Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9017
Revised: January 15, 2014
Accepted: April 8, 2014
Published online: July 21, 2014
Processing time: 269 Days and 7.9 Hours
The worldwide interest in the gut microbiome and its impact on the upstream liver highlight a critical upside to breath research: it can uniquely measure otherwise unmeasurable biology. Bacteria make gases [volatile organic compounds (VOCs)] that are directly relevant to pathophysiology of the fatty liver and associated conditions, including obesity. Measurement of these VOCs and their metabolites in the exhaled breath, therefore, present an opportunity to safely and easily evaluate, on both a personal and a population level, some of our most pressing public health threats. This is an opportunity that must be pursued. To date, however, breath analysis remains a slowly evolving field which only occasionally impacts clinical research or patient care. One major obstacle to progress is that breath analysis is inherently and emphatically mutli-disciplinary: it connects engineering, chemistry, breath mechanics, biology and medicine. Unbalanced or incomplete teams may produce inconsistent and often unsatisfactory results. A second impediment is the lack of a well-known stepwise structure for the development of non-invasive diagnostics. As a result, the breath research landscape is replete with orphaned single-center pilot studies. Often, important hypotheses and key observations have not been pursued to maturation. This paper reviews the rationale and requirements for breath VOC research applied to the gut-fatty liver axis and offers some suggestions for future development.
Core tip: The biology of the gut-liver axis has always been fascinating and exceedingly difficult to study. With the rapidly expanding interest in the gut microbiome, however, finding better measurement techniques to evaluate this biology has never been more relevant. Breath volatile organic compounds (VOCs) measurement presents the unmatched potential to address this critical unmet need. Breath measurement can be challenging, however, and requires coherent teams including engineers, breath chemists, and clinical researchers. It also requires long term vision and strategy. This paper describes the rationale for breath VOCs, critically reviews the history of breath VOC development, and offers suggestions for progress.
- Citation: Solga SF. Breath volatile organic compounds for the gut-fatty liver axis: Promise, peril, and path forward. World J Gastroenterol 2014; 20(27): 9017-9025
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9017
The gut flora microbiome and the gut-liver axis are exceptionally difficult to evaluate. However, the now universal appreciation of the microbiome’s impact on upstream fatty liver and associated disorders such as obesity compels an even greater interest in improved measurement techniques.
Breath researchers have measured gut flora activity in exhaled breath for decades. However, there has been little sustained success. This paper critically reviews the experience to date and offers suggestions for future progress.
Breath analysis still holds the unique and possibly unmatched potential to better measure this challenging and highly significant physiology.
The role of gut flora in fatty liver pathogenesis has been studied for decades. Alcohol fatty liver research, for example, demonstrated that gut flora were necessary but not sufficient for liver disease, and explored the use of gut flora therapy (poorly absorbed antibiotics) using animal models[1,2]. Various lines of evidence also pointed to a key role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD)[3-5]. Gut flora, via various mechanisms such as altered small bowel motility and impaired mucosal barrier function, have also been long appreciated to affect the clinical course of cirrhosis, regardless of liver disease etiology[6].
Research connecting the gut flora to the liver has been particularly challenging and fascinating because gut bacterial biology and liver disease are distinct disciplines connected anatomically via a nearly inaccessible portal venous system. And although there is a history of gut flora therapies (i.e., prebiotics, probiotics, dietary interventions) for liver disease[7,8], progress has been slow because the science, especially the details of the gut flora, is underdeveloped. Nevertheless, the potential impact was evident: non-alcohol fatty liver, alcohol fatty liver, and cirrhosis affect many people.
However, with the now-familiar association of gut flora dysbiosis to obesity[9,10] and insulin resistance[11-13] interest in gut flora biology and, along with it, the gut-liver axis, has grown and today would be difficult to overstate. The gut flora is now regarded as a newly discovered metabolic organ. Many essential questions persist and have triggered a worldwide effort to better understand this new organ[14-16]. Multiple comprehensive reviews have addressed the impact of gut flora on fatty liver and/or obesity[17-20].
The major studies which have propelled these advances have generally used detailed fecal analysis. These analyses can include a variety of techniques including DNA sequencing, culture, and metabolic profiling[21]. The emerging data indicate several possible mechanisms of gut flora influence: fermentation, effects on metabolism, inflammatory signaling, or a combination. Notably, it is understood the gut microbiome is personal; one’s gut flora, as well as their metabolic response to diet, and upstream liver effects cannot be predicted a priori[22]. Thus, since exogenous ethanol is metabolized to acetaldehyde at a variable and unpredictable rate[23], the same should follow for endogenous ethanol produced from gut flora. Furthermore, it is acknowledged that there remain many additional unknowns that exist about the gut-liver axis (i.e., motility, mucosal barrier, immune system interactions, molecular mechanisms within the hepatocyte). However, despite both these known differences and true unknowns, there is a rapidly growing interest in the gut flora therapies and dietary interventions premised on these mechanisms[24].
Therefore, notwithstanding the usefulness of fecal analysis to date, is not clear that it will prove as successful for wide scale clinical research[25]. Fecal analysis, by virtually any method, has a number of drawbacks: samples are collected infrequently and episodically, are expensive to run, and result in large amount data that nevertheless remains challenging to interpret in the setting of multiple, interrelated physiologic variables: i.e., gut flora modulate mucosal integrity and immune function with differential impact on the liver, and vice-versa[26,27]. Fecal analysis cannot readily account for a number of factors in the gastrointestinal tract, including transit time, presence or absence of mucosal disease, and the possible differential impact bacterial subpopulations (e.g., distal small bowel vs colonic, and so on).
Breath volatile organic compound (VOC) measurement, therefore, may serve to complement fecal analysis[28]. Individual VOCs can be measured for specific hypothesis driven goals tailored to match the present understanding of the role of gut flora in the gut-liver axis.
Since the pathogenesis of fatty liver (Table 1) is multifactorial and there are many variables which impact the gut-liver axis, the most successful research will likely simultaneously measure multiple VOCs.
It is presumed that some of these metabolites (e.g., ethanol) are produced only by gut flora, whereas others (e.g., acetaldehyde) are produced by both gut flora and human metabolism. Notably, some of these VOCs may potentiate others. For example, ethanol and acetaldehyde can increase the growth of gram negative bacteria and intestinal permeability, respectively, and thereby may promote uptake of inflammatory mediators[37]. Hydrogen sulfide may reduce gastrointestinal motility and thereby lead to bacterial stasis and overgrowth[38]. Other VOCs have multiple affects that overlap multiple categories. For example, some gut flora metabolize choline efficiently and their over-abundance can lead both to choline deficiency and an overproduction of the toxic metabolites dimethylamine and trimethylamine[39]. Both mechanisms have been implicated in the pathogenesis of fatty liver and non-alcoholic steatohepatitis[40,41]. Each of these VOCs have been measured in exhaled breath, though usually separately. However, much like the standard twelve lead electrocardiogram or lipid panels, it is likely that the most meaningful VOC breath data would come from the simultaneous measurement and interpretation of multiple VOCs and/or profiles.
In contrast to fecal analysis, exhaled breath VOC analysis can measure the global activity of the entire gut-liver axis. Because breath measurement is non-invasive, safe, and potentially inexpensive, it easily enables studies with repeated measures. For example, it is simple and highly relevant to envision evaluating the immediate differential effect of various oral challenges (e.g., high/low fiber, high/low fructose) in various subjects (e.g., lean/obesity, fatty liver/cirrhosis) using timed VOC measurements over several hours, days, or longer.
The gut liver axis (Table 2) includes many important, highly variable factors that are difficult to measure physiologically. While fecal analysis is inherently limited, breath VOC measurement may evaluate the global activity of the entire system.
| Gut flora | Lumen factors | Hepatic factors | Host |
| Bacterial diversity and function | Barrier integrity | Enzyme heterogeneity (e.g., alcohol dehydrogenase) | Diet |
| Mucosal or lumen associated | Immune defense | Liver disease | Medications |
| Location | Mucosal disease (e.g., celiac, crohns) | Cirrhosis and porto-systemic shunting | Co-morbid conditions (e.g., diabetes) |
| (e.g., small bowel, right colon) | |||
| Transit time | Age, gender, body mass index |
In summary, the microbiome and gut-liver axis are a major research emphasis world-wide, and studies employing fecal analysis are appropriately credited with many advances. However, even if fecal analysis was fully validated, free, easy to perform, and always yielded interpretable results, it still cannot measure many “upstream” factors germane to both fatty liver and the metabolic syndrome and the marked heterogeneity between subjects. Studies using breath VOC analysis, in contrast, can uniquely evaluate the entire organism in real time. The simple capability of repeated measures greatly expands options in clinical research.
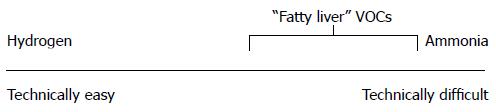
Breath analysis is appealing because it enables the potential for non-invasive, real time, easy to use, point of care measurement of metabolites that are, in some cases, difficult or impossible to measure by blood assays or other means. Previous attempts to apply breath analysis to gut physiology, however, have not been met with great success. Two examples, hydrogen and ammonia, are illustrative.
Breath hydrogen testing has been available for decades[42]. The monitors are relatively inexpensive, portable, and simple to operate. Aside from the addition of methane (to capture preferential methane producers) and carbon dioxide (for quality control), the instrumentation and breath collection process have not significantly changed in many years. Hydrogen measurement is technically easy: it is relatively inert; its measurement is not affected by background ambient air; and it is present at high concentrations (parts per million)[43]. Breath hydrogen testing has been incorporated into hundreds of published research studies.
The most widely accepted clinical use is in the evaluation of small intestine bacterial overgrowth (SIBO) and carbohydrate mal-absorption. Regarding the former, SIBO has emerged as a possible important and modifiable factor in the pathogenesis of irritable bowel syndrome (IBS) for some patients[44]. As a result, the use of hydrogen breath testing has surged over the last decade to evaluate SIBO in IBS, including responsiveness to putative gut flora therapy (i.e., rifaximin, a poorly absorbed antibiotic)[45,46]. Because SIBO or “gut dysbiosis” is challenging to measure by other means, breath hydrogen testing had the potential to fulfill an important unmet need.
However, there remain serious concerns about its validity. An excellent recent review noted many problems, including lack of standardized instructions regarding testing substrates, doses and time intervals, as well as varying definitions of positive vs negative tests persist[47]. Thus, notwithstanding a surging scientific and public interest in the possible role of gut flora in IBS, the American College of Gastroenterology does not endorse routine breath testing[48].
The results of a recent meeting of the United States Food and Drug Administration (FDA) Gastrointestinal Drugs Advisory Committee (GIDAC) provide additional insight[49]. The meeting’s purpose was the design of clinical trials to evaluate the safety, efficacy, and durability of response of repeat cycles of Xifaxan (rifaximin). To the author’s knowledge, this was the first time a breath test was seriously considered in the drug evaluation and approval process for a gut disease. But despite its long history, lack of technical issues, and the unmet need, GIDAC and the sponsor (Salix) easily agreed that breath hydrogen testing fails to meet criteria as a valid biomarker for any purpose and should not utilized[50]. Future developments seem unlikely.
In contrast to hydrogen, ammonia is highly volatile and difficult to measure by any method[51,52]. Due to its relevance to gut flora and various disease states[53], breath researchers have aspired to measure it for greater than thirty years[54]. A progression of highly sophisticated measurement platforms (e.g., GC/MS, quantum cascade lasers[55]) have been used in the hopes that ever faster and more precise equipment modifications will finally yield accurate and reproducible results usable for clinical research and patient care. Many technical factors must be considered (e.g., temperature, humidity, flow, and mode of breathing) alongside complex biologic concerns (e.g., contamination from oral bacteria)[56,57]. Despite these major challenges, many small studies were published purporting to demonstrate the utility of breath ammonia measurement for a specific disease or condition (e.g., hepatic encephalopathy, renal dialysis, exercise[58-60]). However, it now appears from work published by highly experienced groups, that exhaled breath may not reflect systemic levels, at least not be by the methods described to date. Aspirations repeatedly exceeded reality. Not surprisingly, therefore, the current ammonia literature has nearly completely ignored breath research[53,61,62].
In summary, breath hydrogen is easy to measure and has an established role in clinical research and patient care. However, it is not a valid biomarker and its impact has not grown with in parallel with the rise in interest in gut flora. Breath ammonia is difficult to measure and, notwithstanding intense efforts by multiple breath research groups, has had little influence on clinical ammonia research. Thus, both the easy and difficult extremes of the breath metabolite spectrum reveal that, at times, the breath enterprise exists as only a tangential contributor to overall human research. The literature is replete with orphaned pilot studies. While hydrogen and ammonia serve as prototypical examples, this pattern has been duplicated with many metabolites (Table 3).
| Technical/scientific factors | |
| Monitor/interface/biology | Too many interrelated unknowns |
| Unique data: uncertain utility | Relevance difficult to establish |
| Relevance may not exist | |
| Non-technical factors | |
| Inadequate teams | Engineers, chemists, doctors, statisticians |
| Inadequate synergy | Single center efforts |
| Lack of focus | Too many diseases, too little strategy |
| Lack of common languages | Device development is not drug development |
| Few models of commercial success | Difficult to envision endgame |
Notably, most of the candidate “fatty liver” VOCs are also quite difficult to measure (Figure 1).
By definition, VOCs are dynamic and changeable. Furthermore, they are present only in trace quantities and are subject to multiple confounders, including environmental factors. Therefore, studies of VOCs carry an exceptional burden of validation that requires the demonstration of reproducibility. Ideally, this includes at least three kinds of reproducibility: immediate (paired samples back to back), day to day, and location to location. The latter is needed because of ambient air influences, especially if human breath is collected in proximity to medical or research offices and facilities. For example, it must be proven that a subject’s breath ethanol at 200 ppb would be measured the same in low (e.g., 50 ppb) and high (e.g., 5000 ppb) ambient air environments. Once established, then other important influences should evaluated, including time of day, mode of breathing, mouth rinses, food intake including composition and timing, and so on.
It must be acknowledged that such studies are often tedious, have poor publication value and short term return on investment. However, they are essential. Historians note that when the United States Food and Drug Administration (FDA) first promoted the basic drug safety expectations that evolved into present day preclinical and phase I studies (i.e., the Food, Drug, and Cosmetic Act of 1938), most pharmaceutical companies simply folded[63]. The survivors, e.g., Merck, not only responded by drastically increasing their research enterprise, their leadership specifically assigned only their best scientists to these early stage efforts in acknowledgement of both their critical importance and tedium.
Breath research has to date failed to uniformly meet these requirements. Breath research papers often detail monitor mechanics and the ability of the monitor to reproducibly measure a targeted VOC against a known laboratory reference gas standard. Without further evaluation, small cross-sectional human studies are then performed purportedly to evaluate a disease state. Unfortunately, this pattern ultimately results in an unconvincing and inherently limited literature, as illustrated above for both breath hydrogen and ammonia. Breath VOC researchers have, therefore, earned skepticism from the broader research community.
Fecal VOC analysis should also meet these standards. For example, a recently published study evaluated fecal VOCs in NAFLD[64]. Using home stool kits, subjects produced samples once, froze them, and later transported them to the lab. Fecal VOCs were then measured and compared to DNA analysis. Given the large number of VOCs measured (two hundred twenty), small sample size (thirty cases and controls) and observational case-control study design, the strength of the study’s conclusions is largely determined by the confidence in the measurement process. However, while the authors and accompanying editorial carefully and appropriately discuss multiple other important influences and limitations of the study, neither substantively addresses this more basic issue[65]. Even for analyses that may be exploratory and descriptive, more complete methods discussion is imperative to build a confidence foundation for additional studies.
Finally, it is noteworthy that while blood VOC analysis may also have important potential, it has similar downsides. For example, Zhu et al[66], recently reported that specific gut flora compositions may drive an elevated endogenous ethanol production in a pediatric population with non-alcoholic steatohepatitis. However, blood assays for VOCs can also be challenging[67]; for example, despite the fact that ammonia has been measured in the blood for over one hundred years, the proper blood source (venous vs arterial)[68] and state (partial pressure NH3vs NH4+)[69,70] remain debated. Furthermore, phlebotomy makes studies requiring multiple repeated measures difficult.
In the 1950’s and 1960’s, the United States FDA promulgated a three phase strategy to evaluate the safety and efficacy of new drugs[63]. The phases became familiar worldwide and created a uniform path for drug development. It is relatively easy, therefore, to interpret and compare clinical trials as they evolve through the phases. This is helpful not only for medical researchers, scientists, and regulators, but also for other stakeholders including investors and the broader public. Furthermore, drugs are developed and approved for a specific disease indication. Because this process is slow and highly resource intensive, progression through the phases occurs only after careful and continuous consideration of an unmet need and competing alternatives[71]. As a result of this stepwise structure, regulatory approval, at least in the United States, is a milestone that is almost always associated with at least some commercial potential.
Unfortunately for breath research, an analogous path does not exist for non-invasive diagnostics or biomarker development[72]. While the FDA indeed regulates non-invasive medical devices, the requirements for approval are much different, generally lower, and not as well known. Furthermore, they are not nearly as meaningful. Therefore, while biomarkers researchers may have lower apparent initial development costs and greater latitude than drug researchers, they risk misunderstanding and misdirection amongst members of the development team.
It is essential, however, that an overall strategy exists. This begins with an extensive and thorough validation of a putative biomarker applied to a particular application, e.g., risk estimation, screening, diagnosis, monitoring, and so on. Moreover, biomarkers should also be characterized by purpose, e.g., predictive, prognostic, and so on[73-75]. This compass must guide testing. Poorly designed studies in the wrong population are destined to yield uninterpretable results; this is especially true in breath analysis, where experimental monitors are often touted to measure experimental metabolites via experimental interface samplers to describe unknown biology.
Successful breath VOC research requires (1) multiple disciplinary teams; (2) extensive early stage validation studies; and (3) a clear clinical research strategy.
Coherent teams require, at a minimum, the ongoing participation of engineers, breath measurement experts, clinical researchers with experience in gut biology, gastroenterology, hepatology, and statistics. The process should begin with a foundation of knowledge and experience with breath VOCs resulting in focused testable hypotheses that can be transformed into monitors with specific performance specifications and operational capacities. Ideally, multiple monitors are built and are tested clinically side by side first at a single site and then at multiple sites for accuracy and reproducibility. After these are clearly established and normative data are generated, disease specific hypothesis can be pursued. Finally, a clear long term clinical research strategy grounded in the requirements for biomarker development should be articulated. Outside of a few centers of excellence, (e.g., the Austrian Breath Research Institute, ISTM Keele University) such a comprehensive approach would be novel for breath research. The recent publication of comprehensive breath research books[76], growing interest in breath research conferences, and the development and greater use a specially designed interface sampler[77] are positive steps.
The breath VOC metabolites of interest shown in Figure 1 are nearly as technically challenging as ammonia. Each of them, however, have been measured in breath with the generation of some normative data[33,78]. Many innovative and useful small, single center studies have been published, as has been recently reviewed[28].
A few studies have specifically focused on the gut liver axis and demonstrated some physiologic insights. For example, Cope et al[79], evaluated the effect of an intervention (neomycin, a poorly absorbed antibiotic) on exhaled breath ethanol in an obese murine model of fatty liver compared to lean littermates. In addition to utilizing an intervention, this convincing study also reported repeated measures and thereby accounted for diurnal ethanol variations. The follow up human studies did not have these strengths and were therefore less persuasive[80,81]. At present, though, breath VOCs are most developed not for fatty liver but for use in diabetes monitoring, where multiple groups have many significant recent advances[82,83].
Finally, it must be acknowledged that liver disease, especially fatty liver, is difficult to accurately measure by any means, including blood assays, imaging, or biopsy[84,85]. Moreover, the pathophysiology of fatty liver, its relationship to steatohepatitis, cirrhosis, and associated conditions like obesity is complex, and there are many important mechanisms that do not involve VOCs. Thus, even if a well validated breath VOC panel existed now, it would be difficult to definitely tie such a profile to a clinical outcome of interest, and multiple measurement modalities are likely needed. As a result, breath research groups might aspire to participate in established long term fatty liver research programs (e.g., the United States based Non-Alcoholic Steatohepatitis Clinical Research Network[86]) as ancillary studies.
Many of the pioneers of breath research have creatively adapted existing measurement platforms to breath measurement[87]. Even now, specifically designed breath monitors are usually built as one-of-a-kind prototypes. The obvious legacy has been the small, single center fundamentally limited studies described above. For the same straightforward reasons, clinical researchers need multiple identical monitors that are portable and measure multiple VOCs simultaneously and accurately. Because clinical research is most convincing when multi-center studies involve large numbers of subjects, the need for multiple identical monitors is imperative. This is especially true due to extra reproducibility requirements of breath VOC research. While engineers may be understandably reluctant to commit the resources to build five monitors (especially after the results of a single prototype may have been equivocal), that is the prescription.
Given engineering advances and the right vision, this is achievable. Breath measurement experts, “breathologists,” should be involved at every stage, from design through maturation. Like drug development, this process will likely require a collaborative effort between academia and industry, but could occur at a fraction of the cost.
Breath analysis continues its infancy, and is almost always discussed in terms of its potential. But the rationale for breath has never been greater: breath affords the almost unique opportunity to quickly, cheaply, and non-invasively measure important markers that reflect the global gut-liver axis biology not measurable in other ways. Engineers, if they are willing, are ever more capable of making fast, portable, ultra-sensitive monitors. Comprehensive breath research teams should thoroughly address reproducibility to build a foundation for specific hypothesis driven goals. Those willing to invest in a long term strategy for breath VOC development may yet transform and revolutionize gut-liver axis research and patient care, with major payoffs in diseases such as fatty liver, obesity, and the metabolic syndrome.
P- Reviewers: Assy N, Kawaguchi T, Rosa H S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Wall C, Raleigh JA, Frankenberg MV. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13 Suppl:S39-S50. [PubMed] |
| 2. | Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2609-2618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Compare D, Coccoli P, Rocco A, Nardone OM, De Maria S, Cartenì M, Nardone G. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 5. | Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35:14S-20S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol. 2012;18:337-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Quigley EM, Monsour HP. Targeting the microbiota in the management of gastrointestinal and liver disease. Rev Gastroenterol Peru. 2013;33:139-144. [PubMed] |
| 8. | Kirpich IA, McClain CJ. Probiotics in the treatment of the liver diseases. J Am Coll Nutr. 2012;31:14-23. [PubMed] |
| 9. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6411] [Article Influence: 337.4] [Reference Citation Analysis (0)] |
| 10. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8764] [Article Influence: 461.3] [Reference Citation Analysis (1)] |
| 11. | Vanni E, Bugianesi E. The gut-liver axis in nonalcoholic fatty liver disease: Another pathway to insulin resistance? Hepatology. 2009;49:1790-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Siebler J, Galle PR, Weber MM. The gut-liver-axis: endotoxemia, inflammation, insulin resistance and NASH. J Hepatol. 2008;48:1032-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Mehal WZ. The gut-liver axis: a busy two-way street. Hepatology. 2012;55:1647-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4573] [Cited by in RCA: 3727] [Article Influence: 207.1] [Reference Citation Analysis (0)] |
| 15. | Available from: http://www.human-microbiome.org/. |
| 16. | National Institutes of Health. Microbiome Project. Available from: http://www.hmpdacc.org/. |
| 17. | Duseja A, Chawla YK. Obesity and NAFLD: The role of bacteria and microbiota. Clin Liver Dis. 2014;18:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28 Suppl 4:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 20. | Park JS, Seo JH, Youn HS. Gut microbiota and clinical disease: obesity and nonalcoholic Fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Paliy O, Agans R. Application of phylogenetic microarrays to interrogation of human microbiota. FEMS Microbiol Ecol. 2012;79:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3651] [Article Influence: 280.8] [Reference Citation Analysis (0)] |
| 23. | Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 795] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 24. | Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: How prebiotic can work? Br J Nutr. 2013;109 Suppl 2:S81-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2012;302:G1-G9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 27. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1103] [Article Influence: 68.9] [Reference Citation Analysis (1)] |
| 28. | Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res. 2012;6:027108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Bikov A, Paschalaki K, Logan-Sinclair R, Horváth I, Kharitonov SA, Barnes PJ, Usmani OS, Paredi P. Standardised exhaled breath collection for the measurement of exhaled volatile organic compounds by proton transfer reaction mass spectrometry. BMC Pulm Med. 2013;13:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Alkhouri N, Cikach F, Eng K, Moses J, Patel N, Yan C, Hanouneh I, Grove D, Lopez R, Dweik R. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur J Gastroenterol Hepatol. 2014;26:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Hanouneh IA, Zein NN, Cikach F, Dababneh L, Grove D, Alkhouri N, Lopez R, Dweik RA. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol. 2014;12:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Spaněl P, Dryahina K, Rejšková A, Chippendale TW, Smith D. Breath acetone concentration; biological variability and the influence of diet. Physiol Meas. 2011;32:N23-N31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Wang T, Pysanenko A, Dryahina K, Spaněl P, Smith D. Analysis of breath, exhaled via the mouth and nose, and the air in the oral cavity. J Breath Res. 2008;2:037013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Wzorek B, Mochalski P, Sliwka I, Amann A. Application of GC-MS with a SPME and thermal desorption technique for determination of dimethylamine and trimethylamine in gaseous samples for medical diagnostic purposes. J Breath Res. 2010;4:026002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Mochalski P, Wzorek B, Sliwka I, Amann A. Improved pre-concentration and detection methods for volatile sulphur breath constituents. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1856-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Miekisch W, Schubert JK, Noeldge-Schomburg GF. Diagnostic potential of breath analysis--focus on volatile organic compounds. Clin Chim Acta. 2004;347:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 620] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 37. | Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 38. | Gallego D, Clavé P, Donovan J, Rahmati R, Grundy D, Jiménez M, Beyak MJ. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511-12516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 804] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 40. | Vance DE. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr Opin Lipidol. 2008;19:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 545] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 42. | Pimentel M, Mathur R, Chang C. Gas and the microbiome. Curr Gastroenterol Rep. 2013;15:356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Saad RJ, Chey WD. Breath Testing for Small Intestinal Bacterial Overgrowth: Maximizing Test Accuracy. Clin Gastroenterol Hepatol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 45. | Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther. 2012;36:1084-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Majewski M, Reddymasu SC, Sostarich S, Foran P, McCallum RW. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 48. | Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 49. | Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM283448.pdf. |
| 50. | Available from: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/gastrointestinaldrugsadvisorycommittee/ucm279646.pdf. |
| 51. | Goggs R, Serrano S, Szladovits B, Keir I, Ong R, Hughes D. Clinical investigation of a point-of-care blood ammonia analyzer. Vet Clin Pathol. 2008;37:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Blanco Vela CI, Bosques Padilla FJ. Determination of ammonia concentrations in cirrhosis patients-still confusing after all these years? Ann Hepatol. 2011;10 Suppl 2:S60-S65. [PubMed] |
| 53. | Adeva MM, Souto G, Blanco N, Donapetry C. Ammonium metabolism in humans. Metabolism. 2012;61:1495-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 54. | Lovett AM, Reid NM, Buckley JA, French JB, Cameron DM. Real-time analysis of breath using an atmospheric pressure ionization mass spectrometer. Biomed Mass Spectrom. 1979;6:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Tittel FK, Lewicki R. Tunable mid-infrared laser absorption spectroscopy. Semiconductor Lasers Fundamentals and Applications, Woodhead Publishing Series in Electronic and Optical Materials. Oxford: Woodhead Publishing Ltd 2013; 579-629. |
| 56. | Solga SF, Mudalel M, Spacek LA, Lewicki R, Tittel F, Loccioni C, Russo A, Risby TH. Factors influencing breath ammonia determination. J Breath Res. 2013;7:037101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Hibbard T, Crowley K, Killard AJ. Direct measurement of ammonia in simulated human breath using an inkjet-printed polyaniline nanoparticle sensor. Anal Chim Acta. 2013;779:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Adrover R, Cocozzella D, Ridruejo E, García A, Rome J, Podestá JJ. Breath-ammonia testing of healthy subjects and patients with cirrhosis. Dig Dis Sci. 2012;57:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Endre ZH, Pickering JW, Storer MK, Hu WP, Moorhead KT, Allardyce R, McGregor DO, Scotter JM. Breath ammonia and trimethylamine allow real-time monitoring of haemodialysis efficacy. Physiol Meas. 2011;32:115-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Senthilmohan ST, Milligan DB, McEwan MJ, Freeman CG, Wilson PF. Quantitative analysis of trace gases of breath during exercise using the new SIFT-MS technique. Redox Rep. 2000;5:151-153. [PubMed] |
| 61. | Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012;49:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Tranah TH, Vijay GK, Ryan JM, Shawcross DL. Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis. 2013;28:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Carpenter D. Repuation and Power. Organizational Image and Pharmaceutical Regulation at the FDA. Princeton: Princeton University Press 2010; . |
| 64. | Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868-75.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 522] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 65. | Boursier J, Rawls JF, Diehl AM. Obese humans with nonalcoholic fatty liver disease display alterations in fecal microbiota and volatile organic compounds. Clin Gastroenterol Hepatol. 2013;11:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1283] [Article Influence: 106.9] [Reference Citation Analysis (1)] |
| 67. | Aylward LL, Kirman CR, Blount BC, Hays SM. Chemical-specific screening criteria for interpretation of biomonitoring data for volatile organic compounds (VOCs)--application of steady-state PBPK model solutions. Regul Toxicol Pharmacol. 2010;58:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, Arroliga AC, Mullen KD. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 69. | He Y, Li G, Song H, Luo T, Gao B, Xu J. Partial pressure of NH3 in cirrhotic patients with and without hepatic encephalopathy. J Gastrointestin Liver Dis. 2011;20:169-174. [PubMed] |
| 70. | Lin CH, Chi CH, Wu SY, Hsu HC, Chang YH, Huang YY, Chang CJ, Hong MY, Chan TY, Shih HI. Prognostic values of blood ammonia and partial pressure of ammonia on hospital arrival in out-of-hospital cardiac arrests. Am J Emerg Med. 2013;31:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Kramer DB, Xu S, Kesselheim AS. Regulation of medical devices in the United States and European Union. N Engl J Med. 2012;366:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, Keller S, Weinryb I, Green M, Duan L. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006;23:312-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 74. | Colburn WA, Lee JW. Biomarkers, validation and pharmacokinetic-pharmacodynamic modelling. Clin Pharmacokinet. 2003;42:997-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054-1061. [PubMed] |
| 76. | Amann A, Smith D. Volatile Biomarkers. 1st ed. Amsterdam: Elsevier 2013; . |
| 78. | Spaněl P, Dryahina K, Smith D. A quantitative study of the influence of inhaled compounds on their concentrations in exhaled breath. J Breath Res. 2013;7:017106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340-1347. [PubMed] |
| 80. | Nair S, Cope K, Risby TH, Diehl AM. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:1200-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Solga SF, Alkhuraishe A, Cope K, Tabesh A, Clark JM, Torbenson M, Schwartz P, Magnuson T, Diehl AM, Risby TH. Breath biomarkers and non-alcoholic fatty liver disease: preliminary observations. Biomarkers. 2006;11:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Minh Tdo C, Blake DR, Galassetti PR. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res Clin Pract. 2012;97:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Dowlaty N, Yoon A, Galassetti P. Monitoring states of altered carbohydrate metabolism via breath analysis: are times ripe for transition from potential to reality? Curr Opin Clin Nutr Metab Care. 2013;16:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Baršić N, Lerotić I, Smirčić-Duvnjak L, Tomašić V, Duvnjak M. Overview and developments in noninvasive diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3945-3954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Heeringa M, Hastings A, Yamazaki S, de Koning P. Serum biomarkers in nonalcoholic steatohepatitis: value for assessing drug effects? Biomark Med. 2012;6:743-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | National Institute of Diabetes and Digestive and Kidney Diseases. NASH CRN. Available from: http://jhuccs1.us/nash/. |
| 87. | Konvalina G, Haick H. Sensors for breath testing: from nanomaterials to comprehensive disease detection. Acc Chem Res. 2014;47:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |