | Atherosclerosis was assessed as absent, mild (aorta only), moderate (coronary artery streaks/plaques), or severe (coronary artery narrowing) | For the entire cohort, mild atherosclerosis was present in 21% and moderate to severe in 2%. Atherosclerosis was significantly more common in children with fatty liver than those without (30% vs 19%, P < 0.001) |
| Schwimmer et al[9] | Overweight children with (n = 150), and without (n = 150) NAFLD, matched for gender, age, and severity of obesity | Liver biopsy | Prevalence of cardiovascular risk factors (abdominal obesity, dyslipidemia, hypertension, IR, and glucose abnormalities) | NAFLD was strongly associated with multiple cardiovascular risk factors independently of both BMI and hyperinsulinemia |
| Pacifico et al[22] | Obese children with (n = 29), and without NAFLD (n = 33); healthy lean controls (n = 30) | Liver ultrasound | cIMT, mean (95%CI) | NAFLD vs no NAFLD and controls: 0.58 (0.54-0.62) mm vs 0.49 (0.46-0.52) mm and 0.40 (0.36-0.43) mm; P < 0.01 and P < 0.0005, respectively Log cIMT was associated with NAFLD severity in a multiple linear regression analysis adjusted for age, gender, Tanner stage, and cardiovascular risk factors Coefficient b, 0.08; P < 0.0005 |
| Demircioğlu et al[23] | Obese children with mild (n = 32), moderate-severe NAFLD (n = 22), and without NAFLD (n = 26); healthy lean controls (n = 30) matched for age and gender | Liver ultrasound | cIMT, mean ± SD | All obese vs controls: |
| Left CCA, 0.414 ± 0.071 mm vs 0.352 ± 0.054 mm, P < 0.0001 | ||||
| Left CB, 0.412 ± 0.067 mm vs 0.350 ± 0.058 mm, P < 0.0001 | ||||
| Left ICA, 0.324 ± 0.068 mm vs 0.266 ± 0.056 mm, P < 0.0001 | ||||
| NAFLD was significantly associated with left CCA, CB, ICA in multiple regression linear analyses adjusted for age, gender, weight, mean ALT level, TC, obesity, and grade of hepatosteatosis | ||||
| CCA = standardized β, 0.451; P = 0.01 | ||||
| CB = standardized β, 0.627; P < 0.0001 | ||||
| ICA = standardized β, 0.501; P = 0.020 | ||||
| Kelishadi et al[24] | Obese adolescents with (n = 25), and without (n = 25) components of MetS; normal weight adolescents with (n = 25) and without (n = 25) components of MetS | Liver ultrasound and elevated ALT | cIMT, mean ± SD | NWMN vs NWMA vs POMN vs POMA: 0.29 ± 0.02 mm vs 0.37 ± 0.04 mm vs 0.41 ± 0.05 mm; the differences were significant between groups with the exception of NWMA vs POMN |
| cIMT was significantly associated with NAFLD in a logistic regression analysis after adjustment for age, gender and pubertal status | ||||
| Odds ratio, 1.2 (95%CI: 1.03-2.1) | ||||
| Manco et al[25] | Overweight and obese children with (n = 31), and without (n = 49) NAFLD, matched for age, gender, and BMI | Liver biopsy | cIMT, median (IQR) | NAFLD vs no NAFLD: |
| Right cIMT, 0.47 (0.07) mm vs 0.48 (0.05) mm, P = 0.659 | ||||
| Left cIMT, 0.49 (0.12) mm vs 0.47 (0.05) mm, P = 0.039 | ||||
| NAFLD was not associated with cIMT in a multivariate analysis | ||||
| Caserta et al[26] | Randomly selected adolescents (n = 642) of whom 30.5% and 13.5% were, respectively, overweight and obese. Overall prevalence of NAFLD, 12.5% | Liver ultrasound | cIMT, mean (95%CI) | NAFLD vs no NAFLD: 0.417 (0.409-0.425) mm vs 0.395 (0.392-0.397) mm, P < 0.001 |
| NAFLD was significantly associated with cIMT in a multivariate analysis after adjustment for age, BP, BMI, TG, c-HDL, TC, IR, MetS, grade of steatosis | ||||
| Standardized β, 0.0147 (95%CI: 0.0054-0.0240); P = 0.002 | ||||
| Pacifico et al[14] | Obese children with (n = 100), and without (n = 150) NAFLD; healthy lean controls (n = 150) | Liver ultrasound and elevated ALT | cIMT and FMD, mean (95%CI) | Controls and no NAFLD vs NAFLD: cIMT, 0.47 (0.46-0.48) mm and 0.52 (0.50-0.54) mm vs 0.55 (0.53-0.54) mm, P < 0.0001 and P < 0.01, respectively |
| FMD, 15.0 (13.9-17.3) and 11.8 (10.1-13.7) vs 6.7 (5.0-8.6) %, P < 0.01 and P < 0.001 respectively | ||||
| NAFLD was associated with low FMD and increased cIMT in a multiple logistic regression analysis after adjustment for age, gender, Tanner stage, and MetS | ||||
| Odds ratio, 2.31 (95%CI: 1.35-3.97); P = 0.002 and 1.99 (95%CI: 1.18-3.38); P = 0.010, respectively | ||||
| Nobili et al[27] | Children with NAFLD (n = 118) | Liver biopsy | Atherogenic lipid profile (TG/HDL-c, TC/HDL-c and LDL-c/HDL-c ratios) | The severity of liver injury was strongly associated with a more atherogenic profile, independently of BMI, insulin resistance, and the presence of MetS |
| Weghuber et al[28] | Obese children with (n = 14), and without (n = 14) NAFLD | Proton MR spectroscopy | FMD, mean ± SD | NAFLD vs no NAFLD: 108.6% ± 11.8% vs 110.7% ± 9.0%; P = 0.41 |
| El-Koofy et al[29] | Overweight/obese children (n = 33) | Liver biopsy | Atherogenic lipid profile (TC, LDL-c, HDL-c, TG) | Children with NAFLD had significantly higher TC, LDL-c, TG and lower HDL-c compared to patients with normal liver histology (P < 0.05) |
| Sert et al[30] | Obese children with (n = 44), and without (n = 36) NAFLD; lean subjects (n = 37) | Liver ultrasound and elevated ALT | cIMT, mean ± SD | Lean and no NAFLD vs NAFLD: 0.0359 ± 0.012 mm vs 0.378 ± 0.017 mm vs 0.440 ± 0.026 mm, P < 0.05 and P < 0.05, respectively |
| Akın et al[31] | Obese children with (n = 56), and without (n = 101) NAFLD | Liver ultrasound | cIMT, mean (95%CI) | NAFLD vs no NAFLD: 0.48 (0.47-0.49) mm vs 0.45 (0.44-0.45) mm, P < 0.001 |
| NAFLD was the only variable associated with increased cIMT in a multiple regression adjusted for age and gender | ||||
| β, 0.031 [SE (β) = 0.008]; P < 0.001 | ||||
| Gökçe et al[32] | Obese children with (n = 50), and without (n = 30) NAFLD; healthy lean controls (n = 30) | Liver ultrasound | cIMT, mean ± SD | NAFLD vs no NAFLD vs control group: |
| Right cIMT, 0.46 ± 0.21 mm vs 0.35 ± 0.09 mm vs 0.30 ± 0.13 mm, P < 0.01 | ||||
| Left cIMT, 0.44 ± 0.09 mm vs 0.35 ± 0.08 mm vs 0.27 ± 0.04 mm, P < 0.01 | ||||
| NAFLD was the only variable associated with increased cIMT in a multiple regression adjusted for age, gender, BMI, BP, TG, HDL-c, IR and MetS | ||||
| Right cIMT = β, 0.241; P < 0.05 | ||||
| Left cIMT = β, 0.425; P < 0.01 | ||||
| Sert et al[33] | Obese children with (n = 97), and without (n = 83) NAFLD; lean subjects (n = 68) | Liver ultrasound and elevated ALT | cIMT, mean ± SD | Lean and no NAFLD vs NAFLD: 0.354 ± 0.009 mm vs 0.383 ± 0.019 mm vs 0.437 ± 0.028 mm; P < 0.05 and P < 0.05, respectively |
| Alp et al[34] | Obese children with (n = 93), and without (n = 307) NAFLD; healthy lean controls (n = 150) | Liver ultrasound | cIMT, mean ± SD | Severe NAFLD vs mild NAFLD vs no NAFLD vs controls: 0.09 ± 0.01 cm vs 0.10 ± 0.01 cm vs 0.09 ± 0.01 cm vs 0.06 ± 0.01 cm, P < 0.001 |
| Huang et al[35] | Adolescents (n = 964) | Liver ultrasound | PWV, mean ± SD | No NAFLD, low metabolic risk vs NAFLD, low metabolic risk vs no NAFLD, high metabolic risk vs NAFLD, high metabolic risk: males, 6.6 ± 0.7 m/s vs 6.7 ± 0.6 m/s vs 6.9 ± 1.0 m/s; females, 6.2 ± 0.7 m/s vs 6.3 ± 0.7 m/s vs 6.5 ± 0.7 m/s vs 6.4 ± 0.6 m/s |
| Males and females who had NAFLD in the presence of the metabolic cluster had greater PWV | ||||
| b, 0.20 (95%CI: 0.01-0.38); P = 0.037 | ||||
| Jin et al[36] | Obese children (n = 71), and healthy controls (n = 47) | Liver ultrasound | PWV, mean ± SD | Obese vs controls: 4.54 ± 0.66 m/s vs 3.70 ± 0.66 m/s, P < 0.001 |
| Fatty liver was positively correlated with PWV (P < 0.01) |
In the Bogalusa Heart study in children, investigators found that the extent to which the intimal surface was covered with atherosclerotic lesions was significantly associated with elevation of concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), triglycerides (TG), and lower concentration of high-density lipoprotein cholesterol (HDL-c)[37]. In particular, the TC/HDL-c, LDL-c/HDL-c, and TG/HDL-c ratios have been reported as useful markers of atherogenic lipid abnormalities, as well as of insulin resistance, MetS, and high cardiovascular risk[38,39]. Schwimmer et al[9] in a case-control study involving a large clinical sample of overweight and obese children and adolescents, showed that children with a biopsy-proven NAFLD had a significantly higher fasting glucose, insulin, TC, LDL-c, TG, systolic and diastolic blood pressure than age-, gender-, and BMI-matched peers without NAFLD. Thus, obese children and adolescents with a definitive diagnosis of NAFLD had a more severe cardiovascular risk profile. Nobili et al[27], in a large group of consecutively recruited children with liver biopsy-proven NAFLD, found that the NAFLD activity and fibrosis scores had significant positive correlations with TG/HDL-c, TC/HDL-c, and LDL-c/HDL-c ratios. After adjusting for potential confounders including BMI, insulin resistance, impaired glucose tolerance, and presence of MetS, both NAFLD activity score and stage of fibrosis remained independent predictors of an atherogenic lipid profile. The lipid ratios were found to be markedly higher in children with established NASH compared with those with simple steatosis or borderline disease, indicating that severity of liver injury in children with NAFLD is strongly associated with increased atherogenic risk. Very recently, El-Koofy et al[29] studied the prevalence of MetS, insulin resistance, and NAFLD in a small group of overweight/obese children presenting with hepatomegaly and/or raised alanine aminotransferase. Laboratory analysis included fasting blood glucose, serum insulin, TG, HDL-c, LDL-c and liver biochemical profile in addition to liver biopsy. They found a close association between obesity, MetS, insulin resistance and NAFLD. Children with NAFLD had significantly higher TC, LDL-c, TG, fasting insulin, and lower HDL-c compared to patients with normal liver histology.
Recent improvements in imaging technology have identified early vascular changes that can be assessed noninvasively using ultrasonography[40-42]. These early changes include impairment of FMD, arterial stiffness, and increased cIMT. Measurement of these changes have been shown to be good surrogate markers for atherosclerosis disease identification and progression as well as of future clinical cardiovascular events[43,44].
Pacifico et al[22] first showed that severity of ultrasonographycally diagnosed NAFLD in obese children was significantly associated with cIMT, independently of anthropometric and metabolic features. Similarly, Demircioğlu et al[23], in a subsequent study, observed an association between ultrasonographically detected NAFLD and cIMT measured at the common artery, carotid bulb and internal carotid artery. Moreover, there was an increase in cIMT values of each segment with the increase in grades of hepatosteatosis. Kelishadi et al[24] reported a significant association between cIMT and NAFLD in children and adolescents, suggesting that the liver and the vessels share common mediators. In a case-control study involving a mixed population of 80 overweight and mildly obese children of whom 31 had biopsy-proven NAFLD, and 49 had no ultrasound evidence of NAFLD as well as no abnormal levels of aminotransferases, Manco et al[25] found that cIMT were significantly higher on the left side in NAFLD cases, though there was a substantial overlap of cIMT values between cases and controls. No association was found between cIMT and histologic severity of steatosis, NAFLD activity score, and fibrosis.
The association between NAFLD and carotid atherosclerosis has also been determined in a large, randomly selected adolescent population from Reggio Calabria, a town in southern Italy[26]. The authors found that NAFLD, as well as BMI, waist circumference, and systolic blood pressure were independent markers of increased cIMT. In a study involving a large sample size, Pacifico et al[14] showed that obese children with ultrasound-diagnosed NAFLD have a significantly lower FMD response and increased cIMT compared to obese children without NAFLD independently of other cardiovascular risk factors and MetS, and that obese children exibit more functional and morphologic vascular changes than healthy lean controls, regardless of liver involvement. The large number of subjects in that study may in part account for the associations the authors were able to identify between NAFLD and functional vascular changes, in contrast to the study by Weghuber et al[28] in which a very small sample of obese children with NAFLD [diagnosed by nuclear magnetic resonance spectroscopy (MRS)] had a FMD response similar to those without NAFLD. In a recent study, Akın et al[31] demonstrated that obese children and adolescents with ultrasonographycally detected NAFLD had higher cIMT than those without NAFLD regardless of association with elevated liver enzymes. Moreover, there was a statistically significant correlation between cIMT values and the grade of NAFLD. Similar results were obtained by Gökçe et al[32] and by Sert et al[30,33] in obese children with ultrasound diagnosed NAFLD. Gökçe et al[32] found that cIMT was significantly higher in obese with NAFLD than in obese children without NAFLD and in a control group. After adjusting for potential confounders (age, blood pressure, BMI, TG, HDL-c, insulin resistance, and MetS), NAFLD was observed to be strongly correlated with cIMT. Sert and colleagues[30,33] confirmed that children with NAFLD have significantly higher cIMT values than those without NAFLD and the lean group. Components of MetS, such as dyslipidemia, elevated fasting glucose levels and insulin resistance did not show a significant association with increased cIMT. Finally, in a very recent study, Alp et al[34] demonstrated that cIMT was significantly higher in children with ultrasonographycally detected NAFLD than the control subjects. Additionally, there was an increase in cIMT values with the increase in grades of liver steatosis.
A few pediatric studies have analyzed the relationship between NAFLD and carotid artery stiffness. In a well-defined community-based cohort of Australian adolescents, Huang et al[35] aimed to examine the association between NAFLD (diagnosed by ultrasound), MetS, and arterial stiffness as measured by applanation tonometry. The enrolled subjects were identified at “high metabolic risk” and at “low metabolic risk” according to systolic blood pressure, homeostasis model assessment of insulin resistance (HOMA-IR), TG, and BMI. The authors found that NAFLD is associated with increased arterial stiffness only in the presence of the “high risk” metabolic cluster. Jin et al[36], in an attempt to identify a marker of early vascular functional change in obese children, compared carotid artery stiffness parameters (i.e., compliance coefficient, stiffness index, and pulse wave velocity, obtained with ultrasound radiofrequency technology) in obese children and healthy controls. Arterial stiffness was higher in obese children than in healthy subjects; in addition the authors demonstrated that in obese children the carotid arterial stiffness parameters, especially the pulse wave velocity, were correlated with obesity-related risk factors, the systolic blood pressure, and the presence of fatty liver.
Information regarding abnormalities in cardiac function among NAFLD patients is limited in both adults and children. Moreover, the data are conflicting.
Table 2 summarizes the studies on the effects of NAFLD on cardiac metabolism, structure and function in adults[15-17,45-50]. Goland et al[17] have shown a markedly impaired diastolic function and mild alterations in LV structure in 38 adult patients with (ultrasound-diagnosed, n = 27; biopsy-proven, n = 11) NAFLD, in the absence of diabetes, hypertension, and morbid obesity. Using multivariate analysis, early diastolic myocardial velocity on tissue Doppler imaging (TDI) was the only independent index able to characterize patients with NAFLD. Similar findings have been later reported by Fotbolcu et al[18] in 35 nondiabetic, normotensive adult patients with ultrasound-diagnosed NAFLD. However, independent predictors of LV impairment were not determined. Recently, in a study examining cardiac status by high resolution magnetic resonance imaging (MRI) in a clinical group of 19 adult patients with NAFLD (defined as > 5% intrahepatic lipid on MRS), Hallsworth et al[49] demonstrated significant changes in cardiac structure and evidence of early diastolic dysfunction in the 19 patients with NAFLD compared to the 19 age-, gender-, and BMI-matched controls, in the absence of cardiac metabolic changes or overt cardiac disease. There was no correlation between blood pressure and cardiac parameters. Finally, Karabay et al[50] found that patients with biopsy-proven NAFLD have evidence of subclinical myocardial dysfunction. However, no significant differences were found among NAFLD groups (i.e., simple steatosis, borderline NASH, and definite NASH). The absence of differences in cardiac function between subgroup patients may be explained by similar HOMA-IR values. Conversely, Perseghin et al[16] showed that 21 men with higher intrahepatic fat content, as measured by MRS, had excessive fat accumulation in the epicardial area and abnormal LV energy metabolism compared to the 21 men matched for anthropometric features with lower intrahepatic fat content. These alterations were detected despite normal LV morphology and function by cardiac MRI. Similarly, in a study using cardiac MRI and involving 61 diabetic male subjects, Rijzewijk et al[46] found that, compared with the 29 men with lower intrahepatic fat content on MRS, the 32 patients with higher intrahepatic fat content had decreased myocardial perfusion, glucose uptake, and high-energy phosphate metabolism but similar values of LV function and morphology.
| Ref. | Study population and sample size | Diagnosis | Outcomes | Main results |
| Lautamäki et al[15] | T2DM and coronary artery disease patients with (n = 27), and without (n = 28) fatty liver. The 2 groups were matched for age, BMI, and fasting plasma glucose | Hepatic MRS | Myocardial insulin resistance and perfusion (PET) | In patients with T2DM and coronary artery disease, liver fat is an indicator of myocardial insulin resistance and reduced coronary functional capacity |
| Goland et al[17] | Nondiabetic, normotensive patients with NAFLD (n = 38), and age and gender-matched controls (n = 25) | Liver ultrasound and liver biopsy in a subgroup of 11 NAFLD patients | LV structure and function (M-mode echocardiography; and pulsed Doppler echocardiography) | Patients with NAFLD had mild changes in cardiac geometry (thickening of the interventricular septum and posterior wall, and increased LV mass) as well as significant differences in parameters of diastolic function compared with the control group |
| Perseghin et al[16] | Young nondiabetic men matched for anthropometric features with (n = 21) or without (n = 21) fatty liver | MRS | LV morphology and function; Intrapericardial and extrapericardial fat content; and resting LV energy metabolism (Cardiac MRI and cardiac 31P-MRS) | Newly found young individuals with fatty liver had excessive fat accumulation in the epicardial area and abnormal LV energy metabolism despite normal LV morphological features and systolic and diastolic functions |
| Fallo et al[45] | Never-treated essential hypertensive patients with (n = 48) or without (n = 38) fatty liver. The 2 groups were similar as to gender, age and blood pressure levels | Liver ultrasound | LV structure and function (M-mode echocardiography; and pulsed Doppler echocardiography) | NAFLD patients had similar prevalence of LV hypertrophy compared to subjects without NAFLD, but a higher prevalence of LV diastolic dysfunction |
| Rijzewijk et al[46] | T2DM patients with (n = 32) and without (n = 29) fatty liver | MRS | Cardiac perfusion and substrate metabolism; LV morphology and function (PET, cardiac MRI and cardiac 31P-MRS) | T2DM patients with fatty liver showed decreased myocardial perfusion, glucose uptake, high-energy phosphate metabolism compared with similar patients without hepatic steatosis |
| Fotbolcu et al[18] | Nondiabetic, normotensive patients with NAFLD (n = 35) and control subjects (n = 30). The 2 groups were similar as to gender and age | Liver ultrasound | LV structure and function (M-mode echocardiography; Pulsed and Tissue Doppler echocardiography) | Patients with NAFLD had changes in cardiac geometry (thickening of the interventricular septum and posterior wall, and increased LV mass) as well as significant differences in parameters of systolic and diastolic function compared with the control group |
| Bonapace et al[47] | T2DM patients with (n = 32) and without (n = 18) fatty liver. The 2 groups were similar as to gender, age, BMI, waist circumference, and diabetes duration | Liver ultrasound | LV structure and function (M-mode echocardiography; Pulsed and Tissue Doppler echocardiography) | T2DM patients with fatty liver showed LV diastolic dysfunction, even if the LV morphology and systolic function were preserved |
| Mantovani et al[48] | Hypertensive T2DM patients with (n = 59) and without (n = 57) fatty liver | Liver ultrasound | LV structure (M-mode echocardiography) | Hypertensive T2DM patients with NAFLD have a remarkably higher frequency of LV hypertrophy than hypertensive diabetic patients without NAFLD |
| Hallsworth et al[49] | Adult subjects matched for anthropometric features with (n = 19) or without (n = 19) fatty liver | MRS | Cardiac structure, function, and metabolism (cardiac MRI, cardiac tagging, and cardiac 31P-MRS) | The major findings in NAFLD patients compared to controls were: thickening of the cardiac wall, independent of changes in LV mass; altered myocardial strains; concentric remodeling; evidence of diastolic dysfunction; but no significant difference in cardiac energetics |
| Karabay et al[50] | NAFLD patients (n = 55) and healthy controls (n = 21; normal laboratory values and liver ultrasound) | Liver biopsy | LV structure and function (M-mode echocardiography; Pulsed and Tissue Doppler echocardiography; and speckle tracking echocardiography) | Patients with NAFLD had changes in cardiac geometry (thickening of the interventricular septum and posterior wall, and increased LV mass) as well as significant differences in parameters of diastolic function compared with the control group |
| LV global longitudinal strain and strain rate in systole were lower in NAFLD group as compared to controls; however no significant differences were found among NAFLD groups (i.e., simple steatosis, borderline NASH, and definite NASH) |
In the pediatric population, information on the relationship between NAFLD and cardiac structure and function is very scant (Table 3)[30,33,34,51-53]. In the earliest study by Sert et al[30], increased LV mass was found in adolescents with NAFLD compared to both lean controls and obese subjects without liver involvement. Similar results were obtained in a subsequent study by the same authors[33]. In a study including 93 obese children with ultrasound-diagnosed NAFLD, 307 obese subjects without liver involvement, and 150 age- and gender-matched healthy controls, Alp et al[34] showed that subclinical systolic and diastolic impairment could be detected by TDI in obese children with NAFLD. Also, cardiac dysfunction was correlated with the increase in grades of liver steatosis. Recently, Singh et al[51] measured by 2-dimensional speckle tracking echocardiography myocardial function in 3 groups of age-, gender-, and Tanner stage-matched adolescents [lean (n = 14); obese with normal (n = 15) or increased (n = 15) intrahepatic triglyceride (IHTG) content (≥ 5.6%)]. The authors showed that obese adolescents with increased IHTG had greater impairment of systolic and diastolic function, manifested by decreased systolic and diastolic myocardial strain and strain rate than BMI-SD score matched obese adolescents with normal IHTG content. The cardiac functional abnormalities were independently associated only with HOMA-IR, after adjustment for BMI, conventional cardiovascular risk factors, and intra-abdominal, intra-cardiac, and intra-hepatic fat content. However, given the small number of adolescents included in their study, the possibility of a type 2 error raised by the authors themselves is possible. In a more recent study, Pacifico et al[52] showed that obese children with NAFLD have features of early LV diastolic and systolic dysfunction, as measured by two-dimensional echocardiography using TDI, compared to obese children without NAFLD and lean controls. Notably, when the group of obese subjects was divided according to the presence of NASH, it was evident that some functional cardiac differences were more pronounced in the group of NASH. A major finding of this study was that the echocardiographic features of early LV diastolic and systolic dysfunction were significantly associated with NAFLD independently of several metabolic variables.
| Ref. | Study population and sample size | Diagnosis | Outcomes | Main results |
| Sert et al[30] | Obese adolescents with (n = 44), and without (n = 36) NAFLD; and control subjects (n = 37) | Liver ultrasound and elevated serum alanine aminotransferase | LV structure (M-mode echocardiography) | Increased LV mass was found in NAFLD group compared to both lean controls and obese subjects without NAFLD |
| Alp et al[34] | Obese children and adolescents with (n = 93), and without (n = 307) NAFLD matched for gender and age; and control subjects (n = 150) | Liver ultrasound | LV structure and function; Epicardial fat (M-mode echocardiography; Pulsed and Tissue Doppler echocardiography) | Increased end-systolic thickness of the interventricular septum, and larger LV mass, as well as LV systolic and diastolic dysfunction were found in NAFLD group. In addition, obese children with NAFLD had increased epicardial fat thickness |
| Singh et al[51] | Obese children and adolescents with (n = 15), and without (n = 15) NAFLD matched for age, gender, Tanner stage, and BMI z score; and control subjects (n = 15) matched for gender, age, and Tanner stage | Hepatic MRS | LV structure and function; Intracardiac triglyceride content (Integrated backscatter ultrasonography and speckle tracking echocardiography; cardiac MRS) | LV global longitudinal strain and early diastolic strain rates were significantly decreased in obese children with NAFLD compared to both lean controls and obese subjects without NAFLD. Intracardiac triglyceride content was not different among the 3 groups |
| Sert et al[33] | Obese adolescents with (n = 97), and without (n = 83) NAFLD; and control subjects (n = 68) | Liver ultrasound and elevated serum alanine aminotransferase | LV structure and function (M-mode echocardiography; Pulsed and Tissue Doppler echocardiography) | Obese adolescents with NAFLD exhibited increased LV dimensions and mass, as well as LV diastolic dysfunction |
| Pacifico et al[52] | Obese children and adolescents with (n = 54), and without (n = 54) NAFLD matched for age, gender, pubertal status, and BMI-SD score; and healthy control subjects (n = 18) matched for gender, age, and pubertal status | Hepatic magnetic resonance imaging; and liver biopsy in a subgroup of 41 NAFLD patients | LV structure and function; Epicardial fat (M-mode echocardiography; Pulsed and Tissue Doppler echocardiography) | Increased interventricular septum thickness at end-diastole and at end-systole, as well as LV systolic and diastolic dysfunction were found in NAFLD group. Children with more severe liver histology had worse LV dysfunction than those with more mild liver changes. NAFLD group had also increased epicardial fat thickness |
| Fintini et al[53] | Children with biopsy-proven NAFLD (n = 50). No patients without NAFLD, and no healthy control children were included | Liver biopsy | LV structure and function (M-mode echocardiography; and pulsed Doppler echocardiography) | About 35% (n = 18) of the 50 children with NAFLD had LV hypertrophy. Children with NASH showed, almost invariably, the presence of clear cut LV hypertrophy |
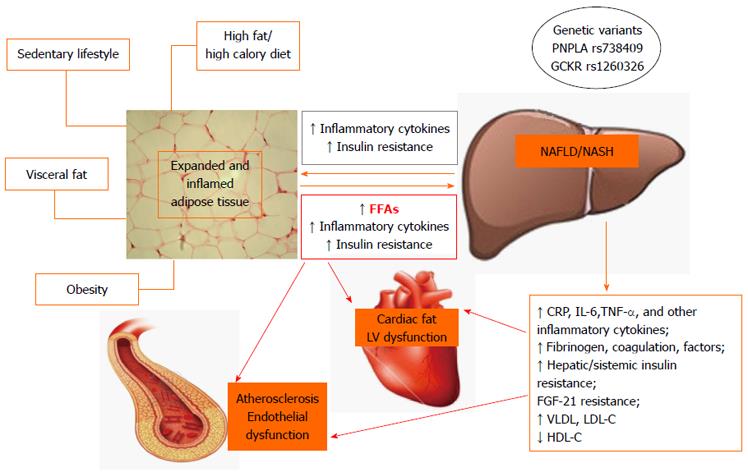
The pathophysiological mechanisms of CVD in NAFLD are still poorly understood. Probably they involve insulin resistance, an abnormal ectopic fat storage as well as atherogenic dyslipidemia, and a low-grade inflammatory state in the presence of genetic susceptibility (Figure 1).
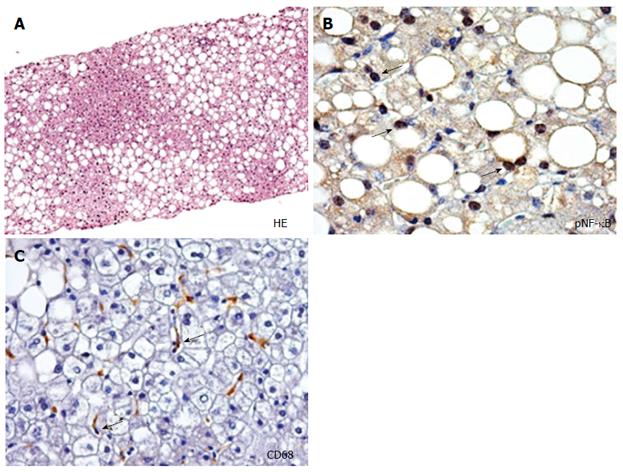
The expansion and inflammation of visceral adipose tissue mass, with consequent release of multiple molecules, is one of the earliest steps in the chain of events involved in the development of insulin resistance and NAFLD, as well as atherosclerosis, including free fatty acids (FFAs), hormones and proinflammatory adipocytokines, particularly in obese and overweight persons[54-57]. In this situation, the liver might function both as the target organ and the source of the resulting systemic abnormalities that can promote increased risk of CVD. Increased insulin resistance occurs when the advanced forms of NAFLD develop, which potentially sets up a vicious cycle of insulin resistance, increased influx of FFAs into the liver and increased hepatic steatosis[11]. In turn, hepatic steatosis (Figure 2A) leads to subacute intrahepatic inflammation through activation of nuclear factor-κB (NF-κB) pathways (Figure 2B) that exacerbate insulin resistance both locally in the liver and systematically including cardiac insulin resistance[55-58]. Indeed, Lautamäki et al[15] demonstrated that, in patients with type 2 diabetes and coronary artery disease, liver fat content was an independent indicator of myocardial insulin resistance and reduced coronary functional capacity. The consequences of insulin resistance in the heart are incompletely understood. Using genetically engineered mice with deletion of insulin receptors in cardiomyocites, Boudina et al[59] have shown that impaired myocardial insulin signaling leads to multiple mitochondrial defects that include reduced oxygen consumption and adenosine triphosphate synthesis, reduced levels of mitochondrial enzymes that regulate pyruvate and fatty acid metabolism, and decreased content of citric acid cycle proteins. Insulin signaling also regulates the expression of genes such as peroxisome proliferator-activated receptor-α in the heart, which controls the capacity of mitochondria to oxidize fatty acids. In addition, mitochondria from hearts with defective insulin signaling demonstrate evidence of oxidative stress. These mechanisms could potentially contribute to myocardial dysfunction when the heart becomes insulin resistant.
In patients with NAFLD, the increased FFAs may induce myocardial lipid accumulation, which is detrimental to LV function[60-63]. In fact, myocardial steatosis may cause alterations in myocardial substrate metabolism and efficiency (cardiac work/myocardial oxygen consumption) that occur early in the cascade of events leading to impaired LV contractility. Rijzewijk et al[46] showed that intramyocardial fat content, as detected by 1H-MRS was significantly higher in uncomplicated type 2 diabetic men than in nondiabetic control subjects and was associated with impaired cardiac metabolism[46]. Moreover, using cardiac MRI and 31P-MRS, Perseghin et al[16] demonstrated that individuals with fatty liver had an increased amount of fat in the epicardial area and displayed abnormal cardiac metabolism. Epicardial fat is a metabolically active organ that generates proatherogenic, proinflammatory and prothrombotic adipo-cytokines[64-67]. Its anatomic location, without any barrier to the adjacent myocardium, enables local paracrine interaction between epicardial fat and the myocardium[64]. Thus, epicardial and myocardial fat represent abnormal ectopic fat storage and may be a marker of the cumulative effects of NAFLD and insulin resistance in the setting of pathological adiposity, with consequent adverse associated cardiovascular outcome[65,68].
Cardiac lipotoxicity is a well-described phenomenon in insulin resistance, and is generally attributed to products of FFA excess metabolism[62,69]. Ceramide is a sphingolipid that is a key mediator of cellular stress pathways that induce apoptosis and mitochondrial dysfunction. In normal physiology, ceramide is derived from de novo synthesis or can be derived from sphingomyielin hydrolysis. Ceramide acts as a lipotoxic intermediate when it builds up as a result of elevated circulating FFAs. Structural alterations in mitochondria can reduce cardiac function by providing an insufficient supply of ATP to cardiac myocytes or by increasing reactive oxygen species production, which has been associated with increased apoptosis, DNA damage, and DNA repair[70]. Recently, it has been shown that ceramide also plays an important role in the pathogenesis of obesity-mediated vascular dysfunction via a mechanism that involves protein phosphatase 2A-mediated dephosphorylation of nitric oxide synthase III[71]. In addition, the treatment of mice with lipotoxic cardiomyopathy with the inhibitor of ceramide synthesis myriocin reversed contractile dysfunction in a mouse model of lipotoxic cardiomyopathy[72]. Taken together, it is reasonable that ceramide accumulation may contribute to the pathogenesis of cardiac and vascular dysfunction in insulin-resistant states.
NAFLD is also characterized by an atherogenic lipid profile, consisting of high TG levels, low HDL-c, an increase in small, dense LDL-c particles, increased very low-density lipoprotein (VLDL) cholesterol levels and elevated apolipoprotein B100 concentration[58]. This type of atherogenic dyslipidemia is strongly linked to adverse cardiovascular outcome[37,58]. The increased hepatic production of TG-rich VLDL provides a limited compensatory mechanism for IHTG[58,73]. However, this also results in abnormal HDL-c metabolism causing HDL-c reduction as well as compositional alterations. In fact, the amount of liver fat has a significant negative correlation with subfractions of HDL-c known to be antiatherogenic, which are reduced in NAFLD independently of peripheral insulin sensitivity[74].
In the presence of increased FFA flux and chronic, low grade inflammation, the liver is again both the target of and a contributor to systemic inflammatory changes. The steatotic and inflamed liver releases several mediators including C reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and other inflammatory cytokines that amplify the systemic, low-grade inflammation[11,55-57]. Increased intrahepatic cytokine expression results from local activation of the NF-κB pathway (Figure 2B), as mediated by hepatocellular damage and fat-derived factors, and is likely to play a key role in the progression of NAFLD and CVD[9,55-57]. Several studies have shown that a number of genes involved in fatty acid metabolism, lipolysis, monocyte and macrophage recruitment, coagulation, and inflammation are overexpressed in patients with NAFLD[75,76]. In particular, NASH presents a distinct panel of regulatory genes which are dysregulated compared to controls and subjects with simple steatosis. Indeed, circulating levels of several inflammatory markers (CRP, IL-6, monocyte chemotactic protein 1, and TNF-α), procoagulant factors (plasminogen activator inhibitor 1, fibrinogen, and factor VII), and oxidative stress markers are highest in patients with NASH, intermediate in those with simple steatosis, and lowest in control subjects without steatosis, and the differences are independent of obesity and other potentially confounding factors[76,77].
There is much evidence to suggest that macrophage infiltration (Figure 2C) could play an essential role in the pathogenesis of NAFLD and atherosclerosis by communicating inflammatory signals by scavenging modified lipids[78]. In this light, the systemic inflammation, which is exacerbated by steatohepatitis, could have a dual role on the progression of atherosclerotic plaque and steatohepatitis. Macrophages were the first inflammatory cells to be associated with atherosclerosis[79]; recently, the process of macrophage polarization has been a subject of interest[80,81]. Two distinct modes of macrophage activation were proposed to differentiate between inflammatory M1 and anti-inflammatory M2 macrophages[82]. M1-macrophages exert definitive pro-inflammatory roles and M1-derived cytokines may be involved in further activating myofibroblasts and fibrogenetic cells; M2-macrophages have been described as wound-healing, based on their ability to promote wound healing through matrix remodeling and the recruitment of fibroblasts[83]. The process of macrophage polarization during atherogenesis has been a subject of interest as macrophage subsets have been demonstrated to display some degree of plasticity and heterogeneity within atherosclerotic lesions[80,81]. In parallel, in NAFLD, the exacerbated release of M1 macrophages derived mediators contributes to the pathogenesis of several liver lesions, namely hepatocyte steatosis and apoptosis, inflammatory cell recruitment, and activation of fibrogenesis[84,85].
The term “adipokines” (adipose tissue cytokines) comprises polypeptide factors which are expressed significantly, although not exclusively, by adipose tissue in a regulated manner. Besides adipocytes, accounting for one-third of the cells, adipose tissue is composed of stromal cells, including macrophages, fibroblasts, and infiltrating monocytes, all of which contribute to adipokine production[78,86]. The major adipokines (leptin, adiponectin, resistin) exert several metabolic actions and have a role in cellular and animal models of liver injury[87]. Leptin has several immune and metabolic functions. Obesity is associated with high circulating leptin levels and leptin resistance in the central nervous system as leptin fails to correct hyperglycemia in patients with obesity[86]. Several in vitro and in vivo studies have identified a close connection between leptin and liver fibrosis. These show that leptin modulates the biology of different cell types participating in the response to liver injury, such as Kupffer cells, sinusoidal endothelial cells, and myofibroblast-like cells[88]. Leptin is also a potential mediator of cardiac hypertrophy in obesity, possibly by causing an increase in sympathetic vasoconstrictor tone and arterial blood pressure, or through direct stimulation of protein synthesis in cardiomyocytes[89].
Adiponectin exerts insulin-sensitizing effects in the liver, skeletal muscle, and adipose tissue. Adiponectin improves insulin signaling and profoundly affects glucose metabolism. Adiponectin and leptin have divergent effects on inflammation; adiponectin reduces inflammation, stimulating secretion of anti-inflammatory cytokines (i.e., IL-10), and inhibiting release of TNF-α, IL-6, and chemokines[86,87]. Resistin may represent a link between obesity and insulin resistance; its action determinates reduction of peripheral insulin sensitivity, increase in endogenous glucose production by the liver, induction of insulin resistance and stimulation of proinflammatory cytokines (i.e., IL-6 and TNF-α)[87].
Recently, hepatocytes and hepatic stem/progenitor cells (HPC) have been indicated as a source of adiponectin and resistin in the course of NAFLD[90,91]. In NASH, the expression of adiponectin by liver parenchymal cells (hepatocytes and HPCs) was down-regulated and it was inversely correlated with steatohepatitis grade. This is in agreement with the current understanding of this adipokine. In fact, adiponectin has anti-inflammatory and anti-fibrogenic properties and, in steatotic liver, has been shown to ameliorate necroinflammation and steatosis when administered in experimental NASH[87,92]. On the other hand, HPCs up-regulated their expression of resistin in correlation with progression towards NASH and fibrosis[93]. Several lines of evidence link the biology of resistin with hepatic inflammation, fibrogenesis and macrophage polarization. In rats, resistin administration significantly worsens inflammation after lipopolysaccharide injection[94], and activates fibrogenetic cells through the activation of NF-κB pathway[93,94]. Moreover, hepatic resistin expression increases in NASH; is correlated with inflammatory cell infiltration, and has been associated with macrophage recruitment within the liver[91].
Widespread research has been conducted on the relationship of adiponectin, as well as of resistin, with cardiovascular risk. Adiponectin exerts a protective effect against endothelial dysfunction induced by advanced glycation end-products[95]. This process is, in part, mediated by a decrease in the expression of adhesion molecules, and provides evidence of the protective role of adiponectin in the pathogenesis of the vascular complications of obesity/MetS. Low adiponectin levels may impair the ability of the heart to adapt to acute and chronic stress, as suggested by studies of adiponectin deficiency in mice[61]. On the other hand, resistin can act as an effector molecule that leads to an atherosclerotic state, possibly through several mechanisms. It has been shown that resistin has direct effects on endothelial cell activation by inducing the expression of cell adhesion molecules, thereby enhancing leukocyte adhesion[96,97]. Previous “in vitro” experimental studies on endothelial cells and atherosclerotic plaque progression also showed that resistin can impair endothelium-dependent relaxation, promote angiogenesis[98], and induce vascular inflammation[99]. An increase in resistin concentration significantly decreases endothelial nitric oxide synthase expression and nitric oxide production through oxidative stress in cultured human coronary artery endothelial cells[100], suggesting that the effects of resistin can be mediated by oxidative stress. However, the precise role of resistin in the clinical scenario remains to be fully elucidated.
Fibroblast growth factor-21 (FGF-21) has emerged as important endocrine factor involved in glucose and lipid metabolism and energy regulation[101]. FGF-21 is primarily expressed by liver, adipose tissue, and pancreas. FGF-21 stimulates glucose uptake in adipocytes and regulates energy metabolism and enhanced mitochondrial oxidative function through the activation of AMP-activated protein kinase and sirtuin 1[101]. FGF-21 has a hepato-protective action, but subjects with NAFLD show a condition of “FGF-21 resistance”, which worsens in subjects with NASH[102]. A recent study has demonstrated that FGF-21 knockout mice exhibit an increased relative heart weight and develop enhanced signs of dilation and cardiac dysfunction[103]. In addition, in vitro treatment of cardiomyocytes with FGF-21 reverses these cardiac alterations[103]. Thus, FGF-21 resistance or reduced levels observed in subjects with NAFLD/NASH, might play a role in cardiac anatomic and functional abnormalities.
In recent years, genetic studies have highlighted several single nucleotide polymorphisms (SNPs) that may characterize children with a high risk for NAFLD development and progression[104-110]. In particular, a common missense variant (rs738409), characterized by a C-to-G substitution encoding an isoleucine-to-methionine substitution at amino acid position 148 (I148M), in the patatin-like phospholipase 3 (PNPLA3) gene has been associated not only with hepatic fat content and increased serum liver enzymes but also with increased risk of NASH and fibrosis progression[104,111-114]. The I148M PNPLA3 variant influences liver triglyceride content without apparently affecting body mass, serum lipid levels and systemic insulin resistance[113,115]. The association between I148M variant and both liver enzymes and steatosis has been confirmed in obese children of different ethnicities[111,112,116,117]. More recently, a SNP (rs1260326) in the glucokinase regulatory protein (GCKR) gene has been associated with fatty liver and with higher serum triglycerides and large VLDL levels in obese children and adolescents[109]. This association was independent of ethnicity, age, gender, z-score BMI, and glucose tolerance[109].
Because of the limited knowledge of the molecular pathogenesis of NAFLD, the current therapies consist of strategies aimed at decreasing the incidence of the known risk factors. Prevention and control of modifiable risk factors such as overweight and unhealthy lifestyle can have an impact on the overall health of children and adolescents as well as on the prevention and control of pediatric NAFLD and the related MetS[2,3,10]. Lifestyle changes and pharmacological treatment of pediatric NAFLD have extensively been discussed elsewhere[2,3,10]. However, it is not known how treatment of NAFLD modulates the risk of CVD.
It has been established that preclinical atherosclerosis is not an irreversible but rather a dynamic process. Different interventions on cardiovascular risk factors (dyslipidemia, hypertension, diabetes mellitus, and obesity) have been shown to slow or even regress the progression of atherosclerosis[118-123]. However, data on the reversibility of subclinical atherosclerotic markers in children with NAFLD are scant. In a study evaluating a 1-year intervention program with diet and physical exercise in children and adolescents with NAFLD, Pacifico et al[124] showed favorable changes in vascular function as estimated by FMD. In the same study, the authors failed to demonstrate a significant regression of cIMT, though there was a trend after the lifestyle intervention. Studies in adults and children have shown that lifestyle interventions can halt the progression of cIMT[118-120,122,123], but others have shown no such effect[125,126]. Possible reasons for such conflicting results include the age of population (prepubertal children, adolescents, or adults), the type of population (healthy, otherwise healthy obese, or obese subjects with obesity related comorbidities), the length of intervention, the type and intensity of lifestyle intervention, the degree of weight and visceral fat loss, and different analyses of cIMT measurements (i.e., maximum or mean value of cIMT). In this context, of great interest is the study by Koskinen et al[127] who showed that in young adults recovery from the MetS was associated with reduced cIMT progression during a 6-year follow-up period. Thus, it is possible that a longer lifestyle intervention may be necessary to regress cIMT in children with NAFLD. Of note, Pacifico et al[124] demonstrated that higher cIMT values in obese children with fatty liver as well as in those with MetS were related to impaired brachial FMD, supporting the idea that endothelial dysfunction is a necessary step before the development of structural arterial disease. The restoration of FMD observed in such patients might be the initial step to halt the progression of the atherosclerotic disease.
In a recent systematic review on the effect of current non-surgical treatments on liver disease and cardio-metabolic risk in NAFLD, Musso et al[128] found that weight loss is safe and may ameliorate both liver and cardio-metabolic disease in NAFLD. Although a ≥ 5% weight loss improves steatosis and cardio-metabolic variables, a ≥ 7% weight loss improves also histological disease activity in NASH; however, the latter goal was achieved by < 50% individuals even in randomized controlled studies adopting intensive multidisciplinary lifestyle interventions, making patient compliance a concern. No studies have yet examined the effect of reducing liver fat and inflammation on cardiac function and geometry both in children and adults.
Although cross-sectional studies have shown that children with NAFLD are at risk for early atherosclerotic changes and cardiac abnormalities, long-term longitudinal studies are required to determine more definitively the extent to which pediatric NAFLD and its severity influence long-term cardiovascular outcomes in the general population. In particular, follow-up studies may clarify whether the increased risk of atherosclerotic changes and cardiac alterations might reflect the clustering of underlying metabolic risk factors, or NAFLD per se, especially NASH, might confer a risk of adverse cardiovascular outcome above and beyond that associated with the individual components of MetS. In children, the cardiovascular system remains plastic and damage-reversible if early and appropriate interventions are established effectively. Therapeutic goals for NAFLD should address nutrition, physical activity and avoidance of smoking to prevent not only end-stage liver disease but also CVD. Future studies should also examine the long-term effect of reducing liver fat and inflammation on vascular functional and structural changes as well as on cardiac function and geometry in children.
P- Reviewers: Atanasov G, Gong NQ, Najimi M S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3717] [Article Influence: 161.6] [Reference Citation Analysis (2)] |
| 2. | Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282-1293. [PubMed] |
| 3. | Mencin AA, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Pediatr Clin North Am. 2011;58:1375-1392, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Byrne CD. Ectopic fat, insulin resistance and non-alcoholic fatty liver disease. Proc Nutr Soc. 2013;72:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 922] [Cited by in RCA: 858] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 6. | Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, Dziura J, Taksali SE, Kursawe R, Shaw M. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | D’Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, Caprio S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817-1822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 9. | Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 10. | Pacifico L, Nobili V, Anania C, Verdecchia P, Chiesa C. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J Gastroenterol. 2011;17:3082-3091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 11. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1484] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 12. | Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473-480. [PubMed] |
| 13. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Pacifico L, Anania C, Martino F, Cantisani V, Pascone R, Marcantonio A, Chiesa C. Functional and morphological vascular changes in pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Lautamäki R, Borra R, Iozzo P, Komu M, Lehtimäki T, Salmi M, Jalkanen S, Airaksinen KE, Knuuti J, Parkkola R. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291:E282-E290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Perseghin G, Lattuada G, De Cobelli F, Esposito A, Belloni E, Ntali G, Ragogna F, Canu T, Scifo P, Del Maschio A. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology. 2008;47:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949-955. [PubMed] |
| 18. | Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, Kurtoglu U, Dindar I. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J. 2010;17:457-463. [PubMed] |
| 19. | Bland J, Skordalaki A, Emery JL. Early intimal lesions in the common carotid artery. Cardiovasc Res. 1986;20:863-868. [PubMed] [DOI] [Full Text] |
| 20. | Stary HC. Lipid and macrophage accumulations in arteries of children and the development of atherosclerosis. Am J Clin Nutr. 2000;72:1297S-1306S. [PubMed] |
| 21. | Schwimmer JB, Deutsch R, Behling C, Lavine JE. Fatty liver as a determinant of atherosclerosis. Hepatology. 2005;42:610A. |
| 22. | Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, Dvisic G, Chiesa C. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Demircioğlu F, Koçyiğit A, Arslan N, Cakmakçi H, Hizli S, Sedat AT. Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2008;47:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Kelishadi R, Cook SR, Amra B, Adibi A. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis. 2009;204:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Manco M, Bedogni G, Monti L, Morino G, Natali G, Nobili V. Intima-media thickness and liver histology in obese children and adolescents with non-alcoholic fatty liver disease. Atherosclerosis. 2010;209:463-468. [PubMed] |
| 26. | Caserta CA, Pendino GM, Amante A, Vacalebre C, Fiorillo MT, Surace P, Messineo A, Surace M, Alicante S, Cotichini R. Cardiovascular risk factors, nonalcoholic fatty liver disease, and carotid artery intima-media thickness in an adolescent population in southern Italy. Am J Epidemiol. 2010;171:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Nobili V, Alkhouri N, Bartuli A, Manco M, Lopez R, Alisi A, Feldstein AE. Severity of liver injury and atherogenic lipid profile in children with nonalcoholic fatty liver disease. Pediatr Res. 2010;67:665-670. [PubMed] [DOI] [Full Text] |
| 28. | Weghuber D, Roden M, Franz C, Chmelik M, Torabia S, Nowotny P, Gruber S, Waldhäusl W, Klingler A, Bieglmayer C. Vascular function in obese children with non-alcoholic fatty liver disease. Int J Pediatr Obes. 2011;6:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | El-Koofy NM, Anwar GM, El-Raziky MS, El-Hennawy AM, El-Mougy FM, El-Karaksy HM, Hassanin FM, Helmy HM. The association of metabolic syndrome, insulin resistance and non-alcoholic fatty liver disease in overweight/obese children. Saudi J Gastroenterol. 2012;18:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Sert A, Pirgon O, Aypar E, Yilmaz H, Odabas D. Relationship between left ventricular mass and carotid intima media thickness in obese adolescents with non-alcoholic fatty liver disease. J Pediatr Endocrinol Metab. 2012;25:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Akın L, Kurtoglu S, Yikilmaz A, Kendirci M, Elmalı F, Mazicioglu M. Fatty liver is a good indicator of subclinical atherosclerosis risk in obese children and adolescents regardless of liver enzyme elevation. Acta Paediatr. 2013;102:e107-e113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Gökçe S, Atbinici Z, Aycan Z, Cınar HG, Zorlu P. The relationship between pediatric nonalcoholic fatty liver disease and cardiovascular risk factors and increased risk of atherosclerosis in obese children. Pediatr Cardiol. 2013;34:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Sert A, Aypar E, Pirgon O, Yilmaz H, Odabas D, Tolu I. Left ventricular function by echocardiography, tissue Doppler imaging, and carotid intima-media thickness in obese adolescents with nonalcoholic fatty liver disease. Am J Cardiol. 2013;112:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Alp H, Karaarslan S, Selver Eklioğlu B, Atabek ME, Altın H, Baysal T. Association between nonalcoholic fatty liver disease and cardiovascular risk in obese children and adolescents. Can J Cardiol. 2013;29:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Huang RC, Beilin LJ, Ayonrinde O, Mori TA, Olynyk JK, Burrows S, Hands B, Adams LA. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology. 2013;58:1306-1314. [PubMed] |
| 36. | Jin Y, Chen Y, Tang Q, Xue M, Li W, Jiang J. Evaluation of carotid artery stiffness in obese children using ultrasound radiofrequency data technology. J Ultrasound Med. 2013;32:105-113. [PubMed] |
| 37. | Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 775] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 444] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 39. | Quijada Z, Paoli M, Zerpa Y, Camacho N, Cichetti R, Villarroel V, Arata-Bellabarba G, Lanes R. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes. 2008;9:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Raitakari OT. Imaging of subclinical atherosclerosis in children and young adults. Ann Med. 1999;31:33S-40S. [PubMed] |
| 41. | Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3348] [Cited by in RCA: 3465] [Article Influence: 150.7] [Reference Citation Analysis (0)] |
| 42. | Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1455] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 43. | Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1261] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 44. | O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14-22. [PubMed] |
| 45. | Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, Ermani M, Catena C, Soardo G, Di Piazza L. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Rijzewijk LJ, Jonker JT, van der Meer RW, Lubberink M, de Jong HW, Romijn JA, Bax JJ, de Roos A, Heine RJ, Twisk JW. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. J Am Coll Cardiol. 2010;56:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Mantovani A, Zoppini G, Targher G, Golia G, Bonora E. Non-alcoholic fatty liver disease is independently associated with left ventricular hypertrophy in hypertensive Type 2 diabetic individuals. J Endocrinol Invest. 2012;35:215-218. [PubMed] |
| 49. | Hallsworth K, Hollingsworth KG, Thoma C, Jakovljevic D, MacGowan GA, Anstee QM, Taylor R, Day CP, Trenell MI. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J Hepatol. 2013;58:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Karabay CY, Kocabay G, Kalayci A, Colak Y, Oduncu V, Akgun T, Kalkan S, Guler A, Kirma C. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: a speckle-tracking echocardiography study. Eur J Gastroenterol Hepatol. 2014;26:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, Klein S. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;162:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, Chiesa C. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Fintini D, Chinali M, Cafiero G, Esposito C, Giordano U, Turchetta A, Pescosolido S, Pongiglione G, Nobili V. Early left ventricular abnormality/dysfunction in obese children affected by NAFLD. Nutr Metab Cardiovasc Dis. 2014;24:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 54. | Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 55. | Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 56. | Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 57. | Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 58. | Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 59. | Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Bugianesi E. Nonalcoholic fatty liver disease (NAFLD) and cardiac lipotoxicity: Another piece of the puzzle. Hepatology. 2008;47:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Bugianesi E, Gastaldelli A. Hepatic and cardiac steatosis: are they coupled? Heart Fail Clin. 2012;8:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Abel ED, O’Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 63. | Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 64. | Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536-543. [PubMed] |
| 65. | Kankaanpää M, Lehto HR, Pärkkä JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689-4695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 66. | Dutour A, Achard V, Sell H, Naour N, Collart F, Gaborit B, Silaghi A, Eckel J, Alessi MC, Henegar C. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J Clin Endocrinol Metab. 2010;95:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 68. | Iacobellis G, Pellicelli AM, Grisorio B, Barbarini G, Leonetti F, Sharma AM, Barbaro G. Relation of epicardial fat and alanine aminotransferase in subjects with increased visceral fat. Obesity (Silver Spring). 2008;16:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord. 2000;24:28S-32S. [PubMed] |
| 70. | Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1105] [Cited by in RCA: 1171] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 71. | Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848-1859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 72. | Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 73. | Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430-15435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 74. | Kantartzis K, Rittig K, Cegan A, Machann J, Schick F, Balletshofer B, Fritsche A, Schleicher E, Häring HU, Stefan N. Fatty liver is independently associated with alterations in circulating HDL2 and HDL3 subfractions. Diabetes Care. 2008;31:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Sookoian S, Gianotti TF, Rosselli MS, Burgueño AL, Castaño GO, Pirola CJ. Liver transcriptional profile of atherosclerosis-related genes in human nonalcoholic fatty liver disease. Atherosclerosis. 2011;218:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, Muggeo M, Day CP. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring). 2008;16:1394-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 78. | Gaudio E, Nobili V, Franchitto A, Onori P, Carpino G. Nonalcoholic fatty liver disease and atherosclerosis. Intern Emerg Med. 2012;7:297S-305S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol. 2012;3:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 431] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 81. | Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2133] [Cited by in RCA: 2035] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 82. | Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3531] [Cited by in RCA: 3820] [Article Influence: 201.1] [Reference Citation Analysis (0)] |
| 83. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3290] [Cited by in RCA: 3114] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 84. | Van Hul N, Lanthier N, Español Suñer R, Abarca Quinones J, van Rooijen N, Leclercq I. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am J Pathol. 2011;179:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 438] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 86. | Tilg H. Adipocytokines in nonalcoholic fatty liver disease: key players regulating steatosis, inflammation and fibrosis. Curr Pharm Des. 2010;16:1893-1895. [PubMed] |
| 87. | Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 88. | Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, de Divitiis O, Varricchio M. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, Onori P, Alvaro D, Gaudio E. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56:2142-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 91. | Carpino G, Renzi A, Onori P, Gaudio E. Role of hepatic progenitor cells in nonalcoholic fatty liver disease development: cellular cross-talks and molecular networks. Int J Mol Sci. 2013;14:20112-20130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 92. | Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1520] [Cited by in RCA: 1709] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 93. | Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Beier JI, Guo L, von Montfort C, Kaiser JP, Joshi-Barve S, Arteel GE. New role of resistin in lipopolysaccharide-induced liver damage in mice. J Pharmacol Exp Ther. 2008;325:801-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Del Turco S, Navarra T, Gastaldelli A, Basta G. Protective role of adiponectin on endothelial dysfunction induced by AGEs: a clinical and experimental approach. Microvasc Res. 2011;82:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736-740. [PubMed] |
| 97. | Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415-419. [PubMed] |
| 98. | Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 99. | Cho Y, Lee SE, Lee HC, Hur J, Lee S, Youn SW, Lee J, Lee HJ, Lee TK, Park J. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2011;57:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 100. | Chen C, Jiang J, Lü JM, Chai H, Wang X, Lin PH, Yao Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299:H193-H201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 101. | Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1243] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 102. | Giannini C, Feldstein AE, Santoro N, Kim G, Kursawe R, Pierpont B, Caprio S. Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. J Clin Endocrinol Metab. 2013;98:2993-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 103. | Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019. [PubMed] |
| 104. | Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, Dongiovanni P, Fargion S, Nobili V. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 105. | Valenti L, Motta BM, Alisi A, Sartorelli R, Buonaiuto G, Dongiovanni P, Rametta R, Pelusi S, Fargion S, Nobili V. LPIN1 rs13412852 polymorphism in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2012;54:588-593. [PubMed] |
| 106. | Rossi F, Bellini G, Alisi A, Alterio A, Maione S, Perrone L, Locatelli F, Miraglia del Giudice E, Nobili V. Cannabinoid receptor type 2 functional variant influences liver damage in children with non-alcoholic fatty liver disease. PLoS One. 2012;7:e42259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Lin YC, Chang PF, Chang MH, Ni YH. A common variant in the peroxisome proliferator-activated receptor-γ coactivator-1α gene is associated with nonalcoholic fatty liver disease in obese children. Am J Clin Nutr. 2013;97:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 108. | Adams LA, White SW, Marsh JA, Lye SJ, Connor KL, Maganga R, Ayonrinde OT, Olynyk JK, Mori TA, Beilin LJ. Association between liver-specific gene polymorphisms and their expression levels with nonalcoholic fatty liver disease. Hepatology. 2013;57:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 109. | Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, Dykas DJ, Bale AE, Giannini C, Pierpont B. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2012;55:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 110. | Al-Serri A, Anstee QM, Valenti L, Nobili V, Leathart JB, Dongiovanni P, Patch J, Fracanzani A, Fargion S, Day CP. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: evidence from case-control and intra-familial allele association studies. J Hepatol. 2012;56:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 111. | Romeo S, Sentinelli F, Cambuli VM, Incani M, Congiu T, Matta V, Pilia S, Huang-Doran I, Cossu E, Loche S. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol. 2010;53:335-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 112. | Santoro N, Kursawe R, D’Adamo E, Dykas DJ, Zhang CK, Bale AE, Calí AM, Narayan D, Shaw MM, Pierpont B. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 113. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 739] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 114. | Valenti L, Alisi A, Nobili V. I148M PNPLA3 variant and progressive liver disease: a new paradigm in hepatology. Hepatology. 2012;56:1883-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 116. | Lin YC, Chang PF, Hu FC, Yang WS, Chang MH, Ni YH. A common variant in the PNPLA3 gene is a risk factor for non-alcoholic fatty liver disease in obese Taiwanese children. J Pediatr. 2011;158:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 117. | Goran MI, Walker R, Le KA, Mahurkar S, Vikman S, Davis JN, Spruijt-Metz D, Weigensberg MJ, Allayee H. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes. 2010;59:3127-3130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 119. | Wunsch R, de Sousa G, Toschke AM, Reinehr T. Intima-media thickness in obese children before and after weight loss. Pediatrics. 2006;118:2334-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 120. | Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Rönnemaa T, Niinikoski H, Lagström H, Talvia S, Jula A, Heinonen OJ. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study). Circulation. 2013;127:2088-2096. [PubMed] |
| 121. | Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 122. | Shai I, Spence JD, Schwarzfuchs D, Henkin Y, Parraga G, Rudich A, Fenster A, Mallett C, Liel-Cohen N, Tirosh A. Dietary intervention to reverse carotid atherosclerosis. Circulation. 2010;121:1200-1208. [PubMed] |
| 123. | Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Pacifico L, Arca M, Anania C, Cantisani V, Di Martino M, Chiesa C. Arterial function and structure after a 1-year lifestyle intervention in children with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2013;23:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 125. | Karason K, Wikstrand J, Sjöström L, Wendelhag I. Weight loss and progression of early atherosclerosis in the carotid artery: a four-year controlled study of obese subjects. Int J Obes Relat Metab Disord. 1999;23:948-956. [PubMed] |
| 126. | Anderssen SA, Hjelstuen AK, Hjermann I, Bjerkan K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis. 2005;178:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Koskinen J, Magnussen CG, Taittonen L, Räsänen L, Mikkilä V, Laitinen T, Rönnemaa T, Kähönen M, Viikari JS, Raitakari OT. Arterial structure and function after recovery from the metabolic syndrome: the cardiovascular risk in Young Finns Study. Circulation. 2010;121:392-400. [PubMed] |
| 128. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |