Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.8910
Revised: January 24, 2014
Accepted: April 21, 2014
Published online: July 21, 2014
Processing time: 301 Days and 12 Hours
Mast cells (MCs), located ubiquitously near blood vessels, are descended from CD34+ hematopoietic stem cells. Initially, although their role has been well defined in hypersensitivity reactions, the discovery of their sharing in both innate and adaptive immunity has allowed to redefine their crucial interplay on the regulatory function between inflammatory and tumor cells through the release of mediators granule-associated (mainly tryptase and vascular endothelial growth factor). In particular, in several animal and human malignancies it has been well demonstrated that activated c-Kit receptor (c-KitR) and tryptase (an agonist of the proteinase-activated receptor-2) take pivotal part in tumor angiogenesis after the MCs activation, contributing to tumor cells invasion and metastasis. In this review, we focused on crucial MCs density (MCD) role in colorectal cancer (CRC) development and progression angiogenesis-mediated; then, we will analyze the principal studies that have focused on MCD as possible prognostic factor. Finally, we will consider a possible role of MCD as novel therapeutic target mainly by c-KitR tyrosine kinase inhibitors (imatinib, masitinib) and tryptase inhibitors (gabexate and nafamostat mesylate) with the aim to prevent CRC progression.
Core tip: In several malignancies it has been well demonstrated that mast cell (MC), activated c-Kit receptor (c-KitR) and tryptase secreted after MC degranulation play a pivotal role in tumor angiogenesis, helping tumor cell invasion and metastasis. The close relationship between MC density, angiogenesis and tumor progression could suggest a role for MCs as a possible prognostic factor in colorectal cancer (CRC). Moreover, considering MC-mediated CRC development, c-KitR tyrosine kinase inhibitors (imatinib, masitinib) and tryptase inhibitors (gabexate and nafamostat mesylate) could be used to block MC activation/degranulation and the tryptase/proteinase-activated receptor-2 axis respectively, and may be evaluated in future clinical trials in CRC patients.
- Citation: Marech I, Ammendola M, Gadaleta C, Zizzo N, Oakley C, Gadaleta CD, Ranieri G. Possible biological and translational significance of mast cells density in colorectal cancer. World J Gastroenterol 2014; 20(27): 8910-8920
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/8910.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.8910
In 1869 Nettleship and Tay[1] described a particular form of pigmented rash (“urticaria pigmentosa”), which presented a dermographism entirely similar to some urticaria forms. Mast cells (MCs) were identified by Ehrlich[2] in 1879 and named “mastzellen”(from the German mast = well-fed) because it was believed that they were particularly numerous in overfed animals. It was subsequently shown that cutaneous lesions observed in these animals were characterized by a focal accumulation of some of these mast cells[2]. In 1949 Ellis[3] described a form of systemic mastocytosis characterized by an abnormal infiltration of MCs into extracutaneous organs. Historically, “mastocytosis” is a morbid condition characterized by a marked increase (usually about ten times compared to normal) of the density of tissue MCs in specific anatomical sites[4]. Currently, “mastocytosis” includes a wide spectrum of clinical disorders (with an extremely heterogeneous clinical course and prognosis) sharing particular tyrosine kinase c-Kit receptor (c-KitR) mutations that confer its increased activation, determining stem cell factor (SCF)-independent MC proliferation[5,6].
MCs are the progeny of CD34+ hematopoietic stem cells and require SCF for their differentiation, activation and proliferation[7]. MCs are located throughout the body; on the epithelial surface, in blood vessels, nerves and glands[8]. Classically, MCs are divided into three subgroups according to the protease expression in their granules: the first type of MC contains only tryptase, the second only chymase, and the third tryptase, chymase and other proteases[8,9].
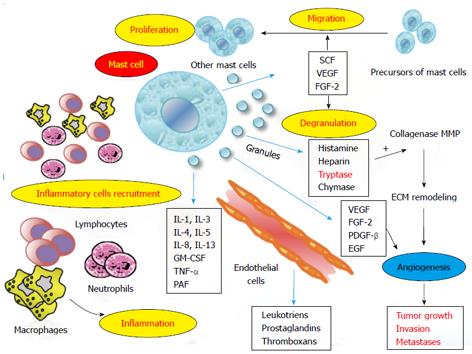
Although the role of mast cells has long been well defined in hypersensitivity reactions, since 1990[10,11] it has been discovered that they also have a role in both innate and adaptive immunity. This has allowed us to redefine their crucial interplay on the regulatory function between inflammatory and tumor cells[12-15] by means of the release of various granule-associated mediators [histamine, serotonin, heparin, tryptase, chymase, thymidine phosphorylase, tumour necrosis factor, vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), platelet-derived growth factor-β (PDGF-β), epidermal growth factor (EGF)]; lipid-derived mediators (leukotrienes, prostaglandins, platelet-activating factor); cytokines (transforming growth factor-β, interleukins, IL-6); and chemokines[16-19].
MCs express many types of receptors allowing them to recognize different stimuli and to respond accordingly[8,9]. For the fragment crystallisable portion of Immunoglobulin (Ig)G and IgE, MCs express various receptors, and in response to several antigens they release preformed (e.g., histamine, tryptase) and synthesized de novo mediators (i.e., leukotrienes, prostaglandins)[10,20]. Regarding innate immunity, MCs express some receptors for components of complement (CR3, CR4, CR5), and others belonging to the Nod-like receptors family. The recognition of pathogens by the innate immune cells and the link between innate and adaptive immunity however are via toll-like receptors (TLR type 1, 2, 3, 4, 6, 7 and 9)[21].
Many experimental studies have assessed MCs as protagonists both in inflammation and angiogenesis[20,22,23], processes closely interconnected and related to tumor development and progression[24-27]. Following the above-mentioned synthetic review of the various functions of MCs, in the upcoming sections we focus on the crucial role of MCs in angiogenesis-mediated tumor development and progression and illustrate the most common identification methods of MCs. In particular, as well as playing a role in tumor angiogenesis, it has been demonstrated that the number of MCs, so-called MC density (MCD), increases in several human and animal malignancies, and this increased MCD correlates with increased angiogenesis. On this basis, we analyze the principal studies that have focused on MCD as a possible prognostic factor, considering the MC as a possible novel therapeutic target in colorectal cancer (CRC).
During inflammatory reactions, immune cells (MCs, macrophages, neutrophils, and lymphocytes) synthesize pro-angiogenic factors that induce first neovascularization, then the further migration of inflammatory cells to the site of inflammation, amplifying the process[25,28]. At the same time, there is well-established evidence that tumor cells are surrounded by an infiltrate of inflammatory cells, which synergize with stromal cells and malignant cells in a paracrine manner[29-31]. As a consequence, there is a stimulation of endothelial cell proliferation and blood vessel formation[32-34]. It is important to underline that MCs are located near blood vessels and regulate many functions of endothelial cells[35-37].
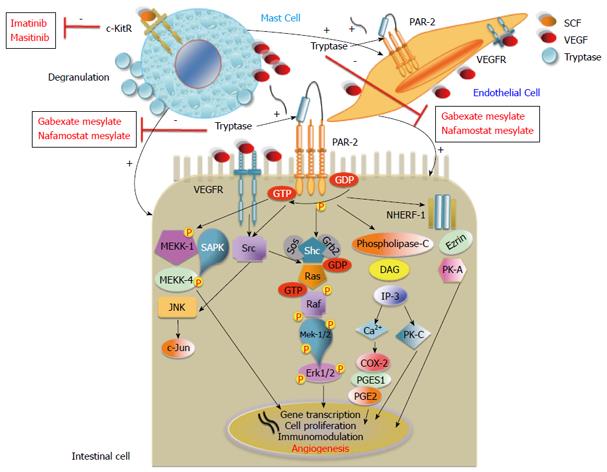
In particular, the c-KitR activated by SCF and tryptase after MC degranulation play pivotal part in tumor angiogenesis[38,39].
The increased activation of the c-KitR pathway leads to MC activation, which induces pro-angiogenic cytokines (such as VEGF, PDGF, FGF-2) and tryptase degranulation[38,39]. MC c-KitR activation induces cross-talk between MCs and the tumor cell microenvironment (endothelial and other cells), leading consequentially to the strengthening of pro-angiogenic signaling[6].
Tryptase is also an agonist of proteinase-activated receptor-2 (PAR-2)[40], which is expressed in epithelial and endothelial cells with proteolytic activities. It belongs to the unique superfamily of G-protein-coupled receptors and is activated by tryptase. Tryptase activation leads to cell proliferation and the release of IL-6 and granulocyte-macrophage colony-stimulating factor, which act as pro-angiogenic molecules[41]. Moreover, tryptase degrades extracellular matrix components[42], activating in its stored matrix metalloproteinases[43] and plasminogen activators that together help the invasion and metastasis of tumor cells[44] (Figure 1). In vitro studies on matrigel and in vivo studies on the chick embryo chorioallantoic membrane displayed the capillary growth induced by tryptase and, conversely, suppressed by tryptase inhibitors[45,46].
Apart from the above biological background, the role of MCs in tumor development has emerged from observation of a strong correlation between an increase of MCD and an increase of microvascular density (MVD) in many human and animal malignancies such as oral squamous carcinoma[13,47], breast cancer[11,12,16], gastrointestinal cancer[26,48-50], hepatocarcinoma[51], pancreatic adenocarcinoma[52], renal cell carcinoma[53], non-small cell lung cancer[54,55], melanoma[56], endometrial carcinoma[27,57], non-Hodgkin’s lymphomas[58], and multiple myeloma[59]. With particular reference to hematological disorders, some evidence suggest that high MCD infiltration is directly correlated with tumor progression and worse disease outcome[60-62].
Conversely, a few studies have shown that high MCD is linked to good prognosis[63,64].
To further emphasize that MC activation plays a pivotal role in tumor progression, it was shown in breast cancer that degranulated MCs (MCs-Try) are mainly present in peri-tumoral tissue (to strengthen the hypothesis that they are tumor-reactive), unlike those rich in granules MCs (MCs-TB) which are especially present in tumor infiltration and contribute to stromal remodeling and differentiation of myofibroblasts (through tryptase released in stromal microenvironment)[11].
The close relationship between MCD, angiogenesis and tumor progression could suggest a role for MCs and the pro-angiogenic factors released from them as novel therapeutic targets in cancer. In particular, it is possible to block MC activation/degranulation by means of c-KitR tyrosine kinase inhibitors (TKI) such as imatinib and masitinib, and also to block the tryptase released from MCs by means of tryptase inhibitors (gabexate and nafamostat mesylate)[12,65-67].
MCs can be classically or conventionally identified by means of histochemical methods. Among these, Toluidine blue histochemistry (Undritz Stain) metachromatically stains MC granules, making them appear red or blue-red due to the presence of sulphated proteoglycans (heparin)[68]. With the above histochemistry, MCs appear as rather large oval or elongated cells (diameter of 20-30 μm) containing numerous basophilic granules in their cytoplasm that can hide the nucleus[12,69].
By immunohistochemistry MCs can be stained with antibodies towards c-KitR (e.g., human-specific monoclonal antibodies anti-CD117), towards the content of their granules, i.e., tryptase or chymase[68]. With a primary anti-c-KitR antibody, a membrane, cytoplasmic or mixed staining is observed[68]. With primary anti-chymase and anti-tryptase antibodies a diffuse cytoplasmic staining is observed[68].
Under the electron microscope MCs present a small, round nucleus, few mitochondria, some meandering tanks of rough endoplasmic reticulum and a small Golgi complex. The numerous specific granules (some hundreds) measure 0.3-0.8 μm in diameter and appear bordered by a membrane showing a variable fine granular or lamellar structure[70,71].
Following their activation, MCs degranulate and exocytose the content into the surroundings. Piecemeal degranulation is typified by variable losses of the granule content[71-73].
Normally, MCs are present in the mucosa and submucosa of the gastrointestinal tract in humans and mice[74].
In a preclinical study in mice, MCs played a crucial role in epithelial tumorigenesis, appearing in early dysplastic tissue and expanding in polyps[75]. However, when analysing the potential role of MCs in tumor development in several mice studies, Heijmans et al[76] were unable to draw certain conclusions due to a lack of a suitable animal model to study CRC. In fact, in IL-10-deficient mice with MCs Chichlowski et al[77] showed a reduced risk of development of inflammatory bowel disease (IBD) compared to in that of IL-10-deficient mice without MCs. Thus, this result emphasizes the protective role of MCs within the colonic microenvironment by enhancing the efficacy of the mucosal barrier. In reality, these data suggest that MCs can play a dual and opposite function, and this is probably due to the presence in the intestinal tract of different types of MCs, each with a specific role, with specific granules, and expressing various receptors[74].
It is noted that patients affected by IBD have an increased cumulative incidence of CRC than the general population and that this incidence increases with the duration of the bowel disease[78]. In particular, it was found that high MCD in intestinal adenomatous polyps[75,79-81] could drive a cascade of events to boost the progressive growth of adenomatous polyps, the immediate precursors of CRC[75].
In this regard, Taweevisit, considering 192 CRC patients, displayed a direct correlation between MCD, tumor development and grading[82].
With the aim to find a correlation between MCD and stage/prognosis in CRC patients, many studies (summarized in Table 1) have been conducted with mixed results. One Author showed no correlation between MCD and prognosis[83,84]. Other Authors have shown a direct and significant correlation between high MCD and improved prognosis[85-87]. The majority of studies however have shown that high MCD is related to tumor aggressiveness[48-50] and reduced survival[88-90].
| Ref. | Disease stage/main stages | Neoadjuvant therapy | Patients (n)/site | Methods of MCs identification | Correlation with overall survival/stage | P value |
| Xia et al[83] | All TNM stages | No | 155 | Immunohistochemistry | No with OS | NS |
| (mainly II-III) | CC | primary anti-tryptase and anti-chymase abs | ||||
| Xia et al[84] | Stage IIIB | No | 93 | Immunohistochemistry | No with OS | NS |
| CC | primary anti-tryptase ab | |||||
| Nielsen et al[85] | All Dukes’ stage | No | 584 | Immunohistochemistry | Yes, high MCD with high OS | 0.02 |
| (mainly B-C) | CRC | primary anti-tryptase ab | ||||
| Tan et al[86] | All TNM stages | NR | 60 | Immunohistochemistry | Yes, high MCD with high OS | < 0.01 |
| CRC | primary anti-tryptase and anti-chymase abs | |||||
| Fisher et al[88] | All Dukes’ stage | No | 331 | Giemsa method | Yes, high MCD with low OS | NE |
| (mainly B-C) | RC | |||||
| Yodavudh et al[89] | All TNM stages | No | 130 | Immunohistochemistry | Yes, high MCD with low OS | < 0.0001 |
| (mainly II-III) | CRC | primary anti-tryptase ab | ||||
| Elezoğlu et al[87] | All TNM stages | NR | 204 | Toluidine blue histochemistry | Yes, high MCD with high OS | 0.035 |
| (mainly II-III) | CRC | |||||
| Acikalin et al[49] | All TNM stages | No | 60 | Giemsa method | Yes, high MCD with low OS | 0.0013 |
| (mainly II-III) | CRC | |||||
| Gulubova et al[50] | All TNM stages | No | 106 | Immunohistochemistry | Yes, high MCD with low OS | 0.038 |
| (mainly II) | CRC | primary anti-tryptase ab; toluidine blue histochemistry |
Xia et al[83] studied MCD in 39 patients with colon adenoma and in 155 colon cancer (CC) patients of all TNM stages, evaluating a relationship between MCD (positive to both tryptase and chymase) and tumor progression. Interestingly, a significant increase of MCD localized in adjacent normal colon mucosa in CC patients was noted compared to those with colon adenomas (P < 0.05)[83]. Moreover, MCD located in adjacent normal colon mucosa in CC patients was significantly related to pathologic classification (i.e., papillary plus tubular or other), depth of penetration (i.e., high T according to TNM), distant metastases (i.e., M1 according to TNM), and hepatic metastases (P = 0.029, P = 0.054, P = 0.008, P = 0.027)[83]. Instead, there is no correlation between MCD located in the invasive margin or in adjacent normal colon mucosa and survival (P = 0.092 and P = 0.003)[83]. Similarly, in 93 CC patients only in stage IIIB (according to TNM staging), the same Author observed a higher MCD positive to tryptase in non-metastatic regional-draining lymph nodes than in metastatic lymph nodes (P = 0.000)[84].
In 1999, Nielsen et al[85] analysis in a large cohort of CRC patients (n = 584) of all Dukes’ stages displayed a significant correlation between high MCD positive to tryptase and good prognosis (P = 0.02); 50% of all patients with high MCD positive to tryptase were still alive at 3 years.
Subsequently, Tan et al[86] observed that high MCD (positive to tryptase and chymase) is also related to a significantly higher 5-year survival rate (SR). In their study on 60 CRC patients of all TNM stages, a 59% SR was recorded for patients with high MCD compared to 33.3% in those with low MCD (P < 0.01). Curiously, low MCD was significantly related to deeper depth of invasion, but also to low rates of lymph node and distant metastases[86].
Recently, Elezoğlu and Tolunay[87] displayed a significant correlation between MCD positive to tryptase, MVD, and survival in 204 CRC patients of all TNM stages. In the MC group, for values < 10, the five-year SR was 48%, whereas for values > 10 it rose to 58% (P = 0.035). In the MVD arm for values < 10, the five-year SR was 46%, while for values ≥ 10 it was 58% (P = 0.042)[87].
In 1989 Fisher et al[88] was one of the first researchers to identify high MCD as an unfavorable prognostic factor independent from disease stage or lymph nodal status in 331 rectal cancer patients of all Dukes’ stages.
In 60 patients with CRC of all TNM stages Acikalin et al[49] showed that MCD (evaluated by means of the Giemsa stain) was higher in patients with disease recurrence compared to those patients who had been disease free for at least 24 mo (P < 0.001), and that it was correlated to short disease-free survival (P = 0.0013), vascular invasion (P = 0.06), depth of penetration (P = 0.05), lymph nodes metastases (P = 0.05), liver metastases (P = 0.05) and high TNM stage (P = 0.05).
Yodavudh et al[89] confirmed Elezoğlu and Tolunay[87]’s report of a strong correlation between MCD positive to tryptase, MVD, and survival in 130 CRC patients of all TNM stages. Contrarily however, they showed that low MVD (hypovascular tumor tissue) and low MCD are related to significantly longer survival rates (P < 0.0001).
Gulubova and Vlaykova[50] also confirmed a significant correlation between MCD positive to tryptase, MVD, and survival in 106 CRC patients of all TNM stages. Patients with low MCD had a significantly better prognosis compared to those with high MCD (P = 0.038)[50]. In the same way, hypovascular tumor tissue was related to highly significantly longer survival than hypervascular tumor tissue (P < 0.0001)[50].
In a recent series of 41 gastrointestinal cancer patients (of whom 22 had CRC of TNM stage IIIC), Ammendola et al[30] showed a significant correlation between MCD positive to tryptase and the number of metastatic lymph nodes harvested (P = 0.01), and between MCD in primary tumor tissue and in metastatic lymph node tissue (P = 0.02). These data suggest that MCD in primary tumor tissue could be a useful prognostic marker[30,49], surrogating the number of postoperative metastatic lymph nodes after surgical treatment in gastrointestinal cancer patients[91-94].
Even more recently, Malfettone et al[90] showed in 115 CRC patients of all TNM stages that high MCD positive to tryptase correlates with the advanced stages of CRC (P = 0.025). In particular, the expression of PAR-2 (especially at the sites most infiltrated by MCs) is related to MCD expression[90]. Due to the pro-angiogenic activity of tryptase, which stimulates PAR-2 on endothelial cells, it is possible to suggest an involvement of tryptase in CRC angiogenesis[90].
Ducroc et al[95] demonstrated a pivotal role of MC tryptase in inducing PAR-2 activation in several human CC cell lines (T84, Caco-2, HT-29, Cl.19A), promoting their proliferation.
Yoshii et al[96] investigated the distribution of MCD (positive to tryptase) in 30 human CC, showing the prevalence of MCD in the invasive front rather than in either the central tumor part or the normal tissue. In addition, the Authors showed a higher density of PAR-2 in the tumor tissue compared to the normal tissue[96].
Interestingly, two Authors explored the tryptase/PAR-2 axis in one human colon carcinoma cell line (DLD-1)[96,97]. Specifically, the proliferation signal induced by tryptase on DLD-1 cells is mediated by PAR-2, that in turn leads to the increase of calcium[98] and transient phosphorylation of mitogen-activated protein kinase/extracellular signal-related kinase (MEKK) and the mitogen-activated protein kinase (MAPK) pathway[96]. In addition, the increase of calcium PAR-2/Phospholipase C-mediated led to the activation of CycloOXygenase-2 (COX-2) and prostaglandin E2 (PGE2) synthesis, suggesting that the MEKK and MAPK pathway activation and PGE2 synthesis were together essential for DLD-1 proliferation[96] (Figure 2).
Sodium-hydrogen antiporter 3 regulator 1 (NHERF-1) is a cytoplasmic adaptor protein present in various cellular types (including intestinal cells). NHERF-1 regulates several transmembrane receptors, transporters and other proteins localized near the plasma membrane, and via the Ezrin/protein kinase-A- mediated network seems to lead to CRC progression[99,100].
Interestingly, Malfettone et al[90], having confirmed the close interplay between MCD and PAR-2 in tumour progression and invasiveness, showed that the PAR-2(+)/cytoplasmic NHERF-1(+) expression immunophenotype is an unfavourable prognostic factor in CRC patients, as it is associated with the presence of lymph nodal and distant metastasis, poor differentiation grade and lymphovascular invasion. If further studies conducted in stage II CRC patients should confirm the role of the PAR-2(+)/cytoplasmic NHERF-1(+) expression immunophenotype as a negative prognostic biomarker, it will become a prerequisite to the treatment of patients with adjuvant chemotherapy.
Finally, if future studies demonstrate that high MCD positive to tryptase is an independent unfavourable prognostic factor[30,49,50,88,89] related to a significant and increased risk of tumor progression, this parameter could be considered in the decision to give chemotherapy associated with tryptase inhibitors (gabexate and nafamostat mesylate).
Clearly, before being able to use MC targeted agents, a more in-depth knowledge of MC-mediated angiogenic mechanisms and the complex hierarchical relationships between the various angiogenesis signaling pathways will be necessary[101-104].
In this regard, tryptase may induce angiogenesis mainly by the increase of VEGF expression mediated via PAR-2, which is expressed also on endothelial cells as well as intestinal cells[12,27,45,54]. Moreover, VEGF and its receptors are widely expressed in intestinal carcinoma cells, and VEGF stimulates VEGFR-2-positive tumor, mast and endothelial cells directly, leading to tumor growth and angiogenesis by paracrine and autocrine stimulation signals[26,105,106].
Considering the central role of MCs in the activation of gastrointestinal and endothelial cells which contribute to tumor angiogenesis and progression, c-KitR could also be a potential therapeutic target for inhibiting their pro-angiogenic cytokine degranulation (VEGF, PDGF, FGF, tryptase) and activation[6,38,67,107]. In fact, MC c-KitR activation potentiates the cross-talk between MCs and endothelial cells (Figure 2), leading to the strengthening of pro-angiogenic signaling. Therefore, MCs could represent a possible therapeutic target through tryptase inhibitors (gabexate and nafamostat mesylate) and c-KitR inhibitors (imatinib, masitinib) to arrest angiogenesis-mediated tumor growth in gastrointestinal cancer[108-110].
Although the role of MCs was well defined in hypersensitivity reactions, the discovery of their regulatory function in innate and adaptive immunity has allowed us to understand their complex interplay between inflammatory and tumor cells. In fact, much evidence obtained from in vitro and in vivo studies has demonstrated that common MCs phenotypes, if adequately stimulated by various factors (histamine, heparin, tryptase, chymase, VEGF, FGF-2, PDGF-β, EGF), are able to interfere with tumor cells and the tumor microenvironment inducing tumor angiogenesis and progression[10,12].
Although the majority of studies have reported that several malignancies are associated with an increase of MC infiltration, controversial data about the relationship between MCD and prognosis in CRC have been reported. Considering these studies, conflicting conclusions[48-50], may in part depend on considerable bias related to CRC disease (radical surgical treatment with relative lymph node collection, type of resection, histology or stage tumor, colon plus rectal cancer, small sample size)[83,85,86,88], and different methods of MC evaluation (histochemistry with Toluidine blue, Giemsa stain, primary antibody anti-tryptase or anti-chymase for immunohistochemistry, standardization of MC counts with reference to magnification, MC location, microscopic field of evaluation)[76,84,87,90]. Despite these biases, the majority of the published studies suggest that high MCD in tumors may play a role as an unfavourable prognostic marker. Should this prognostic marker be validated in expected future studies it would be intriguing to conduct clinical trials employing chemotherapy plus tryptase inhibitors or TK inhibitors MC c-KitR.
P- Reviewers: Chen JL, Huang ZH, Stanojevic GZ S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Nettleship T, Tay W. Rare forms of urticaria. Brit Med J. 1869;2:323-330. |
| 2. | Ehrlich P. Beiträge zur Kenntniss der granulirten Bindegewebszellen und der eosinophilen Leukocythen. Arch Anat Physiol (Leipzig). 1879;3:166-169. |
| 3. | Ellis JM. Urticaria pigmentosa; a report of a case with autopsy. Arch Pathol (Chic). 1949;48:426-435. [PubMed] |
| 4. | Marone G, Spadaro G, Genovese A. Biology, diagnosis and therapy of mastocytosis. Chem Immunol. 1995;62:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Horny HP, Sotlar K, Valent P. Mastocytosis: state of the art. Pathobiology. 2007;74:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Orfao A, Garcia-Montero AC, Sanchez L, Escribano L. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Shea-Donohue T, Stiltz J, Zhao A, Notari L. Mast cells. Curr Gastroenterol Rep. 2010;12:349-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Irani AM, Schwartz LB. Human mast cell heterogeneity. Allergy Proc. 1994;15:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464-4468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 665] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 609] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 11. | Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, Resta L. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology. 2011;58:1096-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Ranieri G, Ammendola M, Patruno R, Celano G, Zito FA, Montemurro S, Rella A, Di Lecce V, Gadaleta CD, Battista De Sarro G. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int J Oncol. 2009;35:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Ranieri G, Labriola A, Achille G, Florio G, Zito AF, Grammatica L, Paradiso A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int J Oncol. 2002;21:1317-1323. [PubMed] |
| 14. | Ranieri G, Roccaro AM, Vacca A, Ribatti D. Thymidine phosphorylase (platelet-derived endothelial cell growth factor) as a target for capecitabine: from biology to the bedside. Recent Pat Anticancer Drug Discov. 2006;1:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Passantino L, Patruno R, Valerio P, Penna A, Mazzone F, Zito AF, Catalano V, Pellecchia A, Jirillo E, Ranieri G. Thymidine phosphorylase profiles in nonmalignant and malignant pancreatic tissue. Potential therapeutic role of capecitabine on tumoral and endothelial cells and tumor-infiltrating macrophages. Immunopharmacol Immunotoxicol. 2005;27:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Raica M, Cimpean AM, Ceausu R, Ribatti D, Gaje P. Interplay between mast cells and lymphatic vessels in different molecular types of breast cancer. Anticancer Res. 2013;33:957-963. [PubMed] |
| 17. | Ribatti D, Nico B, Finato N, Crivellato E. Tryptase-positive mast cells and CD8-positive T cells in human endometrial cancer. Pathol Int. 2011;61:442-444. [PubMed] |
| 18. | Nagata M, Shijubo N, Walls AF, Ichimiya S, Abe S, Sato N. Chymase-positive mast cells in small sized adenocarcinoma of the lung. Virchows Arch. 2003;443:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Horny HP, Greschniok A, Jordan JH, Menke DM, Valent P. Chymase expressing bone marrow mast cells in mastocytosis and myelodysplastic syndromes: an immunohistochemical and morphometric study. J Clin Pathol. 2003;56:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 944] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 21. | Oda K, Kitano H. A comprehensive map of the toll-like receptor signaling network. Mol Syst Biol. 2006;2:2006.0015. [PubMed] |
| 22. | Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 673] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 23. | Nakayama T, Yao L, Tosato G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J Clin Invest. 2004;114:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Ribatti D, Crivellato E. Mast cells, angiogenesis and cancer. Adv Exp Med Biol. 2011;716:270-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Ribatti D, Guidolin D, Marzullo A, Nico B, Annese T, Benagiano V, Crivellato E. Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol. 2010;91:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Ribatti D, Finato N, Crivellato E, Marzullo A, Mangieri D, Nico B, Vacca A, Beltrami CA. Neovascularization and mast cells with tryptase activity increase simultaneously with pathologic progression in human endometrial cancer. Am J Obstet Gynecol. 2005;193:1961-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Ranieri G. Hot topic: targeting tumor angiogenesis: an update. Curr Med Chem. 2012;19:937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Saponaro C, Malfettone A, Ranieri G, Danza K, Simone G, Paradiso A, Mangia A. VEGF, HIF-1α expression and MVD as an angiogenic network in familial breast cancer. PLoS One. 2013;8:e53070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Ammendola M, Zuccalà V, Patruno R, Russo E, Luposella M, Amorosi A, Vescio G, Sammarco G, Montemurro S, De Sarro G. Tryptase-positive mast cells and angiogenesis in keloids: a new possible post-surgical target for prevention. Updates Surg. 2013;65:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Ranieri G, Coviello M, Chiriatti A, Stea B, Montemurro S, Quaranta M, Dittadi R, Paradiso A. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435-439. [PubMed] |
| 32. | Ranieri G, Coviello M, Patruno R, Valerio P, Martino D, Milella P, Catalano V, Scotto F, De Ceglie A, Quaranta M. Vascular endothelial growth factor concentrations in the plasma-activated platelets rich (P-APR) of healthy controls and colorectal cancer patients. Oncol Rep. 2004;12:817-820. [PubMed] |
| 33. | Mangia A, Chiriatti A, Ranieri G, Abbate I, Coviello M, Simone G, Zito FA, Montemurro S, Rucci A, Di Leo A. H pylori status and angiogenesis factors in human gastric carcinoma. World J Gastroenterol. 2006;12:5465-5472. [PubMed] |
| 34. | Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13:1845-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 35. | Miyazaki T, Okada N, Ishibashi K, Ogata K, Ohsawa T, Ishiguro T, Nakada H, Yokoyama M, Matsuki M, Kato H. Clinical significance of plasma level of vascular endothelial growth factor-C in patients with colorectal cancer. Jpn J Clin Oncol. 2008;38:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Werther K, Christensen IJ, Nielsen HJ. The association between preoperative concentration of soluble vascular endothelial growth factor, perioperative blood transfusion, and survival in patients with primary colorectal cancer. Eur J Surg. 2001;167:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Ranieri G, Ruggieri E, Falco G, Zizzo N, Mattioli E, Zito AF, Patruno R, Gasparini G. Drug targets to pro-angiogenetic factors with special reference to primary peritoneal mesothelioma. Endocr Metab Immune Disord Drug Targets. 2006;6:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Hassan S, Kinoshita Y, Kawanami C, Kishi K, Matsushima Y, Ohashi A, Funasaka Y, Okada A, Maekawa T, He-Yao W. Expression of protooncogene c-kit and its ligand stem cell factor (SCF) in gastric carcinoma cell lines. Dig Dis Sci. 1998;43:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Ribatti D, Ranieri G, Basile A, Azzariti A, Paradiso A, Vacca A. Tumor endothelial markers as a target in cancer. Expert Opin Ther Targets. 2012;16:1215-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245-282. [PubMed] |
| 41. | Liu Y, Mueller BM. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun. 2006;344:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689-4696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 288] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, Ramamurthy NS. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 241] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Stack MS, Johnson DA. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase). J Biol Chem. 1994;269:9416-9419. [PubMed] |
| 45. | Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 321] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 46. | Ribatti D, Ranieri G, Nico B, Benagiano V, Crivellato E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int J Dev Biol. 2011;55:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Sekiya R, Onitsuka T. Association of mast cells with tumor angiogenesis in esophageal squamous cell carcinoma. Dis Esophagus. 2001;14:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Ammendola M, Sacco R, Donato G, Zuccalà V, Russo E, Luposella M, Vescio G, Rizzuto A, Patruno R, De Sarro G. Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology. 2013;85:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Acikalin MF, Oner U, Topçu I, Yaşar B, Kiper H, Colak E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig Liver Dis. 2005;37:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2009;24:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Peng SH, Deng H, Yang JF, Xie PP, Li C, Li H, Feng DY. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005;11:6521-6524. [PubMed] |
| 52. | Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of mast cells with microvessel density in renal cell carcinomas. Eur Urol. 2006;50:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Ibaraki T, Muramatsu M, Takai S, Jin D, Maruyama H, Orino T, Katsumata T, Miyazaki M. The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2005;28:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Carlini MJ, Dalurzo MC, Lastiri JM, Smith DE, Vasallo BC, Puricelli LI, Lauría de Cidre LS. Mast cell phenotypes and microvessels in non-small cell lung cancer and its prognostic significance. Hum Pathol. 2010;41:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, Sirigu P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest. 2003;33:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49:1061-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Ranieri G, Patruno R, Lionetti A, Di Summa A, Mattioli E, Bufo P, Pellecchia A, Ribatti D, Zizzo N. Endothelial area and microvascular density in a canine non-Hodgkin’s lymphoma: an interspecies model of tumor angiogenesis. Leuk Lymphoma. 2005;46:1639-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Nico B, Mangieri D, Crivellato E, Vacca A, Ribatti D. Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev. 2008;17:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Ribatti D, Molica S, Vacca A, Nico B, Crivellato E, Roccaro AM, Dammacco F. Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia. 2003;17:1428-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Ribatti D, Polimeno G, Vacca A, Marzullo A, Crivellato E, Nico B, Lucarelli G, Dammacco F. Correlation of bone marrow angiogenesis and mast cells with tryptase activity in myelodysplastic syndromes. Leukemia. 2002;16:1680-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Molica S, Vacca A, Crivellato E, Cuneo A, Ribatti D. Tryptase-positive mast cells predict clinical outcome of patients with early B-cell chronic lymphocytic leukemia. Eur J Haematol. 2003;71:137-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Tomita M, Matsuzaki Y, Onitsuka T. Correlation between mast cells and survival rates in patients with pulmonary adenocarcinoma. Lung Cancer. 1999;26:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 65. | Erba F, Fiorucci L, Pascarella S, Menegatti E, Ascenzi P, Ascoli F. Selective inhibition of human mast cell tryptase by gabexate mesylate, an antiproteinase drug. Biochem Pharmacol. 2001;61:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Mori S, Itoh Y, Shinohata R, Sendo T, Oishi R, Nishibori M. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci. 2003;92:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Bai Y, Bandara G, Ching Chan E, Maric I, Simakova O, Bandara SN, Lu WP, Wise SC, Flynn DL, Metcalfe DD. Targeting the KIT activating switch control pocket: a novel mechanism to inhibit neoplastic mast cell proliferation and mast cell activation. Leukemia. 2013;27:278-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Patruno R, Zizzo N, Zito AF, Catalano V, Valerio P, Pellecchia V, D’Errico E, Mazzone F, Ribatti D, Ranieri G. Microvascular density and endothelial area correlate with Ki-67 proliferative rate in the canine non-Hodgkin’s lymphoma spontaneous model. Leuk Lymphoma. 2006;47:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2077] [Cited by in RCA: 2126] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 70. | Crivellato E, Nico B, Vacca A, Ribatti D. Ultrastructural analysis of mast cell recovery after secretion by piecemeal degranulation in B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Crivellato E, Ribatti D, Mallardi F, Beltrami CA. Granule changes of human and murine endocrine cells in the gastrointestinal epithelia are characteristic of piecemeal degranulation. Anat Rec. 2002;268:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Mikkelsen HB. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med. 2010;14:818-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Hiromatsu Y, Toda S. Mast cells and angiogenesis. Microsc Res Tech. 2003;60:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977-19982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Heijmans J, Büller NV, Muncan V, van den Brink GR. Role of mast cells in colorectal cancer development, the jury is still out. Biochim Biophys Acta. 2012;1822:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Chichlowski M, Westwood GS, Abraham SN, Hale LP. Role of mast cells in inflammatory bowel disease and inflammation-associated colorectal neoplasia in IL-10-deficient mice. PLoS One. 2010;5:e12220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2075] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 79. | Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M. Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum. 1995;38:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Ranieri G, Ria R, Roccaro AM, Vacca A, Ribatti D. Development of vasculature targeting strategies for the treatment of chronic inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2005;4:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Kashiwase Y, Inamura H, Morioka J, Igarashi Y, Kawai-Kowase K, Kurosawa M. Quantitative analysis of mast cells in benign and malignant colonic lesions: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Allergol Immunopathol (Madr). 2008;36:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 82. | Taweevisit M. The association of stromal mast cell response and tumor cell differentiation in colorectal cancer. J Med Assoc Thai. 2006;89 Suppl 3:S69-S73. [PubMed] |
| 83. | Xia Q, Ding Y, Wu XJ, Peng RQ, Zhou Q, Zeng J, Hou JH, Zhang X, Zeng YX, Zhang XS. Mast Cells in Adjacent Normal Colon Mucosa rather than Those in Invasive Margin are Related to Progression of Colon Cancer. Chin J Cancer Res. 2011;23:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Xia Q, Wu XJ, Zhou Q, Jing-Zeng JH, Pan ZZ, Zhang XS. No relationship between the distribution of mast cells and the survival of stage IIIB colon cancer patients. J Transl Med. 2011;9:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487-495. [PubMed] |
| 86. | Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol. 2005;11:1210-1214. [PubMed] |
| 87. | Elezoğlu B, Tolunay S. The relationship between the stromal mast cell number, microvessel density, c-erbB-2 staining and survival and prognostic factors in colorectal carcinoma. Turk Patoloji Derg. 2012;28:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Fisher ER, Paik SM, Rockette H, Jones J, Caplan R, Fisher B. Prognostic significance of eosinophils and mast cells in rectal cancer: findings from the National Surgical Adjuvant Breast and Bowel Project (protocol R-01). Hum Pathol. 1989;20:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Yodavudh S, Tangjitgamol S, Puangsa-art S. Prognostic significance of microvessel density and mast cell density for the survival of Thai patients with primary colorectal cancer. J Med Assoc Thai. 2008;91:723-732. [PubMed] |
| 90. | Malfettone A, Silvestris N, Saponaro C, Ranieri G, Russo A, Caruso S, Popescu O, Simone G, Paradiso A, Mangia A. High density of tryptase-positive mast cells in human colorectal cancer: a poor prognostic factor related to protease-activated receptor 2 expression. J Cell Mol Med. 2013;17:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Song YX, Gao P, Wang ZN, Liang JW, Sun Z, Wang MX, Dong YL, Wang XF, Xu HM. Can the tumor deposits be counted as metastatic lymph nodes in the UICC TNM staging system for colorectal cancer? PLoS One. 2012;7:e34087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 92. | Morikawa T, Tanaka N, Kuchiba A, Nosho K, Yamauchi M, Hornick JL, Swanson RS, Chan AT, Meyerhardt JA, Huttenhower C. Predictors of lymph node count in colorectal cancer resections: data from US nationwide prospective cohort studies. Arch Surg. 2012;147:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Liu X, Cai H, Shi Y, Wang Y. Prognostic factors in patients with node-negative gastric cancer: a single center experience from China. J Gastrointest Surg. 2012;16:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Sjo OH, Merok MA, Svindland A, Nesbakken A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis Colon Rectum. 2012;55:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Ducroc R, Bontemps C, Marazova K, Devaud H, Darmoul D, Laburthe M. Trypsin is produced by and activates protease-activated receptor-2 in human cancer colon cells: evidence for new autocrine loop. Life Sci. 2002;70:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Yoshii M, Jikuhara A, Mori S, Iwagaki H, Takahashi HK, Nishibori M, Tanaka N. Mast cell tryptase stimulates DLD-1 carcinoma through prostaglandin- and MAP kinase-dependent manners. J Pharmacol Sci. 2005;98:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Jikuhara A, Yoshii M, Iwagaki H, Mori S, Nishibori M, Tanaka N. MAP kinase-mediated proliferation of DLD-1 carcinoma by the stimulation of protease-activated receptor 2. Life Sci. 2003;73:2817-2829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Sendo T, Sumimura T, Itoh Y, Goromaru T, Aki K, Yano T, Oike M, Ito Y, Mori S, Nishibori M. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cell Signal. 2003;15:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Voltz JW, Weinman EJ, Shenolikar S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene. 2001;20:6309-6314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 100. | Mangia A, Saponaro C, Malfettone A, Bisceglie D, Bellizzi A, Asselti M, Popescu O, Reshkin SJ, Paradiso A, Simone G. Involvement of nuclear NHERF1 in colorectal cancer progression. Oncol Rep. 2012;28:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | De Luisi A, Ferrucci A, Coluccia AM, Ria R, Moschetta M, de Luca E, Pieroni L, Maffia M, Urbani A, Di Pietro G. Lenalidomide restrains motility and overangiogenic potential of bone marrow endothelial cells in patients with active multiple myeloma. Clin Cancer Res. 2011;17:1935-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 102. | Basile A, Moschetta M, Ditonno P, Ria R, Marech I, De Luisi A, Berardi S, Frassanito MA, Angelucci E, Derudas D. Pentraxin 3 (PTX3) inhibits plasma cell/stromal cell cross-talk in the bone marrow of multiple myeloma patients. J Pathol. 2013;229:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Ranieri G, Mammì M, Donato Di Paola E, Russo E, Gallelli L, Citraro R, Gadaleta CD, Marech I, Ammendola M, De Sarro G. Pazopanib a tyrosine kinase inhibitor with strong anti-angiogenetic activity: a new treatment for metastatic soft tissue sarcoma. Crit Rev Oncol Hematol. 2014;89:322-329. [PubMed] |
| 104. | Gnoni A, Marech I, Silvestris N, Vacca A, Lorusso V. Dasatinib: an anti-tumour agent via Src inhibition. Curr Drug Targets. 2011;12:563-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 105. | Yang XP, Li Y, Wang Y, Wang Y, Wang P. beta-Tryptase up-regulates vascular endothelial growth factor expression via proteinase-activated receptor-2 and mitogen-activated protein kinase pathways in bone marrow stromal cells in acute myeloid leukemia. Leuk Lymphoma. 2010;51:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994-998. [PubMed] |
| 107. | Passantino L, Passantino G, Cianciotta A, Ribaud MR, Lo Presti G, Ranieri G, Perillo A. Expression of proto-oncogene C-kit and correlation with morphological evaluations in canine cutaneous mast cell tumors. Immunopharmacol Immunotoxicol. 2008;30:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Ranieri G, Gadaleta CD, Patruno R, Zizzo N, Daidone MG, Hansson MG, Paradiso A, Ribatti D. A model of study for human cancer: Spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies. Crit Rev Oncol Hematol. 2013;88:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Ranieri G, Passantino L, Patruno R, Passantino G, Jirillo F, Catino A, Mattioli V, Gadaleta C, Ribatti D. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol Rep. 2003;10:1189-1193. [PubMed] |
| 110. | Ranieri G, Pantaleo M, Piccinno M, Roncetti M, Mutinati M, Marech I, Patruno R, Rizzo A, Sciorsci RL. Tyrosine kinase inhibitors (TKIs) in human and pet tumours with special reference to breast cancer: a comparative review. Crit Rev Oncol Hematol. 2013;88:293-308. [PubMed] |