Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.8998
Revised: December 9, 2013
Accepted: April 15, 2014
Published online: July 21, 2014
Processing time: 304 Days and 10.7 Hours
Hepatitis B (HB) virus (HBV) infection, which causes liver cirrhosis and hepatocellular carcinoma, is endemic worldwide. Hepatitis B vaccines became commercially available in the 1980s. The World Health Organization recommended the integration of the HB vaccine into the national immunisation programs in all countries. HBV prevention strategies are classified into three groups: (1) universal vaccination alone; (2) universal vaccination with screening of pregnant women plus HB immune globulin (HBIG) at birth; and (3) selective vaccination with screening of pregnant women plus HBIG at birth. Most low-income countries have adopted universal vaccine programs without screening of pregnant women. However, HB vaccines are not widely used in low-income countries. The Global Alliance for Vaccine and Immunization was launched in 2000, and by 2012, the global coverage of a three-dose HB vaccine had increased to 79%. The next challenges are to further increase the coverage rate, close the gap between recommendations and routine practices, approach high-risk individuals, screen and treat chronically infected individuals, and prevent breakthrough infections. To eradicate HBV infections, strenuous efforts are required to overcome socioeconomic barriers to the HB vaccine; this task is expected to take several decades to complete.
Core tip: Hepatitis B (HB) vaccines, which are the first vaccines that have been proven to prevent cancer, have played a crucial role in preventing HB virus (HBV) infection worldwide since their development in the 1980s. In particular, the HB vaccines have been rapidly integrated into the national immunisation programs of low-income countries since the Global Alliance for Vaccine and Immunization was launched in 2000. However, we have still not eradicated HBV. More than 240 million people worldwide are carriers of HBV. The vaccine strategies, current status of HBV infection, and unresolved issues related to controlling HBV infection are discussed in this review.
- Citation: Komatsu H. Hepatitis B virus: Where do we stand and what is the next step for eradication? World J Gastroenterol 2014; 20(27): 8998-9016
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/8998.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.8998
According to the World Health Organization (WHO), two billion people (one-third of the global population) have been infected with the hepatitis B (HB) virus (HBV) worldwide, and more than 240 million are chronic carriers (4%-6% of the world population)[1]. Chronically infected individuals have a 25% risk of dying from the sequelae of chronic HBV infection, such as cirrhosis and hepatocellular carcinoma (HCC)[2]. Approximately 600000 people die every year due to the consequences of HBV infection[1]. Globally, chronic HBV infection accounts for 54.4% of the cases of liver cancer[3]. HBV infection is one of the vaccine-preventable infectious diseases. In 1991, the WHO recommended the integration of the HB vaccine into the national immunisation programs in countries with an HBV carrier prevalence of 8% or higher by 1995 and in all other counties by 1997[4].
In this review, the vaccine strategies for the control of HBV infection and the prospect of HBV eradication are summarised and discussed.
In 1963, Blumberg et al[5-8] unexpectedly identified a protein in the blood of Australian aborigines, which was later named the Australia antigen. The investigators were examining serum from multi-transfused patients with conditions such as leukaemia or thalassemia compared to serum from a variety of healthy individuals from different parts of the world to identify genetic polymorphisms of serum proteins[9]. The Australian antigen was initially thought to be associated with leukaemia and Down’s syndrome[5,10], but further observations revealed that the Australia antigen is a component of the infectious agent for HBV. In fact, two patients with Down’s syndrome and a technician in Blumberg’s laboratory became positive for the antigen after developing hepatitis[6,8,10-12]. The Australia antigen was eventually confirmed to be correlated with viral hepatitis[13-16]. In 1970, Dane et al[17] discovered hepatitis B virions -double-coated particles approximately 42 nm in diameter- in the serum of patients with Australia antigen-associated hepatitis.
After the discovery of the Australian antigen, it took over 10 years to make the HB vaccine commercially available. The virion of HBV (i.e., the Dane particle) consists of an inner core and an outer membranous envelope, which contains the Australian antigen[8,18]. Electronic microscopy analyses revealed that enormous numbers of spherical and tubular particles of 22 nm in diameter, which are clearly distinct from the virions, coexist in the serum of HBV-infected patients[11,17,19]. These particles are empty viral envelopes, containing only the Australian antigen and non-infectious agents[6,8,18]. The first available vaccines consisted of purified and formalin-inactivated small empty HBV envelopes containing the hepatitis B surface antigen (HBsAg), which were harvested from the plasma of chronic HBV carriers[20-23]. These plasma-derived HB vaccines first became commercially available in the United States in 1981 and in France in 1982[24,25]. Since 1981, plasma-derived HB vaccines have been manufactured and used in many countries. However, there were concerns about whether the supply of plasma was adequate to meet the demand for the vaccine and whether the safety of a vaccine derived from human blood could be verified. A recombinant expression system was developed to address these problems. The recombinant expression of HBsAg was achieved in HBV-transfected yeast[26,27]. Electron microscopy revealed that the expressed HBsAg polypeptides showed the same appearance as the particles isolated from human plasma. In 1986, the recombinant HB vaccine (the second-generation vaccine) was approved by the United States Food and Drug Administration[8,24]. Although new recombinant HB vaccines using HBV-transfected mammalian cells containing pre-S2+S envelope proteins (i.e., third generation vaccines) were commercialised in the 1990s[25,28,29], the majority of vaccine manufacturers are now adopting a yeast-system for recombinant expression[30]. The complete vaccine series induces protective levels of anti-HBs antibodies in more than 95% of infants, children, and young adults[1]. Although the duration of protection provided by the HB vaccine is controversial, the protection afforded by three or four doses of a monovalent HB vaccine persists for at least 20 years[1,31].
Two vaccine programs are being conducted worldwide to control and eradicate HBV infection. The first program is universal vaccination, which integrates a three- or four-dose series HB vaccine into routine vaccination programs. The other is selective vaccination, which targets high-risk individuals identified by assessments of chronic diseases, lifestyle, and occupation. The WHO strongly recommends universal vaccination in all countries, and nearly all of the countries throughout the world are adopting such a program[4]. In addition, high-income countries that can afford to perform screening of pregnant women give hepatitis B immune globulin (HBIG) to newborn babies born to HBsAg-positive mothers at birth. Because screening of pregnant women is costly and not feasible in low-income countries, the WHO does not recommend it for all countries. As shown in Table 1, the current HBV prevention strategies are classified into three groups: (1) universal vaccination without screening of pregnant women; (2) universal vaccination with screening of pregnant women plus HBIG; and (3) selective vaccination with screening of pregnant women plus HBIG.
| Universal vaccination alone | Universal vac. + pregnant-women screening + HBIG | Selective vaccination + pregnant-women screening + HBIG | |
| Low- and intermediate-income countries1 | √ | ||
| High-and intermediate-income countries1 | √ | ||
| (e.g., European countries, United States) | |||
| High-income countries (e.g., Scandinavian countries, United Kingdom, Japan) | √ |
In intermediate and highly endemic countries, universal vaccination without screening of pregnant women is clearly cost-effective[32]. However, the protective efficacy rate of a three- or four-dose HB vaccine series alone is 70%-80% in perinatal transmission[33-35]. In contrast, HB vaccine plus HBIG increases the protective efficacy rate to 95% in perinatal transmission[35]. Although aspects of the healthcare infrastructure, such as medical personnel, hospitals, and careful follow-up programs, are indispensable for the screening of pregnant women, the administration of HBIG can definitely improve the prevention rate of perinatal transmission.
Universal vaccination with screening of pregnant women plus HBIG is the most effective strategy to reduce and eradicate HBV, if and when the financial condition of a country allows for the implementation of such a program. In fact, almost all high-income countries are conducting universal vaccination with screening of pregnant women plus HBIG. The number of countries providing selective vaccination with screening of pregnant women plus HBIG is small and has gradually declined. Because the prevalence of HBV carriers is low in these counties, universal vaccination is considered not to be cost-effective; instead, the government’s target high-risk individuals for vaccination.
In 2012, a global survey of the efforts to prevent and control viral hepatitis was conducted on the behalf of the WHO by the World Hepatitis Alliance, a non-government organisation that represents approximately 280 hepatitis B and hepatitis C patient groups around the world[36,37]. Of the 194 WHO member states, 126 (64.9%) completed the surveys, which is not a sufficiently high response rate. The rates of response to WHO regional surveys were 26.1% (12/46) in the African Region, 77.1% (27/35) in the Americas Region, 77.3% (17/22) in the Eastern Mediterranean Region, 83.0% (44/53) in the European Region, 36.4% (4/11) in the South-East Asia Region, and 55.6% (15/27) in the Western Pacific Region.
The WHO member states are classified into five groups: “high income”, “upper-middle income”, “lower-middle income”, “low income”, and “other”. The rates of response to the WHO/World Hepatitis Alliance survey in these groups were 80% (40/50: high income), 64.2% (34/53: upper-middle income), 68.0% (34/50: lower-middle income), 47.4% (18/38: low income), and 0% (0/3: other). The proportion of high- and upper-middle-income countries was 2% (1/46) in the African Region, 60% (21/35) in the Americas Region, 32% (7/22) in the Eastern Mediterranean Region, 68% (36/53) in the European Region, 18% (2/11) in the South-East Asia Region, and 26% (7/27) in the Western Pacific Region.
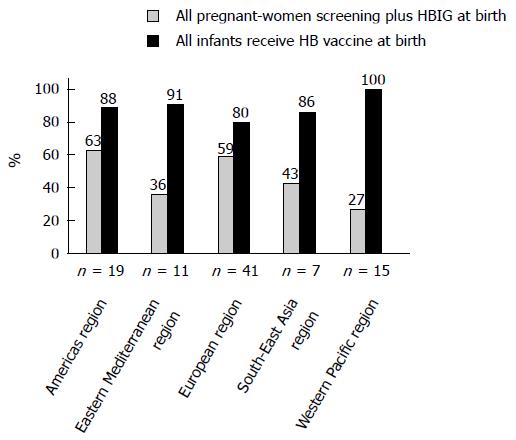
The rates of implementation of screening for all pregnant women plus HBIG at birth and programs administering the HB vaccine to all infants at birth in each WHO region (except for the African Region) are shown in Figure 1. According to this report, the rates of screening of pregnant women plus HBIG administration were 63% (Americas), 36% (Eastern Mediterranean), 59% (Europe), 43% (South-East), and 27% (Western Pacific), with more than half of the countries in the Americas and European Regions adopting HBIG administration after birth. Because high- and upper-middle-income countries are the majority in both the Americas and European regions, these regions can afford to provide screening of all pregnant women plus HBIG administration. In contrast, the Western Pacific Region shows the lowest rate (27%) of screening of pregnant women plus HBIG administration and the second-lowest ratio of high- and upper-middle-income countries.
In light of the low ratio of high- and upper-middle-income countries in the Western Pacific Region, the WHO Western Pacific Office strongly recommends the universal administration of a dose of HB vaccine to all newborn infants rather than a targeted approach for infants born to HBsAg-positive mothers[38]. The coverage of HB vaccine birth dose administration (within 24 h after birth) varies from 80% to 100% among the six WHO regions. The Western Pacific Region achieved 100% birth dose coverage, following sustained efforts by the Western Pacific Office over many years to improve the birth dose coverage in low-income counties[38]. Conversely, the birth dose coverage rate is not high in the regions where high-income counties are implementing screening of all pregnant women, such as the European Region (80%).
Vaccine shortages began to emerge in the late 1990s[39]. Vaccine manufacturers had begun phasing out the production of the traditional, less-expensive vaccines, such as the diphtheria-pertussis-tetanus (DPT) combination used in low-income countries. Between 1998 and 2001, 10 of 14 manufacturers partly or totally stopped their production of traditional vaccines. In 2001, the availability of the traditional DPT combination tuberculosis and measles vaccine dropped to the lowest level in 10 years. Moreover, the prices of these vaccines were increased.
Compared to traditional vaccines (e.g., DPT, oral polio, measles), which cost (USD) $0.06-0.10 per dose, the HB vaccine was expensive in the 1980s and 1990s[40,41]. In 1981, the price of a plasma-derived HB vaccine at introduction was $30 per dose. Although recombinant HB vaccines provide a safe and stable supply of HB vaccine, the price of the recombinant HB vaccine varied from USD $30 to $40, or nearly $100 for the complete series of three shots. Therefore, the benefits of the development of HB vaccines were not experienced by low-income counties, where the vaccines were greatly needed to prevent HBV infection.
The Global Alliance for Vaccine and Immunization (the GAVI Alliance) is a public-private partnership whose partners include United Nations agencies, the WHO, the World Bank, public health institutions, donor and recipient countries, the Bill and Melinda Gates Foundation, pharmaceutical manufacturers, and other members of the philanthropic and financial communities. The GAVI Alliance was launched in 2000 to establish vaccination programs in low-income countries. By bringing together low-income countries, donor governments, research and technical institutes, civil society organisations, vaccine providers, and private philanthropists, the dynamics of the global vaccine market have been changed by the establishment of sustainable supplies of vaccines, research, competition, and price reduction. During the first phase (2000-2005), hepatitis B became one of the three under-utilised vaccines [hepatitis B, Haemophilus influenzae type b (Hib), and yellow fever] immediately available for routine infant immunisation programs through the new and under-utilised vaccine flagship program[42-44]. The HB vaccine was considered to have the greatest potential in the implementation of under-utilised vaccines. The widespread use of new and under-utilised vaccines has the potential to contribute to the United Nations Millennium Development Goal 4 of reducing global childhood mortality by two-thirds by 2015[42,45,46].
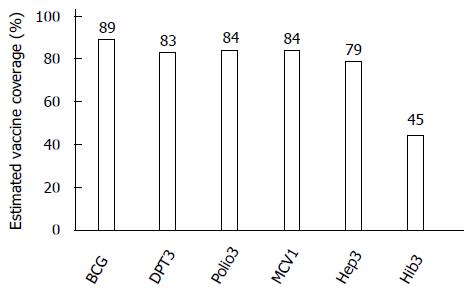
The global coverage in 2012 of traditional vaccines was as follows: Bacille Calmette-Guérin (BCG), 89%; the three-dose DTP (DPT3) vaccine, 83%; the three-dose polio vaccine (Polio3), 84%; and the first-dose measles-containing vaccine (MCV1), 84%[47]. In contrast, as shown in Figure 2, the 2012 coverage of the three-dose HB (Hep3) vaccine and the three-dose Hib vaccine (Hib3) was 79% and 45%, respectively[47]. A slight difference in the coverage between traditional vaccines and under-utilised vaccines remains. In the 2000s, the GAVI Alliance began introducing the combination pentavalent vaccine (DPT-hepB-Hib)[48]. The GAVI Alliance increased the number of manufacturers and reduced the price of the pentavalent vaccine. Most GAVI-eligible countries thus switched to the pentavalent vaccine. By 2015, the GAVI Alliance aims to support the immunisation of an additional 230 million children with the combination pentavalent vaccine[49]. Therefore, the immunisation status of HBV and Hib will soon become the same as that of DPT.
Almost all of the countries classified by the WHO as low-income use universal vaccination without screening of pregnant women. The WHO/United Nations Children’s Fund and the GAVI Alliance have important roles in the prevention of HBV infection in these countries. With the GAVI Alliance’s support, many low-income countries were able to introduce an HB vaccine into their routine vaccine programs. As shown in Figure 3, the global average of the universal three-dose HB vaccine immunisation rate was 30% of the WHO member states in 2000[47]. Very low introduction rates were revealed in the Africa Region (5%) and the South-East Asia Region (10%)[50]. Only the Americas Region achieved above 50% in the universal three-dose HB vaccine immunisation rate in 2000[50]. However, by 2012 the global average of the universal three-dose HB vaccine immunisation rates had more than doubled to 79%[47]. In that year, the routine three-dose HB vaccination coverage was approximately 90% in the Americas Region and Western Pacific Region, and the coverage rates in the African Region and South-East Asia had increased by 14- and 7-fold, respectively.
African region: All of the African Region WHO member states except for Algeria are in Sub-Saharan Africa, which has two-thirds of all of the worldwide cases of human immunodeficiency virus (HIV)[36]. The prevalence of HBV is estimated at 8% in West Africa and 5%-7% in Central, Eastern, and Southern Africa[51,52]. Due to the lower prevalence of the serum hepatitis B e antigen (HBeAg) in Africa compared to that in Asia, HBV infection in Africa is thought to be acquired almost always in early childhood by horizontal transmission rather than by vertical transmission[53-56]. In Sub-Saharan countries, a birth dose is not used; instead, a 6-, 10-, and 14-wk (“6-10-14”) after birth vaccination schedule is common[57,58]. Although universal infant HB vaccination was introduced in only 5% of the African Region member states in 2000, the introduction rate had increased to 72% by 2012[50]. Although a birth dose might be beneficial for African infants[58], no information about the birth dose or regional goals in Africa is available.
Americas region: In the WHO Americas Region, the prevalence of HBV infection varies from low to intermediate[59]. The prevalence of HBV infection is less than 2% in the central and tropical Latin America region, and it has remained between 2% and 4% in the Caribbean, Andean, and south Latin American regions[36,52]. In 2000, the Pan American Health Organization (PAHO) recommended that universal infant HB vaccination should be the primary strategy to prevent HBV transmission[59,60]. The decision to introduce a birth dose of HB vaccine depended on the prevalence of HBV carriers in each country. The PAHO recommended that a birth dose should be added to the vaccine program in the countries and territories where the prevalence of HBsAg exceed 8%[59,61]; the majority of the countries had no birth dose in their routine vaccine schedules. Without a birth dose, a 2-4-6 mo after birth schedule is predominant[59]. In 2012, however, the PAHO advised all of the region’s countries to introduce a birth dose (within 24 h of birth) for universal infant HB vaccination[62]. As shown in Figure 3, the universal three-dose HB vaccine immunisation rate of the Americans Region was 91% in 2012, the highest among the WHO regions.
Eastern Mediterranean region: The epidemiology of HBV infection in this region is complex. Before the introduction of the HB vaccine into the region’s routine immunisation programs, the prevalence of HBV carriers ranged from 2% to 3% in several member states; however, the prevalence was more than 10% in the region members of Somalia and Sudan[63]. Similar to other regions, the universal infant HB immunisation rate increased to 81% by 2012, which is double the figure in 2000[50]. In 2009, universal infant HB vaccination, including a birth dose (within 24 h of birth), was recommended by the Eastern Mediterranean Regional Office, and a reduction in the prevalence of chronic HBV infection to less than 1% among children over 5 years of age by 2015 was set as a time-bound regional goal[63].
European region: Although high- and upper-middle-income countries are the majority in the European Region, the rate of universal HB vaccination was only 79% in 2012, giving the region the rank of fourth highest among the six WHO regions[50]. Although Italy was one of the first countries to begin universal HB vaccination for infants, northern European countries are still using selective vaccination programs. Three countries give the first shot of HB vaccine to school-aged children[64]. It was reported that 13.3 million (1.8%) adults in the European Region had HBsAg; furthermore, two-thirds of the region’s residents who are infected with HBV live in the countries that are not part of the European Union/Free Trade Treaty Association[65], indicating that in the European Region one in 50 adults is an HBV carrier. As long as northern European countries continue selective vaccination, the universal immunisation rate will not reach 100%.
South-East Asia region: The universal HB immunisation rate in the South-East Asia Region in 2000 was 9%, the second lowest figure among the WHO regions. Although universal immunisation reached 72% in 2012, this figure was the lowest among the WHO regions[50]. Mirroring the universal HB vaccination rate, the 2012 DPT coverage rate of the South-East Asia Region was the second lowest (75%) among the WHO regions[66]. These findings suggest that the implementation of the entire panoply of immunisation programs is insufficient in this region. The WHO Regional Office encourages the South-East Asia member states to intensify their routine vaccine immunisation programs[67].
Western Pacific region: Although the Western Pacific Region comprises only 28% of the global population, the region bears a disproportionate burden of HB-related mortality and morbidity, accounting for almost half of all chronic HBV infections worldwide. An estimated 160 million people with chronic HBV infection live in this region, and the regional HBV-related mortality rate is comparable to that of tuberculosis[68,69]. The control and prevention of hepatitis B infection is thus the top priority in this region. In 2005, the Western Pacific region became the first region to set a time-bound goal of reducing the chronic HBV infection rate to less than 2% among five-year-old children by 2012[69]. This milestone influenced the individual countries’ national policies[70] and resulted in the highest coverage rate (91%) for universal HB vaccine immunisation among the WHO regions in 2012[50].
The universal vaccination with screening of all pregnant women plus HBIG strategy is expensive and complicated, but it is the most powerful strategy to prevent and control HBV infection. High-income countries, such as the United States and Italy, are implementing universal vaccination, screening all pregnant women, and administering HBIG to babies born to HBsAg-positive mothers at birth.
In the United States in 1982, the Advisory Committee on Immunization Practices (ACIP) recommended that persons with a substantial risk of HBV infection should be vaccinated[71]. The initial strategies for preventing HBV infection focused on the immunisation of high-risk groups: healthcare workers, men who have sex with men (MSM), drug users, recipients of certain blood products, and close contacts. However, many people who had no identifiable source for infection were infected with HBV[72]. In 1991, the ACIP proposed, for the first time, that hepatitis B vaccination was recommended for all infants regardless of the HBsAg status of the mother[73]. In Italy, an HB vaccine program for the high-risk groups began in 1983[74]. Despite the decreasing circulation of HBV in the late 1980s, a compulsory universal vaccination against HBV was introduced for all newborns and for 12-year-old children (a double cohort policy of mandatory immunisation) in Italy in 1991[75-77]. In some counties, a birth dose (administration within 24 h after birth) is not given if the screened mother is negative for HBsAg[78,79]. In the United States, a birth dose of the monovalent HB vaccine is given to babies born to HBsAg-negative mothers before discharge from the hospital[80]. In Europe, a monovalent or polyvalent vaccine is given to babies born to HBsAg-negative mothers at 2 or 3 mo after birth as the first dose[78,79]. As a birth dose, a monovalent vaccine should be used. In Germany, four doses are recommended for the hexavalent vaccine, and three doses are used for the monovalent vaccine[77,78]. Serum HBsAg is usually used for the screening of pregnant women. However, serum HBeAg is also used for screening in a few countries[81]. In Taiwan, free HBIG is administered to newborn babies born to HBs-positive and HBe-positive mothers[81-83]. Because the risk of mother-to-child transmission is considered low in babies born to HBsAg-positive and HBe-negative mothers, the administration of self-paid HBIG is optional for the families of babies born to HBeAg-negative mothers[82,83].
Denmark, Finland, Iceland, Japan, Norway, Sweden, and the United Kingdom, which are low-endemic countries (prevalence of HBsAg < 1%)[65], use selective vaccination[77,78]. Of these seven countries, in 2012, five were among the 10 countries with the lowest mortality rates for children under 5 years of age, and all seven are among the 24 richest countries in 2013[84,85]. In these countries, selective vaccination is considered more cost-effective than universal vaccination. In 2008, however, Ireland gave up selective vaccination and introduced a universal childhood vaccination program with a hexavalent vaccine[86,87]; the Netherlands decided to implement universal vaccination in 2011[88-90]. Selective vaccination targets individuals at high risk of HBV infection, but the definition of high risk varies from country to country. In 70% or more of the countries with selective immunisation programs, high-risk individuals include the following: injection drug users; non-injection users who are living with current injectors; sexual partners of injection users; children of injectors; MSM; close family contacts; healthcare workers; laboratory staff; police, fire, and rescue services; babies born to mothers who are chronically infected with HBV or to mothers who have had acute hepatitis B during pregnancy; people traveling to or going to reside in an area of high or intermediate prevalence; individuals receiving regular blood or blood products; and individuals in residential accommodations for those with learning difficulties[77]. In these countries, HBIG is given to babies born to HBsAg-positive mothers. In England, the indications for HBIG are as follows: infants of mothers with acute hepatitis, mothers who are HBsAg-positive and HBeAg-positive, mothers who are HBsAg-positive and HBeAg/anti-HBe-negative, mothers whose HBeAg/anti-HBe status is unknown, mothers whose serum HBV DNA levels are ≥ 1 × 106 IU/mL, and infants whose birth weight is less than 1500 g[91,92]. Japan has a unique schedule of HB vaccines plus HBIG. Babies born to HBeAg-positive mothers are administered HBIG twice (48 h and 2 mo after birth) followed by a three-dose HB vaccine series without a birth dose[93].
Since the introduction of infant universal vaccination, successful results of universal vaccination for HBsAg have been reported in many countries, although in almost all of the relevant studies the subjects are children and adolescents (Table 2). The report from China noted in Table 2 was a nationwide survey[94], and the remaining data concern limited areas of the United States, Colombia, Taiwan, Italy, South Africa, and Gambia. With the introduction of infant universal vaccination, the prevalence of HBsAg declined from 1.6% to 0.04% in the United States[95], from 7% to 2% in Colombia[96], from 9.8% to 1.3% in Taiwan[97], from 9%-12% to < 1% in China[94], from 13.4% to 0.91% in Italy[98], from 8%-9% to 0.9% in South Africa[99], from 13.3% to 0.6% in Gambia (Keneba), and from 35% to 1% in Gambia (Mandar)[100]. The highly endemic countries in particular showed a remarkable reduction in the prevalence of HBsAg.
| Ref. | Region | Country | Subjects | Year of the introduction of infant universal vaccination | HBsAg positive rate | ||||
| Before-vaccination program | Year | After vaccination program | Year | ||||||
| 95 | North America | United States | (Hawai) | School children | 1992 | 1.6% | 1989 | 0.04% | 2001-2002 |
| 96 | South America | Colombia | (Colombian Amazon) | 5-9 yr of age | 1992 | 7% | 1992 | 2% | 1999 |
| 97 | Asia | Taiwan | (Taipei) | ≤ 12 yr of age | 1984 | 9.8% | 1984 | 1.3% | 1994 |
| 94 | Asia | China | Young children | 1992 | 9%-12% | 1992 | < 1% | 2005 | |
| 98 | Europe | Italy | (Afragola) | General population | 1991 | 13.4% | 1978 | 0.91% | 2006 |
| 99 | Africa | South Africa | (Gauteng Province) | ≤ 24 mo after birth | 1995 | 8%-9% | 1995-1996 | 0.9% | 2003-2004 |
| 100 | Africa | Gambia | (Keneba) | ≤ 24 yr of age | 1984 | 13.3% | 1984 | 0.6% | 2003 |
| 100 | Africa | Gambia | (Mandar) | ≤ 24 yr of age | 1984 | 35% | 1984 | 1% | 2003 |
The incidence of acute hepatitis B is shown in Table 3. Almost all cases of acute hepatitis B are asymptomatic. All studies except a study that was performed in Alaska were nationwide surveys[101-104]. The study from Taiwan evaluated the mortality from fulminant hepatitis, whose pathogen was not identified[103]. The incidence of acute hepatitis B per 100000 people declined from 3 to 0.3 in the United States[101], from 19 to 0 in Alaska[102], from 5.36 to 1.71 in Taiwan[103], and from 5.1 to 1.3 in Italy[104]. Clear and sizable reductions in the incidence of acute hepatitis B were achieved after the introduction of universal HB vaccination programs.
| Ref. | Region | Country | Subjects | Year of the introduction of universal vaccination | Annual incidence of acute hepatitis B cases per 100000 population | |||
| Before vaccination program | Year | After vaccination program | Year | |||||
| 101 | North America | United States | 0-19 yr of age | 1992 | 3 | 1990 | 0.3 | 2002 |
| 102 | United States (Alaska) | Under 20 yr of age | 1984 | 19 | 1981-1920 | 0 | 1993-1994 | |
| 103 | Asia | Taiwan | Infants | 1984 | 5.36 | 1975-1984 | 1.71 | 1993-1994 |
| 104 | Europe | Italy | General population | 1991 | 5.1 | 1991 | 1.3 | 2005 |
It takes several decades for HCC to develop after an individual becomes an HBV carrier[105]. Only approximately 25 years have passed since the first introduction of universal vaccination for HBV, and thus the number of studies evaluating the incidence of HCC is still limited (Table 4). HCC associated with HBV infection was often observed in young children in Taiwan[106-108]; this finding was very useful for the evaluation of the effectiveness of universal vaccination in a short period. The study from Taiwan showed that nationwide universal vaccination reduced the incidence of HCC in children in 1997[109], which was 13 years after the introduction of the universal vaccine program in that country. The annual incidence of HCC per 100000 people in Taiwan declined from 0.7 to 0.36 in children 6 to 14 years of age and from 0.52 to 0.13 in those 6 to 9 years of age[109]. This was the first report proving that a vaccine could prevent cancer. Moreover, in Alaska, universal newborn vaccination coupled with a simultaneous catch-up vaccination program reduced the annual incidence of HCC per 100000 from 3 to 0[102]. Since 1999, no cases of HCC have occurred in Alaska[102].
| Ref. | Country | Subjects | Year of the introduction of universal vaccination | Annual incidence of HCC cases per 100000 population | |||
| Before vaccination program | Year | After vaccination program | Year | ||||
| 102 | United States (Alaska) | Under 20 yr of age | 1984 | 3 | 1984-1988 | 0 | 1995-1999 |
| 109 | Taiwan | 6-14 yr of age | 1984 | 0.7 | 1981-1986 | 0.36 | 1990-1994 |
| 109 | Taiwan | 6-9 yr of age | 1984 | 0.52 | 1974-1984 | 0.13 | 1984-1986 |
The GAVI Alliance estimated that 3.7 million future deaths from HBV infection were averted by vaccine programs conducted during 2000 and 2011[110]. The GAVI Alliance also estimated that 4.9 million future deaths will be averted during the years 2011 to 2020 in the 73 GAVI-eligible countries, compared to no vaccinations[111]. Although the global coverage of HB vaccine lags behind the global coverage levels for DPT, which is an indicator of expanded immunisation programs, the continued introduction of pentavalent vaccines will improve this situation.
In the WHO Africa and South-East Asia regions, however, the coverage of the HB vaccine was still lower than 80% in 2012[50]. Moreover, these two WHO regions had not reached 80% DTP3 coverage in 2012[50], a coverage minimum set by the Global Immunization Vision and Strategy (GIVS) as a target at the national level to form the framework for strengthening national immunisation programs in 2006 and 2015[112,113]. To improve the poor immunisation coverage and achieve the GIVS target, the South-East Asia Regional director declared 2012 as the year for intensifying routine immunisations[67,114]. In the South-East Asia Region, the coverage of a three-dose HB vaccine increased from 56% in 2011 to 72% in 2012[50].
Although inter-country differences exist, the Africa Region is facing a significant hurdle in its efforts to meet the GIVS goal[52,113,115]. Among HIV-infected individuals in sub-Saharan Africa, the prevalence of HBsAg is 15%[116]. HIV sero-positivity increases the risk of failure to respond to vaccines[117-119]. Many researchers have noted that to increase the HB vaccine coverage rate, it is necessary to secure firm commitments from governments (political commitment), financial flow, consistent scheduling of vaccination outreach programs, stable vaccine supplies, strong infrastructures (e.g., cold chain, stock space for new vaccines, delivery points), inter-sectional coordination, adequate human resources (including educated and trained healthcare providers), the education of women and parents, and surveillance systems to determine the impact of vaccines[120-126]. The GAVI Alliance is expected to have substantial public impacts on vaccination coverage in both WHO regions in 2011 and 2020[111,113].
A mathematical model predicts that 90% of the complete HB vaccine series coverage rate, including a birth dose, can achieve an 84% reduction in HBV-related deaths[127]. On the basis of this mathematical model, a pessimistic view is that the HB vaccine alone might be insufficient to eradicate HBV[128]. In addition, there is concern about the quality of immunisation coverage data. Inconsistencies in immunisation data have been observed in many countries. Although there are no data about such inconsistencies regarding the HB vaccine, the proportion of verified DPT-3 doses was found to be lower than 85% (after over-reporting) in 16 of 27 countries[129]. In many cases, the survey-based DPT-3 immunisation coverage rates have not reached the level suggested by countries’ official reports or WHO/UNICEF estimates[130]. Immunisation data quality audits are needed to obtain accurate and timely data and to determine how best to improve immunisation programs.
Not only the coverage rate but also age-appropriate vaccinations (i.e., timely vaccinations) are important to control infections. However, vaccinations are often delayed after the recommended ages[131-134]. In 31 low- and middle-income countries, the median fraction of timely administered vaccinations was 65% for BCG vaccine, 67% for DPT-1, 41% for DTP-3, 68% for polio-1, 38% for polio-3, and 51% for MCV[131]. The median delay in the 31 countries was 2.1 wk for BCG, 2.4 wk for DPT-1, 6.3 wk for DPT 3, 2.0 wk for polio-1, 6.6 wk for polio-3, and 4.1 wk for MCV[131]. Although that study did not discuss the HB vaccine, an HB vaccination delay could presumably be occurring in low- and middle-income countries. For example, in Argentina only 33% of children were vaccinated on time with the HB vaccine by seven months of age in 2002[135]. In Cambodia, the timely birth dose (within 24 h after birth) coverage was 66%[136]. In the 2000-2002 National Immunization Survey for each state in the United States, the timely administration of a three-dose HB vaccine among children aged 24 to 35 mo ranged from 49.4% (Vermont) to 81.6% (Rhode Island)[137]. Although the HB vaccine birth-dose coverage has increased year after year since 2000 in the United States, the nationwide coverage was still only 61.5% in 2007[138]. These data suggest that delayed vaccinations occur frequently in high-income countries just as in low-income countries.
The situation for programs providing for the screening of pregnant women plus HBIG, which is implemented in high-income countries, is more complicated. The screening rate of pregnant women varies from 96.5% to 98.8% in Puerto Rico, the United States, Italy, and Denmark[139-142]. In the United States, perinatal HBsAg test results were documented in 92.6% of maternal medical records; 13.7% of the infants born to HBsAg-positive mothers were not administered an HB vaccine, and 20.1% of infants were born to mothers with unknown HBsAg status[143]. Because the testing and reporting is incomplete in the United States, the true number of perinatal HBV cases per year is likely to be 10 to 20 times higher[144]. In infants born to HBsAg-positive mothers in the United States between 1994 and 2008, the administration rates of HBIG and the HB vaccine at birth remained at the same levels (from 90.3% to 96.4%), and the rate of completed 3-dose series vaccinations by age 12 mo decreased from 86.0% to 77.7%[145]. The reported rates of missed HBIG administration at birth were 4.0% in Denmark, 5.0% in Italy, 19.7% in the United States, and 62.4% in China[141-143,146]. To eradicate HBV infection, closing and/or filling the gaps between the recommended protocol and routine practices are imperative in all countries.
Because the awareness and knowledge of HBV infection and the HB vaccine are insufficient among people who engage in high-risk behaviours[147], it is important to approach high-risk groups using both selective and universal vaccination strategies[148-150]. The European Centre for Disease Prevention and Control reported that heterosexual transmission (23.4%), nosocomial transmission (23.2%), injected drug use (13.4%), and transmission among MSM (10.3%) were the most common routes for acute hepatitis B transmission in 2011[151]. The coverage rates of HB vaccination among high-risk populations were 6% to 39% (MSM, drug users, commercial sex workers, and heterosexuals; > three doses) in the Netherlands in 2007, 50.5% (> one dose), and 41.8% (> three doses) in the United States in 2009, and 22% (prisoners; > one dose) in England and Wales in 2010[66,152,153]. Even among healthcare workers, the coverage rate of the HB vaccine was 84.9% in Belgium, 85.3% in Italy, 87.5% in Poland, 88.0% in Spain, 93.0% in the United Kingdom, and 75.0% in the United States[154,155]. Household exposure and close family contacts of HBV carriers are another high-risk group. According to a survey of European countries, 90% of the countries with a universal vaccination program and all of the countries with a selective vaccination program recommended HB vaccination for individuals with close family contacts with HBV carriers[77]. However, the vaccine coverage was found to be only 25%-34% among the household contacts of HBV carriers in Italy, the United Kingdom, and Denmark[142,156,157].
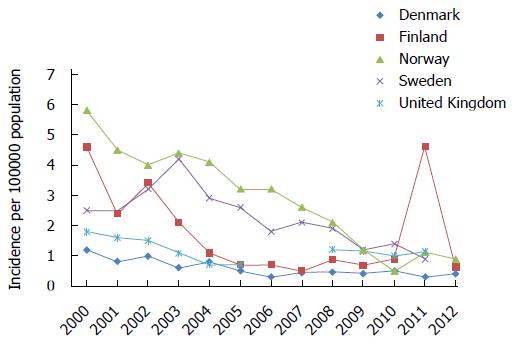
If high-risk adults are not identified or approached, the health of the children in the area will continue to be threatened. This is a particularly serious problem in the countries implementing selective vaccination policies, where almost all children are susceptible to HBV. Thus, whether these countries with selective vaccination will change their policies has become the focal point of increasing attention[158-163]. The incidence of acute hepatitis B in five northern European countries with selective vaccination is illustrated in Figure 4[164-166]. For the past decade in these countries, the incidence of acute hepatitis B continued to decrease. However, there is no obvious trend of decline in the incidence of acute hepatitis B per 100000 people, which fluctuates approximately 0.3-0.6 in Denmark and 0.7 to 1.0 in the United Kingdom. Because the burden of HBV has remained more or less the same over time since 1990 in the Netherlands despite the use of an intensified targeted approach, the Netherlands recognised the failure of the target vaccination strategy and decided to adopt universal vaccination[89]. Many studies have reported that it is difficult to reach high-risk groups[89,159,167-170], and the targeting of vaccinations for adults belonging to high-risk groups must be strengthened to eliminate HBV infection[171].
More than two decades have passed since the first introduction of nationwide universal vaccination, which occurred in Taiwan[172]. Unfortunately, the HB vaccine is not beneficial to individuals who are already chronically infected with HBV, and the majority of HBV carriers might have been born before the introduction of universal vaccination in their country. Some chronically infected individuals might miss the opportunities to receive an infant vaccination or a catch-up vaccination despite the implementation of vaccine programs. Most chronically infected people are unaware of their infection and thus do not receive appropriate treatment.
The WHO global action plan indicated that one of several remaining challenges is the fact that millions of chronically infected individuals do not have timely access to testing, care, and effective treatment to delay the development of the disease and to prevent disability[51]. In the United States, the incidence of acute hepatitis B declined by as much as 80% between 1987 and 2004[173]. However, this decline in acute hepatitis B did not diminish the burden of chronic HBV infection. The burden of HBV infection in the United States, as measured by inpatient and outpatient healthcare utilisation, waitlist registration for liver transplantation, and mortality related to HBV infection, increased substantially throughout the 1990s due to the immigration of a large number of persons from Africa and Asia[173]. In the United States, over half of the individual members of racial/ethnic minority groups have not been tested for HBV, and only one-half of those who tested positive have ever received treatment[174]. Similar to the United States, the United Kingdom also suffers from the burden of chronically infected individuals who were born outside the United Kingdom[175-178]. Chronic HBV infection in migrants has been estimated to account for 96% of all newly added chronic HBV infections in England and Wales between 1996 and 2000[175]. Because screening of the general population is unlikely to be cost-effective, the screening of high-risk populations has been introduced in high-income countries[179-182]. In 2008, the United States Centers for Disease Control updated and expanded the guidelines for testing for chronic HBV infection, in which persons born in geographic regions with an HBsAg-positive prevalence > 2% are recommended for testing[183]. The guidelines recommend the early testing and detection of chronic HBV infection. The 2% screening threshold for the prevalence of chronic HBV infection was demonstrated to be cost-effective[184]. Recent studies in the Netherlands and Canada demonstrated that a selective HBV screening program targeted at all migrants followed by early treatment could be cost-effective[180,185]. This “screen and treat” policy was reported to provide early disease detection, early antiviral treatment, the prevention of HBV-related advanced liver disease, and good quality of life[185,186]. In contrast, low-income countries have no prospect of screening of the high-risk population, diagnosis, or effective treatment[187,188]. The WHO estimates that less than 50% of the blood supply in sub-Saharan Africa is screened for HBV[188]. The budgetary allocation for the implementation of health programs is critically important to identify and treat HBV carriers.
There is no clear definition of “breakthrough infection.” Thus, the term is used in several varying contexts[189,190]. In general, “breakthrough infection” means that an HBV infection occurs despite a history of HB vaccination. In the literature, “breakthrough infection” is often characterised by the seroconversion for anti-HBc antibodies or the detection of HBsAg. The pre-existence of anti-HBs antibodies seems to not always be necessary for the diagnosis of “breakthrough infection”[190]. Therefore, primary vaccine failure (non-responder), waning immunity after vaccination (decline of anti-HBsAb levels over time), the emergence of escape mutants, and inappropriate vaccine schedules can all be causes of “breakthrough infection”.
Although the evidence is insufficient, non-genotype A could be one of the causes of breakthrough infections[191-193]. In the prophylactic treatment of mother-to-child transmission, a high maternal viral load is the highest risk factor for breakthrough infection[82]. Of course, off-schedule treatment and escape mutants can also cause a breakthrough infection during prophylactic treatment in the perinatal period[194]. The frequency of anti-HBc antibodies in vaccinated children born to HBV carrier mothers was 3.3% (HBsAg-positive carriers: 0.6%) in Italy[195], 8.9% (HBsAg-positive carriers: 3.5%) in China[196], 1.7% in the United Kingdom[197], 25.5% (HBsAg-positive carriers: 2.9%) in Thailand[198], and 6% (HBsAg-positive carriers: 2.9%) in Greenland[199]. In all of these countries except Thailand, HBIG was administered at birth.
In vaccinated adolescent general populations, the frequency of anti-HBc antibodies was 1.8% in Alaska, 13.8% in Gambia, and 4.1% in Taiwan[100,200,201]. Although the rate of breakthrough infection might be influenced by the prevalence of chronic HBV infection in each country, at least a few per cent of vaccinated children could experience a breakthrough infection. Nuclear acid amplification testing for the screening of blood donations, which was introduced for detecting the early window period of HBV infection, identified blood donors with vaccine breakthrough infections who had a history of vaccination and were positive for both HBV DNA and anti-HBs antibodies[192,193,202]. The blood donors with vaccine breakthrough infections developed subclinical acute infection but not chronic infection. Similarly, adolescents and young adults vaccinated in infancy showed transient infection but no chronic infection[203,204]. These findings suggested that a completed series of the HB vaccine cannot guarantee that HBV infection would be completely prevented[205].
The need for booster doses in HB vaccine programmes remains controversial. The duration of vaccine-induced immunity is uncertain, but it is definitely long-term (> 15-20 years)[172,203,206]. Several studies have reported that booster doses of infantile immunisation should be considered in adolescence[207-209]. However, numerous studies have demonstrated that booster doses are not needed in immunocompetent individuals who have received a complete series of HB vaccines[31,172,190,196,203,204,206,210,211]. At present, the WHO does not recommend the universal administration of booster doses. However, immunocompromised hosts, such as hemodialysis patients and HIV-positive patients, are known low responders to vaccines. Although routine serologic examinations of anti-HBs antibody levels are not needed after HB vaccination, it is recommended that healthcare providers, chronic hemodialysis patients, HIV-infected patients, and other immunocompromised individuals should be monitored and receive booster doses if their anti-HBs antibody levels decrease to less than 10 mIU/mL[190,211].
In 5%-10% of healthy individuals, a three-intramuscular-dose series of the HB vaccine fails to produce protective antibody levels (> 10 mIU/mL)[212-214]. Increasing age, smoking status, male gender, and obesity are the risk factors for poor or no response to the HB vaccine[212,213,215]. Specific human leukocyte antigen (HLA) types have been reported to be associated with the antibody response to the HB vaccine[216,217]. The HLAs are coded by the major histocompatibility complex (MHC) group of genes located on chromosome six in the human genome. The MHC complex plays a central role in the development of the adaptive immune response to HBsAg.
In efforts to overcome the low and non-responsiveness to the HB vaccine, several approaches have been proposed. An additional dose, an additional three-dose series, an increased vaccine dose, changing the route of administration, new adjuvants, and granulocyte-macrophage colony stimulating factor (GM-CSF) have all been proposed to be influential factors for improving the seroprotection rate. The most common strategy for low responders and non-responders is to give an additional vaccine or a series of vaccines. The best injection site was confirmed to be the deltoid muscle, except in infants[218,219]. Of low and non-responders to the initial three-dose series, 39%-91% and 61%-100% showed good responses after one additional dose (4th dose) and an additional three-dose series, respectively[220,221]. An additional three-high-dose series vaccine could further improve the seroprotection rate[222,223]. Moreover, an additional double dose of the combined hepatitis A and hepatitis B vaccine was shown to increase the seroprotection rate to 59% after the first dose and to 95% after the third dose in non-responders[224]. The hepatitis A component might act as an adjuvant for the hepatitis B response. In the United States, chronic hemodialysis patients who had no response to an initial three-dose series are advised to receive a second series using the same dose and schedule[225]. Clearly, the reduction in the numbers of non-responders depends on the number of additional doses[226,227]. Because it is speculated that intradermal inoculation may activate dermal keratinocytes and Langerhan’s cells, inducing an effective lymphocyte response[226,228], intradermal vaccination is considered to be superior to intramuscular vaccination[229]. However, a meta-analysis showed that intradermal vaccination was almost equivalent to intramuscular vaccination[229]. The HB vaccine using a new adjuvant system (AS04), which is a combination of a fragment of the bacterial lipopolysaccharide and alum, demonstrated more effective seroprotection rate results than a conventional HB vaccine[215,229]. GM-CSF is a candidate cytokine adjuvant, and it is used for the revaccination of non-responders[230]. A standard dose vaccine plus GM-CSF showed almost the same seroprotection rate as that provided by a high-dose vaccine in healthy non-responders[231]. Although there are several options to overcome poor responsiveness to HB vaccines, no consensus protocol has been established.
HB vaccines are very effective against HBV infection and were shown to be the first useful tool for cancer prevention. Financial support for global immunisation, as encouraged by the GAVI Alliance, has become solid and stable. However, the path to the eradication of HBV presents further obstacles. The difficulties to overcome include identifying the best ways to increase coverage rates, closing the gap between recommendations and routine practices, approaching and treating high-risk individuals, screening and treating chronically infected individuals, and preventing breakthrough infections. HBV infection is one of the diseases considered to be a candidate for global eradication, similar to polio, but it is presumed that several decades of effort will be necessary to eradicate HBV. We must acknowledge that the war against HBV will not be over soon. The difference in the prevalence of HBsAg between high- and low-endemic countries is becoming small. Eventually, the countries implementing selective vaccination for HBV will make the wise decision to introduce universal vaccination for global eradication. Although it is uncertain whether the victory against HBV will be gained using only vaccines; at present, vaccines are the most cost-effective method for the control of HBV infections.
P- Reviewer: Zhao HT S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | World Health Organization (2013). Media Centre. Hepatitis B. Accessed by September 4. 2013; Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. |
| 2. | Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA. 1995;274:1201-1208. [PubMed] |
| 3. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1973] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 4. | World Health Organization (2002). Global Alert and Response. Hepatitis B. Prevention and treatment. Accessed by July 24. 2013; Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index5.html. |
| 6. | Alter H. Baruch Blumberg (1925-2011). Nature. 2011;473:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Schmid R. History of viral hepatitis: a tale of dogmas and misinterpretations. J Gastroenterol Hepatol. 2001;16:718-722. [PubMed] |
| 8. | Pérez V. Viral hepatitis: historical perspectives from the 20th to the 21st century. Arch Med Res. 2007;38:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Blumberg BS, Bernanke D, Allison AC. A human lipoprotein polymorphism. J Clin Invest. 1962;41:1936-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Blumberg BS, Gerstley BJ, Hungerford DA, London WT, Sutnick AI. A serum antigen (Australia antigen) in Down‘s syndrome, leukemia, and hepatitis. Ann Intern Med. 1967;66:924-931. [PubMed] |
| 11. | Blumberg BS, Sutnick AI, London WT. Hepatitis and leukemia: their relation to Australia antigen. Bull N Y Acad Med. 1968;44:1566-1586. [PubMed] |
| 12. | Patlak M. The hepatitis B story. Accessed July 23. 2013; Available from: http://www.margiepatlak.com/files/QuickSiteImages/hepatitis.pdf. |
| 13. | Sutnick AI, London WT, Gerstley BJ, Cronlund MM, Blumberg BS. Anicteric hepatitis associated with Australia antigen. Occurrence in patients with Down’s syndrome. JAMA. 1968;205:670-674. [PubMed] |
| 14. | London WT, Sutnick AI, Blumberg BS. Australia antigen and acute viral hepatitis. Ann Intern Med. 1969;70:55-59. [PubMed] |
| 15. | Prince AM. An antigen detected in the blood during the incubation period of serum hepatitis. Proc Natl Acad Sci USA. 1968;60:814-821. [PubMed] |
| 16. | Gitnick GL, Gleich GJ, Schoenfield LJ, Baggenstoss AH, Sutnick AI, Blumberg BS, London WT, Summerskill WH. Australia antigen in chronic active liver disease with cirrhosis. Lancet. 1969;2:285-288. [PubMed] |
| 17. | Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695-698. [PubMed] |
| 18. | Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626-4649. [PubMed] |
| 19. | Bayer ME, Blumberg BS, Werner B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down’s Syndrome and hepatitis. Nature. 1968;218:1057-1059. [PubMed] |
| 20. | Hilleman MR, Buynak EB, Roehm RR, Tytell AA, Bertland AU, Lampson GP. Purified and inactivated human hepatitis B vaccine: progress report. Am J Med Sci. 1975;270:401-404. [PubMed] |
| 21. | Buynak EB, Roehm RR, Tytell AA, Bertland AU, Lampson GP, Hilleman MR. Vaccine against human hepatitis B. JAMA. 1976;235:2832-2834. [PubMed] |
| 22. | Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, Sadovsky R, Morrison JM, Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 677] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981;1:377-385. |
| 25. | Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266-6273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 26. | Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347-350. [PubMed] |
| 27. | McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178-180. [PubMed] |
| 28. | Michel ML, Pontisso P, Sobczak E, Malpièce Y, Streeck RE, Tiollais P. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerized human serum albumin. Proc Natl Acad Sci USA. 1984;81:7708-7712. [PubMed] |
| 29. | Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris). 2010;58:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | World Health Organization (2002). Global Alert and Resopnses. Hepatitis B. Surveillance and control. Accessed July 24. 2013; Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index4.html. |
| 31. | Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine. 2010;28:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Tu HA, Woerdenbag HJ, Kane S, Riewpaiboon A, van Hulst M, Postma MJ. Economic evaluations of hepatitis B vaccination for developing countries. Expert Rev Vaccines. 2009;8:907-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Wong VC, Ip HM, Reesink HW, Lelie PN, Reerink-Brongers EE, Yeung CY, Ma HK. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984;1:921-926. [PubMed] |
| 34. | Lo KJ, Tsai YT, Lee SD, Wu TC, Wang JY, Chen GH, Yeh CL, Chiang BN, Yeh SH, Goudeau A. Immunoprophylaxis of infection with hepatitis B virus in infants born to hepatitis B surface antigen-positive carrier mothers. J Infect Dis. 1985;152:817-822. [PubMed] |
| 35. | Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009;19:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | World Health Organization (2013). Global Alert and Response (GAR) Global policy report on the prevention and control of viral hepatitis in WHO Member States. Accessed by July 29. 2013; Available from: http://www.who.int/csr/disease/hepatitis/global_report/en/index.html. |
| 37. | World Health Organization (2011). Immunization, Vaccines and Biologicals. 2013; Available from: http://www.who.int/immunization/topics/hepatitis_b_survey_2010/en/#. |
| 38. | World Health Organization (2007). Western Pacific regional plan for hepatitis B control through immunization. Accessed by July 30. 2013; Available from: http://www.wpro.who.int/immunization/documents/docs/POA_HepB.pdf. |
| 39. | UNICEF (2002). Vaccines for children: supply at risk. Accessed by July 27. 2013; Available from: http://www.unicef.org/publications/index_4442.html. |
| 41. | Centers for Disease Control and Prevention (CDC). Global progress toward universal childhood hepatitis B vaccination, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:868-870. [PubMed] |
| 42. | Glatman-Freedman A, Cohen ML, Nichols KA, Porges RF, Saludes IR, Steffens K, Rodwin VG, Britt DW. Factors affecting the introduction of new vaccines to poor nations: a comparative study of the Haemophilus influenzae type B and hepatitis B vaccines. PLoS One. 2010;5:e13802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | GAVI alliance. Hepatitis B vaccine support. Accessed by July 38. 2013; Available from: http://www.gavialliance.org/support/nvs/hepb/. |
| 44. | WHO, UNICEF, the World Bank (2009). State of the world's vaccines and immunization. Third edition. Accessed by 28 July. 2013; Available from: http://www.who.int/immunization/sowvi/en/. |
| 45. | World Health Organization. Millennium Development Goals. Accessed by July 28. 2013; Available from: http://www.un.org/millenniumgoals/. |
| 46. | World Health Organization (2010). New and under-utilized vaccines implementation. Accessed by July 28. 2013; Available from: http://www.who.int/nuvi/en/. |
| 47. | World Health Organization (2013). Immunization surveillance, assessment and monitoring.Data, statistics, and graphics. WHO Region and globally. Accessed by Ausust 7. 2013; Available from: http://www.who.int/immunization_monitoring/data/gs_gloprofile.pdf. |
| 48. | GAVI alliance. Investing in immunisation through the GAVI Alliance. The evidence base. Accessed by July 28. 2013; Available from: http://www.gavialliance.org/library/publications/the-evidence-base/. |
| 50. | Word Health Organaization (2013). Immunization surveillance, assessment and monitoring. Reginal summaries. Regional profile. Accessed by August 10. 2013; Available from: http://www.who.int/immunization_monitoring/data/data_regions/en/index.html. |
| 51. | World Health Organization (2012). Prevention Control of Viral Hepatitis: Framework for Global Action. Accessed by August 1. 2013; Available from: http://www.who.int/csr/disease/hepatitis/GHP_framework.pdf. |
| 52. | Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1328] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 53. | Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38 Suppl 2:S5-S12. [PubMed] |
| 54. | Kew MC. Progress towards the comprehensive control of hepatitis B in Africa: a view from South Africa. Gut. 1996;38 Suppl 2:S31-S36. [PubMed] |
| 55. | Nur YA, Groen J, Elmi AM, Ott A, Osterhaus AD. Prevalence of serum antibodies against bloodborne and sexually transmitted agents in selected groups in Somalia. Epidemiol Infect. 2000;124:137-141. [PubMed] |
| 56. | Ott JJ, Stevens GA, Wiersma ST. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC Infect Dis. 2012;12:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Guido Françoisa CD, Jeffrey Mphahlelec M, Burnettd R, Van Hala G, Meheusb A. Hepatitis B vaccination in Africa: mission accomplished? J Epidemiol Infect. 2008;23:24-28. |
| 58. | Ekra D, Herbinger KH, Konate S, Leblond A, Fretz C, Cilote V, Douai C, Da Silva A, Gessner BD, Chauvin P. A non-randomized vaccine effectiveness trial of accelerated infant hepatitis B immunization schedules with a first dose at birth or age 6 weeks in Côte d’Ivoire. Vaccine. 2008;26:2753-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Ropero AM, Danovaro-Holliday MC, Andrus JK. Progress in vaccination against hepatitis B in the Americas. J Clin Virol. 2005;34 Suppl 2:S14-S19. [PubMed] |
| 61. | Pan American Health Organization. PAHO's Regional Immunization Vison and Strategy 2007-2015. Accessed by July 30. 2013; Available from: http://www.who.int/immunization/sage/PAHO_RIVS.pdf. |
| 62. | Pan Americam Health Organization (2012). World Hepatitis Day. Hepatitis vaccination in Latin America and the Caribbean. Accessed by July 30. 2013; Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=18239&Itemid=. |
| 63. | World Health Organization. Regional Office for the Eastern Mediterranean (2009). 2013; Available from: http://applications.emro.who.int/docs/EM_RC56_3_en.pdf. |
| 65. | Hope VD, Eramova I, Capurro D, Donoghoe MC. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: a review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiol Infect. 2014;142:270-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 66. | Lu PJ, Byrd KK, Murphy TV, Weinbaum C. Hepatitis B vaccination coverage among high-risk adults 18-49 years, U.S., 2009. Vaccine. 2011;29:7049-7057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | World Health Organization (2009). Hepatitis B control by 2012 in the WHO Western Pacific Region: rationale and implications. Accessed by July 28. 2013; Available from: http://www.who.int/bulletin/volumes/87/9/08-059220/en/. |
| 70. | Rani M, Yang B, Nesbit R. Hepatitis B control by 2012 in the WHO Western Pacific Region: rationale and implications. Bull World Health Organ. 2009;87:707-713. [PubMed] |
| 71. | Centers for Disease Control (CDC). Recommendation of the Immunization Practices Advisory Committee (ACIP). Inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31:317-322, 327-328. [PubMed] |
| 72. | Alter MJ, Hadler SC, Margolis HS, Alexander WJ, Hu PY, Judson FN, Mares A, Miller JK, Moyer LA. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA. 1990;263:1218-1222. [PubMed] |
| 73. | Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40:1-25. [PubMed] |
| 74. | Mele A, Stroffolini T, Zanetti AR. Hepatitis B in Italy: where we are ten years after the introduction of mass vaccination. J Med Virol. 2002;67:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Bonanni P, Crovari P. Success stories in the implementation of universal hepatitis B vaccination: an update on Italy. Vaccine. 1998;16 Suppl:S38-S42. [PubMed] |
| 76. | Bonanni P, Pesavento G, Bechini A, Tiscione E, Mannelli F, Benucci C, Nostro AL. Impact of universal vaccination programmes on the epidemiology of hepatitis B: 10 years of experience in Italy. Vaccine. 2003;21:685-691. [PubMed] |
| 77. | Mereckiene J, Cotter S, Lopalco P, D’Ancona F, Levy-Bruhl D, Giambi C, Johansen K, Dematte L, Salmaso S, Stefanoff P. Hepatitis B immunisation programmes in European Union, Norway and Iceland: where we were in 2009? Vaccine. 2010;28:4470-4477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | O'Flanagan D, Cotter S, Mereckiene J. Hepatitis B vaccination in Europe.November 2008-March 2009. The Health Protection Surveillance Centre European Centre for disease Control VENICE II Project. Accessed by August 3. 2013; Available from: http://venice.cineca.org/Report_Hepatitis_B_Vaccination.pdf. |
| 79. | O'Flanagan D; EUVAC. NET. National Childhood Vaccination Schedules. Accessed by September 3. 2013; Available from: http://www.euvac.net/graphics/euvac/vaccination/vaccination.html. |
| 80. | Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1-31. [PubMed] |
| 81. | Chen HL, Lin LH, Hu FC, Lee JT, Lin WT, Yang YJ, Huang FC, Wu SF, Chen SC, Wen WH. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142:773-781.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 82. | Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, Chen PJ, Chen DS, Chen HL. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 83. | Chen SC, Toy M, Yeh JM, Wang JD, Resch S. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobin treatment. Pediatrics. 2013;131:e1135-e1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | UNICEF (2012). The State of the World's Children 2012. Accessed by August 5. 2013; Available from: http://www.unicef.org/sowc2012/statistics.php. |
| 85. | Global Finance (2013). The Richest Countries in the World. Accessed by August 14. 2013; Available from: http://www.gfmag.com/component/content/article/119-economic-data/12538-the-richest-countries-in-the-world.html#axzz2YEiEtPjQ. |
| 86. | Ireland's Health Service Executive national immunisation programme. Thornton L (2008). Universal hepatitis B vaccination in Ireland-Why now? Accessed by August 4. 2013; Available from: http://www.immunisation.ie/en/Downloads/PDFFile_15136_en.pdf. |
| 87. | Tilson L, Thornton L, O’Flanagan D, Johnson H, Barry M. Cost effectiveness of hepatitis B vaccination strategies in Ireland: an economic evaluation. Eur J Public Health. 2008;18:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Houweling H, Spaendonck MC, Paulussen T, Verweij M, Ruitenberg EJ. Preparing for the next public debate: universal vaccination against hepatitis B. Vaccine. 2011;29:8960-8964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Houweling H, Wittevrongel CF, Verweij M, Ruitenberg EJ. Public vaccination programmes against hepatitis B in The Netherlands: assessing whether a targeted or a universal approach is appropriate. Vaccine. 2010;28:7723-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | The Health Council of the Netherlands (2009). General vaccination against hepatitis B revisited. Accessed by August 4. 2013; Available from: http://www.gezondheidsraad.nl/sites/default/files/200903E.pdf. |
| 91. | Public Health England (2008). Hepatitis B Guildelines. HBIG for babies born to hep b infected mothers. Accessed by July 29. 2013; Available from: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/HepatitisB/GuidelinesHepatitisB/hepbGuidelines/. |
| 92. | Public Health England (2012). Request form for: Issue of Hepatitis B Immunoglobulin for Infants at Risk of Hepatitis B Infection. Version 7 updated January 2012. Accessed by August 4. 2013;. |
| 93. | Shiraki K. Perinatal transmission of hepatitis B virus and its prevention. J Gastroenterol Hepatol. 2000;15 Suppl:E11-E15. [PubMed] |
| 94. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 95. | Perz JF, Elm JL, Fiore AE, Huggler JI, Kuhnert WL, Effler PV. Near elimination of hepatitis B virus infections among Hawaii elementary school children after universal infant hepatitis B vaccination. Pediatrics. 2006;118:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | de la Hoz F, Perez L, de Neira M, Hall AJ. Eight years of hepatitis B vaccination in Colombia with a recombinant vaccine: factors influencing hepatitis B virus infection and effectiveness. Int J Infect Dis. 2008;12:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Chen HL, Chang MH, Ni YH, Hsu HY, Lee PI, Lee CY, Chen DS. Seroepidemiology of hepatitis B virus infection in children: Ten years of mass vaccination in Taiwan. JAMA. 1996;276:906-908. [PubMed] |
| 98. | Da Villa G, Romanò L, Sepe A, Iorio R, Paribello N, Zappa A, Zanetti AR. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine. 2007;25:3133-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Simani OE, Leroux-Roels G, François G, Burnett RJ, Meheus A, Mphahlele MJ. Reduced detection and levels of protective antibodies to hepatitis B vaccine in under 2-year-old HIV positive South African children at a paediatric outpatient clinic. Vaccine. 2009;27:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, Doherty C, McConkey SJ, Jeffries D, Hall AJ. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193:1528-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Shepard CW, Finelli L, Fiore AE, Bell BP. Epidemiology of hepatitis B and hepatitis B virus infection in United States children. Pediatr Infect Dis J. 2005;24:755-760. [PubMed] |
| 102. | McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 103. | Kao JH, Hsu HM, Shau WY, Chang MH, Chen DS. Universal hepatitis B vaccination and the decreased mortality from fulminant hepatitis in infants in Taiwan. J Pediatr. 2001;139:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Mele A, Tosti ME, Mariano A, Pizzuti R, Ferro A, Borrini B, Zotti C, Lopalco P, Curtale F, Balocchini E. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: areas of improvement and emerging challenges. Clin Infect Dis. 2008;46:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 105. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [PubMed] |
| 106. | Hsu HC, Wu MZ, Chang MH, Su IJ, Chen DS. Childhood hepatocellular carcinoma develops exclusively in hepatitis B surface antigen carriers in three decades in Taiwan. Report of 51 cases strongly associated with rapid development of liver cirrhosis. J Hepatol. 1987;5:260-267. [PubMed] |
| 107. | Chen WJ, Lee JC, Hung WT. Primary malignant tumor of liver in infants and children in Taiwan. J Pediatr Surg. 1988;23:457-461. [PubMed] |
| 108. | Chang MH, Chen DS, Hsu HC, Hsu HY, Lee CY. Maternal transmission of hepatitis B virus in childhood hepatocellular carcinoma. Cancer. 1989;64:2377-2380. [PubMed] |
| 109. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 111. | Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, Reef SE, Schwalbe N, Simons E, Strebel PM. The estimated mortality impact of vaccinations forecast to be administered during 2011-2020 in 73 countries supported by the GAVI Alliance. Vaccine. 2013;31 Suppl 2:B61-B72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 112. | World Health Organaization (2005). Immunization, Vaccines and Biologicals. Global Immunization Vision and Strategy. Goals. Accessed by August 10. 2013; Available from: http://www.who.int/immunization/givs/en/index.html. |
| 113. | Kamara L, Lydon P, Bilous J, Vandelaer J, Eggers R, Gacic-Dobo M, Meaney W, Okwo-Bele JM. Global Immunization Vision and Strategy (GIVS): a mid-term analysis of progress in 50 countries. Health Policy Plan. 2013;28:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Vashishtha VM. Status of immunization and need for intensification of routine immunization in India. Indian Pediatr. 2012;49:357-361. [PubMed] |
| 115. | Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded programme on immunization in Africa: looking beyond 2015. PLoS Med. 2013;10:e1001405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 116. | Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis. 2010;14:e1024-e1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 117. | Bruguera M, Cremades M, Salinas R, Costa J, Grau M, Sans J. Impaired response to recombinant hepatitis B vaccine in HIV-infected persons. J Clin Gastroenterol. 1992;14:27-30. [PubMed] |
| 118. | Arrazola MP, de Juanes JR, Ramos JT, Aragón AJ, Garcia de Codes A. Hepatitis B vaccination in infants of mothers infected with human immunodeficiency virus. J Med Virol. 1995;45:339-341. [PubMed] |
| 119. | Pasricha N, Datta U, Chawla Y, Singh S, Arora SK, Sud A, Minz RW, Saikia B, Singh H, James I. Poor responses to recombinant HBV vaccination in patients with HIV infection. Trop Gastroenterol. 2005;26:178-182. [PubMed] |
| 120. | Clements CJ, Nshimirimanda D, Gasasira A. Using immunization delivery strategies to accelerate progress in Africa towards achieving the Millennium Development Goals. Vaccine. 2008;26:1926-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine. 2011;29:8215-8221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (1)] |
| 122. | Chauke-Moagi BE, Mumba M. New vaccine introduction in the East and Southern African sub-region of the WHO African region in the context of GIVS and MDGs. Vaccine. 2012;30 Suppl 3:C3-C8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Wiysonge CS, Uthman OA, Ndumbe PM, Hussey GD. Individual and contextual factors associated with low childhood immunisation coverage in sub-Saharan Africa: a multilevel analysis. PLoS One. 2012;7:e37905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 124. | Lahariya C, Subramanya BP, Sosler S. An assessment of hepatitis B vaccine introduction in India: Lessons for roll out and scale up of new vaccines in immunization programs. Indian J Public Health. 2013;57:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Wang SA, Hyde TB, Mounier-Jack S, Brenzel L, Favin M, Gordon WS, Shearer JC, Mantel CF, Arora N, Durrheim D. New vaccine introductions: assessing the impact and the opportunities for immunization and health systems strengthening. Vaccine. 2013;31 Suppl 2:B122-B128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 126. | Travasso C. Poor uptake of hepatitis B vaccine in India has several causes, study finds. BMJ. 2013;346:f3596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 127. | Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 477] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 128. | Thursz M, Njie R, Lemoine M. Hepatitis: Global eradication of hepatitis B--feasible or fallacy? Nat Rev Gastroenterol Hepatol. 2012;9:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 129. | Ronveaux O, Rickert D, Hadler S, Groom H, Lloyd J, Bchir A, Birmingham M. The immunization data quality audit: verifying the quality and consistency of immunization monitoring systems. Bull World Health Organ. 2005;83:503-510. [PubMed] |
| 130. | Lim SS, Stein DB, Charrow A, Murray CJ. Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet. 2008;372:2031-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 131. | Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66:e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 132. | Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 133. | Akmatov MK, Kretzschmar M, Krämer A, Mikolajczyk RT. Timeliness of vaccination and its effects on fraction of vaccinated population. Vaccine. 2008;26:3805-3811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Fadnes LT, Nankabirwa V, Sommerfelt H, Tylleskär T, Tumwine JK, Engebretsen IM. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine. 2011;29:3564-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 135. | Dayan GH, Shaw KM, Baughman AL, Orellana LC, Forlenza R, Ellis A, Chaui J, Kaplan S, Strebel P. Assessment of delay in age-appropriate vaccination using survival analysis. Am J Epidemiol. 2006;163:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 136. | Soeung SC, Thiep C, Duncan R, Patel M, Hennessey K. Using data to guide policy: next steps for preventing perinatal hepatitis B virus transmission in Cambodia. Vaccine. 2012;31:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 137. | Luman ET, Barker LE, McCauley MM, Drews-Botsch C. Timeliness of childhood immunizations: a state-specific analysis. Am J Public Health. 2005;95:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Zhao Z, Murphy TV, Jacques-Carroll L. Progress in newborn hepatitis B vaccination by birth year cohorts-1998-2007, USA. Vaccine. 2011;30:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 139. | Dayan GH, Caquías CR, García Y, Malik T, Copeland J, Bi D, Reef S. Medical practices for prevention of perinatal infections in Puerto Rico. Paediatr Perinat Epidemiol. 2008;22:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 140. | Schrag SJ, Arnold KE, Mohle-Boetani JC, Lynfield R, Zell ER, Stefonek K, Noga H, Craig AS, Thomson Sanza L, Smith G. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol. 2003;102:753-760. [PubMed] |
| 141. | Spada E, Tosti ME, Zuccaro O, Stroffolini T, Mele A. Evaluation of the compliance with the protocol for preventing perinatal hepatitis B infection in Italy. J Infect. 2011;62:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 142. | Harder KM, Cowan S, Eriksen MB, Krarup HB, Christensen PB. Universal screening for hepatitis B among pregnant women led to 96% vaccination coverage among newborns of HBsAg positive mothers in Denmark. Vaccine. 2011;29:9303-9307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 143. | Willis BC, Wortley P, Wang SA, Jacques-Carroll L, Zhang F. Gaps in hospital policies and practices to prevent perinatal transmission of hepatitis B virus. Pediatrics. 2010;125:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 144. | Ward JW. Time for renewed commitment to viral hepatitis prevention. Am J Public Health. 2008;98:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Smith EA, Jacques-Carroll L, Walker TY, Sirotkin B, Murphy TV. The national Perinatal Hepatitis B Prevention Program, 1994-2008. Pediatrics. 2012;129:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 146. | Hu Y, Zhang S, Luo C, Liu Q, Zhou YH. Gaps in the prevention of perinatal transmission of hepatitis B virus between recommendations and routine practices in a highly endemic region: a provincial population-based study in China. BMC Infect Dis. 2012;12:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 147. | Schenkel K, Radun D, Bremer V, Bocter N, Hamouda O. Viral hepatitis in Germany: poor vaccination coverage and little knowledge about transmission in target groups. BMC Public Health. 2008;8:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 148. | Hope VD, Ncube F, Hickman M, Judd A, Parry JV. Hepatitis B vaccine uptake among injecting drug users in England 1998 to 2004: is the prison vaccination programme driving recent improvements? J Viral Hepat. 2007;14:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Xiridou M, van Houdt R, Hahné S, Coutinho R, van Steenbergen J, Kretzschmar M. Hepatitis B vaccination of men who have sex with men in the Netherlands: should we vaccinate more men, younger men or high-risk men? Sex Transm Infect. 2013;89:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 150. | Judd A, Hickman M, Hope VD, Sutton AJ, Stimson GV, Ramsay ME, Gill ON, Parry JV. Twenty years of selective hepatitis B vaccination: is hepatitis B declining among injecting drug users in England and Wales? J Viral Hepat. 2007;14:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 151. | European Centre for Disease Prevention and Control (2013). Hepatitis B and C surveillance in Europe 2006-2011. Accessed by August 18. 2013; Available from: http://ecdc.europa.eu/en/publications/Publications/Forms/ECDC_DispForm.aspx?ID=1174. |
| 152. | van Houdt R, Koedijk FD, Bruisten SM, Coul EL, Heijnen ML, Waldhober Q, Veldhuijzen IK, Richardus JH, Schutten M, van Doornum GJ. Hepatitis B vaccination targeted at behavioural risk groups in the Netherlands: does it work? Vaccine. 2009;27:3530-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Beck CR, Cloke R, O’Moore É, Puleston R. Hepatitis B vaccination coverage and uptake in prisons across England and Wales 2003-2010: a retrospective ecological study. Vaccine. 2012;30:1965-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 154. | De Schryver A, Claesen B, Meheus A, van Sprundel M, François G. European survey of hepatitis B vaccination policies for healthcare workers. Eur J Public Health. 2011;21:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 155. | Simard EP, Miller JT, George PA, Wasley A, Alter MJ, Bell BP, Finelli L. Hepatitis B vaccination coverage levels among healthcare workers in the United States, 2002-2003. Infect Control Hosp Epidemiol. 2007;28:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 156. | Scognamiglio P, Girardi E, Fusco M, Piselli P, Spena SR, Maione C, Pisanti FA, Serraino D. Lack of implementation of Hepatitis B Virus (HBV) vaccination policy in household contacts of HBV carriers in Italy. BMC Infect Dis. 2009;9:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Richardson G, Evans MR, Westmoreland D. Hepatitis B immunisation of household contacts: retrospective study of vaccine coverage. J Epidemiol Community Health. 2001;55:934-935. [PubMed] |
| 158. | Zuckerman J, van Hattum J, Cafferkey M, Gjørup I, Hoel T, Rummukainen ML, Weiland O. Should hepatitis B vaccination be introduced into childhood immunisation programmes in northern Europe? Lancet Infect Dis. 2007;7:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 159. | Dawson AJ. An ethical argument in favour of routine hepatitis B vaccination in very low-incidence countries. Lancet Infect Dis. 2005;5:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 160. | English P. Should universal hepatitis B immunisation be introduced in the UK? Arch Dis Child. 2006;91:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 161. | British Medical Association. Hepatitis B vaccination in childhood (2010). Accessed by September 3. 2013; Available from: http://www.hepb.org.uk/news/current/BritishMedicalAssociationcallsforHBVVaccination.pdf. |
| 162. | Louise Heiberg I, Hogh B. Horizontal transmission of hepatitis B virus--why discuss when we can vaccinate? J Infect Dis. 2012;206:464-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 163. | Dannetun E, Tegnell A, Giesecke J. Parents’ attitudes towards hepatitis B vaccination for their children. A survey comparing paper and web questionnaires, Sweden 2005. BMC Public Health. 2007;7:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 165. | Public Health England (2012). Epidemiological Data. Hepatitis B annual report 2011. Accessed by August 16. 2013; Available from: http://www.hpa.org.uk/hpr/archives/2012/hpr3412.pdf. |
| 166. | Rantala M, van de Laar MJ. Surveillance and epidemiology of hepatitis B and C in Europe - a review. Euro Surveill. 2008;13:pii: 18880. [PubMed] |
| 167. | François G, Hallauer J, Van Damme P. Hepatitis B vaccination: how to reach risk groups. Vaccine. 2002;21:1-4. [PubMed] |
| 168. | Rimšelienė G, Nilsen Ø, Kløvstad H, Blystad H, Aavitsland P. Epidemiology of acute and chronic hepatitis B virus infection in Norway, 1992-2009. BMC Infect Dis. 2011;11:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 169. | Papaevangelou G. Hepatitis B immunization programme: lessons learnt in Greece. Vaccine. 1998;16 Suppl:S45-S47. [PubMed] |
| 170. | Baars JE, Boon BJ, Garretsen HF, van de Mheen D. The reach of a free hepatitis B vaccination programme: results of a Dutch study among drug users. Int J Drug Policy. 2010;21:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 171. | Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 172. | Ni YH, Chang MH, Wu JF, Hsu HY, Chen HL, Chen DS. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol. 2012;57:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 173. | Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49:S28-S34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 174. | Hu DJ, Xing J, Tohme RA, Liao Y, Pollack H, Ward JW, Holmberg SD. Hepatitis B testing and access to care among racial and ethnic minorities in selected communities across the United States, 2009-2010. Hepatology. 2013;58:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 176. | Chu JJ, Wörmann T, Popp J, Pätzelt G, Akmatov MK, Krämer A, Reintjes R. Changing epidemiology of hepatitis B and migration--a comparison of six Northern and North-Western European countries. Eur J Public Health. 2013;23:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 177. | Uddin G, Shoeb D, Solaiman S, Marley R, Gore C, Ramsay M, Harris R, Ushiro-Lumb I, Moreea S, Alam S. Prevalence of chronic viral hepatitis in people of south Asian ethnicity living in England: the prevalence cannot necessarily be predicted from the prevalence in the country of origin. J Viral Hepat. 2010;17:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 178. | Williams R, Holt AP. Screening immigrants for tuberculosis--why not for HBV infection? Lancet. 2013;381:2164-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 179. | European Centre for Disease Prevention and Control (2010). Technical Report. Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. Accessed by August 18. 2013; Available from: http://ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_C _EU_neighbourhood.pdf. |
| 180. | Veldhuijzen IK, Toy M, Hahné SJ, De Wit GA, Schalm SW, de Man RA, Richardus JH. Screening and early treatment of migrants for chronic hepatitis B virus infection is cost-effective. Gastroenterology. 2010;138:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 181. | Wong WW, Woo G, Heathcote EJ, Krahn M. Disease burden of chronic hepatitis B among immigrants in Canada. Can J Gastroenterol. 2013;27:137-147. [PubMed] |
| 182. | Fretz R, Negro F, Bruggmann P, Lavanchy D, De Gottardi A, Pache I, Masserey Spicher V, Cerny A. Hepatitis B and C in Switzerland - healthcare provider initiated testing for chronic hepatitis B and C infection. Swiss Med Wkly. 2013;143:w13793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 183. | Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20. [PubMed] |
| 184. | Eckman MH, Kaiser TE, Sherman KE. The cost-effectiveness of screening for chronic hepatitis B infection in the United States. Clin Infect Dis. 2011;52:1294-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 185. | Wong WW, Woo G, Jenny Heathcote E, Krahn M. Cost effectiveness of screening immigrants for hepatitis B. Liver Int. 2011;31:1179-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 186. | Woo G, Tomlinson G, Yim C, Lilly L, Therapondos G, Wong DK, Ungar WJ, Einarson TR, Sherman M, Heathcote JE. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol. 2012;26:445-451. [PubMed] |
| 187. | Nwokediuko SC. Chronic hepatitis B: management challenges in resource-poor countries. Hepat Mon. 2011;11:786-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 188. | Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol. 2013;8:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 189. | FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccination: a completed schedule enough to control HBV lifelong? Milan, Italy, 17-18 November 2011. Vaccine. 2013;31:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 190. | Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 191. | Abushady EA, Gameel MM, Klena JD, Ahmed SF, Abdel-Wahab KS, Fahmy SM. HBV vaccine efficacy and detection and genotyping of vaccineé asymptomatic breakthrough HBV infection in Egypt. World J Hepatol. 2011;3:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 192. | Stramer SL, Wend U, Candotti D, Foster GA, Hollinger FB, Dodd RY, Allain JP, Gerlich W. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 193. | Seed CR, Jones NT, Pickworth AM, Graham WR. Two cases of asymptomatic HBV “vaccine breakthrough” infection detected in blood donors screened for HBV DNA. Med J Aust. 2012;196:651-652. [PubMed] |
| 194. | Chang MH. Breakthrough HBV infection in vaccinated children in Taiwan: surveillance for HBV mutants. Antivir Ther. 2010;15:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 195. | Mele A, Tancredi F, Romanò L, Giuseppone A, Colucci M, Sangiuolo A, Lecce R, Adamo B, Tosti ME, Taliani G. Effectiveness of hepatitis B vaccination in babies born to hepatitis B surface antigen-positive mothers in Italy. J Infect Dis. 2001;184:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 196. | Young BW, Lee SS, Lim WL, Yeoh EK. The long-term efficacy of plasma-derived hepatitis B vaccine in babies born to carrier mothers. J Viral Hepat. 2003;10:23-30. [PubMed] |
| 197. | Boxall EH, A Sira J, El-Shuhkri N, Kelly DA. Long-term persistence of immunity to hepatitis B after vaccination during infancy in a country where endemicity is low. J Infect Dis. 2004;190:1264-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 198. | Poovorawan Y, Chongsrisawat V, Theamboonlers A, Srinivasa K, Hutagalung Y, Bock HL, Hoet B. Long-term benefit of hepatitis B vaccination among children in Thailand with transient hepatitis B virus infection who were born to hepatitis B surface antigen-positive mothers. J Infect Dis. 2009;200:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 199. | Børresen ML, Koch A, Biggar RJ, Ladefoged K, Melbye M, Wohlfahrt J, Krause TG. Effectiveness of the targeted hepatitis B vaccination program in Greenland. Am J Public Health. 2012;102:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 200. | Dentinger CM, McMahon BJ, Butler JC, Dunaway CE, Zanis CL, Bulkow LR, Bruden DL, Nainan OV, Khristova ML, Hennessy TW. Persistence of antibody to hepatitis B and protection from disease among Alaska natives immunized at birth. Pediatr Infect Dis J. 2005;24:786-792. [PubMed] |
| 201. | Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis. 2008;197:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 202. | Linauts S, Saldanha J, Strong DM. PRISM hepatitis B surface antigen detection of hepatits B virus minipool nucleic acid testing yield samples. Transfusion. 2008;48:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Kuriyakose S, Leyssen M, Jacquet JM. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat. 2011;18:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 204. | Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One. 2013;8:e58029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 205. | Boot HJ, van der Waaij LA, Schirm J, Kallenberg CG, van Steenbergen J, Wolters B. Acute hepatitis B in a healthcare worker: a case report of genuine vaccination failure. J Hepatol. 2009;50:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 206. | McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, Khristova M, Zanis C, Peters H, Margolis HS. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142:333-341. [PubMed] |
| 207. | Lu CY, Chiang BL, Chi WK, Chang MH, Ni YH, Hsu HM, Twu SJ, Su IJ, Huang LM, Lee CY. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology. 2004;40:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 208. | Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology. 2010;51:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 209. | Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology. 2013;57:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 210. | Banatvala JE, Van Damme P. Hepatitis B vaccine -- do we need boosters? J Viral Hepat. 2003;10:1-6. [PubMed] |
| 211. | Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561-565. [PubMed] |
| 212. | Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA. 1993;270:2931-2934. [PubMed] |
| 213. | Wood RC, MacDonald KL, White KE, Hedberg CW, Hanson M, Osterholm MT. Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA. 1993;270:2935-2939. [PubMed] |
| 214. | Coates T, Wilson R, Patrick G, André F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23:392-403. [PubMed] |
| 215. | Roukens AH, Visser LG. Hepatitis B vaccination strategy in vaccine low and non-responders: a matter of quantity of quality? Hum Vaccin. 2011;7:654-657. [PubMed] |
| 216. | Kubba AK, Taylor P, Graneek B, Strobel S. Non-responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106-112. [PubMed] |
| 217. | Li ZK, Nie JJ, Li J, Zhuang H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: a meta-analysis. Vaccine. 2013;31:4355-4361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 218. | Leads from the MMWR. Suboptimal response to hepatitis B vaccine given by injection into the buttock. JAMA. 1985;253:1705-1707. [PubMed] |
| 219. | de Lalla F, Rinaldi E, Santoro D, Pravettoni G. Immune response to hepatitis B vaccine given at different injection sites and by different routes: a controlled randomized study. Eur J Epidemiol. 1988;4:256-258. [PubMed] |
| 220. | Clemens R, Sänger R, Kruppenbacher J, Höbel W, Stanbury W, Bock HL, Jilg W. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule--results of a post-marketing surveillance. Vaccine. 1997;15:349-352. [PubMed] |
| 221. | Craven DE, Awdeh ZL, Kunches LM, Yunis EJ, Dienstag JL, Werner BG, Polk BF, Syndman DR, Platt R, Crumpacker CS. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med. 1986;105:356-360. [PubMed] |
| 222. | Bertino JS, Tirrell P, Greenberg RN, Keyserling HL, Poland GA, Gump D, Kumar ML, Ramsey K. A comparative trial of standard or high-dose S subunit recombinant hepatitis B vaccine versus a vaccine containing S subunit, pre-S1, and pre-S2 particles for revaccination of healthy adult nonresponders. J Infect Dis. 1997;175:678-681. [PubMed] |
| 223. | Lin CS, Xie SB, Liu J, Zhao ZX, Chong YT, Gao ZL. Effect of revaccination using different schemes among adults with low or undetectable anti-HBs titers after hepatitis B virus vaccination. Clin Vaccine Immunol. 2010;17:1548-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 224. | Cardell K, Akerlind B, Sällberg M, Frydén A. Excellent response rate to a double dose of the combined hepatitis A and B vaccine in previous nonresponders to hepatitis B vaccine. J Infect Dis. 2008;198:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 225. | Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1-43. [PubMed] |
| 226. | Fabrizi F, Dixit V, Magnini M, Elli A, Martin P. Meta-analysis: intradermal vs. intramuscular vaccination against hepatitis B virus in patients with chronic kidney disease. Aliment Pharmacol Ther. 2006;24:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 227. | Heininger U, Gambon M, Gruber V, Margelli D. Successful hepatitis B immunization in non- and low responding health care workers. Hum Vaccin. 2010;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 228. | Zuckerman AJ. Appraisal of intradermal immunisation against hepatitis B. Lancet. 1987;1:435-436. [PubMed] |
| 229. | Sangaré L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009;27:1777-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 230. | Lin R, Tarr PE, Jones TC. Present status of the use of cytokines as adjuvants with vaccines to protect against infectious diseases. Clin Infect Dis. 1995;21:1439-1449. [PubMed] |