Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.8886
Revised: April 2, 2014
Accepted: June 14, 2014
Published online: July 21, 2014
Processing time: 162 Days and 9 Hours
Irritable bowel syndrome (IBS) is a functional bowel disorder without any structural or metabolic abnormalities that sufficiently explain the symptoms, which include abdominal pain and discomfort, and bowel habit changes such as diarrhea and constipation. Its pathogenesis is multifactorial: visceral hypersensitivity, dysmotility, psychosocial factors, genetic or environmental factors, dysregulation of the brain-gut axis, and altered intestinal microbiota have all been proposed as possible causes. The human intestinal microbiota are composed of more than 1000 different bacterial species and 1014 cells, and are essential for the development, function, and homeostasis of the intestine, and for individual health. The putative mechanisms that explain the role of microbiota in the development of IBS include altered composition or metabolic activity of the microbiota, mucosal immune activation and inflammation, increased intestinal permeability and impaired mucosal barrier function, sensory-motor disturbances provoked by the microbiota, and a disturbed gut-microbiota-brain axis. Therefore, modulation of the intestinal microbiota through dietary changes, and use of antibiotics, probiotics, and anti-inflammatory agents has been suggested as strategies for managing IBS symptoms. This review summarizes and discusses the accumulating evidence that intestinal microbiota play a role in the pathophysiology and management of IBS.
Core tip: Irritable bowel syndrome (IBS) is a functional bowel disorder with multiple pathophysiology, which is not fully understood. Intestinal microbiota has recently been postulated to be involved in the pathophysiology of IBS. Many studies of IBS focus on investigating the efficacy of modulating the microbiota by probiotics and antibiotics. However, the role of the intestinal microbiota in the pathophysiology and management of IBS is not clear. This review provides the accumulating evidence on it.
- Citation: Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol 2014; 20(27): 8886-8897
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/8886.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.8886
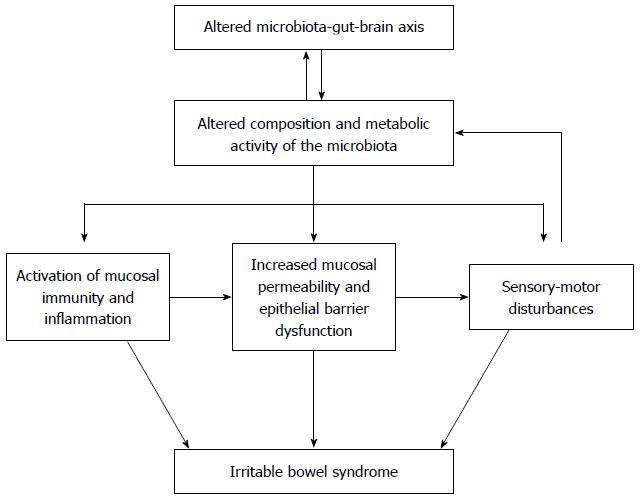
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by abdominal pain or discomfort relieved by defecation, and accompanied by changes in bowel habits such as diarrhea or constipation, which cannot be explained by structural, biochemical, or metabolic abnormalities[1]. The symptoms of IBS have been accounted for as resulting from visceral hypersensitivity, intestinal dysmotility, genetic or environmental factors, psychological factors, or a dysregulated brain-gut axis[2]. In addition to these factors, bacterial infection, dysregulated intestinal immune function, and chronic low-grade mucosal inflammation have all been suggested as putative pathogenetic mechanisms, in which the intestinal microbiota might play an important role, but their role in IBS cannot be fully explained (Figure 1)[3,4].
Intestinal microbiota is a collective term for a complex ecosystem of microbes inhabiting the intestine[5]. In the human intestine, this ecosystem may include any one of over 1000 microbial species, and 1014 cells (i.e., about 10 times more than the number of human cells in the body[6]), containing 150-fold more genes than the human genome[7]. The microbiota can be divided into mucosal and luminal subtypes[8], and it was previously thought to comprise three predominant enterotypes: Bacteroides, Prevotella, and Ruminococcus[9], although such a strict categorization is no longer widely accepted[10].
To evaluate the composition and metabolic activity of the intestinal microbiota, culture-dependent and -independent tests have been developed[11]. It has been shown that size and diversity of the microbiota increase distally from the upper to the lower gastrointestinal (GI) tract[12] and are modulated by gastric acid, intestinal motility, and the function of the ileocecal valve. Their distribution also varies according to the region of the GI tract with gram-positive facultative anaerobic bacteria in the proximal small intestine and gram-negative anaerobes in the distal small intestine. Although the composition and diversity of the microbiota are genetically controlled from birth and become stable after weaning and throughout life, qualitative and quantitative changes can occur over the longitudinal and cross-sectional axes of the intestine: changes in bacterial enzymes and metabolic activity, as well as in microbial populations. The composition and metabolic activity of the microbiota vary between, but also within, individuals due to many factors including mode of delivery at birth, diet, sanitation, antibiotics, and ageing[13]. At birth, contamination from the vaginal canal provides the intestine with the maternal microbiome, while during a delivery by cesarean-section, the gut comes into contact with commensals from the skin and the surgical environment[14]. The composition of the microbiota can also be altered by the feeding method: bifidobacteria increase in breast-fed babies (i.e., babies receiving a high-carbohydrate and high-fiber diet), and Bacteroides spp. increase in formula-fed babies (babies receiving a high-fat diet)[15]. Lastly, it can vary across geographical regions, e.g., between rural Africa and urban Europe[16].
The intestinal microbiota is essential for maintaining individual health, including normal GI function. In this context, its main functions are metabolic, protective, and trophic: it can help to digest and absorb nutrients, and produces a variety of beneficial compounds such as short-chain fatty acids (SCFA)[17], it can act as a barrier against pathogens by adhering to the mucosa, generating immune responses, and interacting with components of the epithelial layer, it can also influence the differentiation and proliferation of the intestinal epithelial cells and the development of the enteric immune system.
In parallel with the beneficial effects of microbial activity on the gut, bacterial fermentation may give rise to large amounts of gas and thus contribute to the symptoms of bloating, flatulence, and abdominal distension, which are commonly reported by patients with IBS[18]. An association between the microbiota and IBS has been supported by the evidence of modulation of mucosal immunity: IBS symptoms were found to be more frequent after an episode of gastroenteritis, and some IBS symptoms were found to improve after antibiotic treatment targeting the intestinal microbiota[19]. This putative link was also demonstrated in studies of probiotics, which modulated the intestinal microbiota in IBS patients. Finally, mucosal immunity-gut microbiota-brain axis is being suggested as a possible pathway for the development of IBS due to altered intestinal microbiota. This review article explores the role of the microbiota in the pathophysiology and management of IBS, and provides a comprehensive summary of the evidence for the concept of IBS as a microbiota-related disorder.
Despite the large volume of studies of the intestinal microbiota, our understanding of its role in health and disease is still in its infancy. In studying the microbiota, culture-based methods are being replaced by advanced, culture-independent, molecular techniques. However, these two approaches are complementary: culture studies of fecal matter or colonic mucosa are valuable for identifying functional groups and for selective enumeration, whereas advanced molecular study are a powerful tool for monitoring changes in microbial composition. The molecular methodology includes sequencing of the small-subunit ribosomal RNA genes through amplification of nucleic acids extracted from fecal or mucosal samples, fingerprinting methods such as denaturing gradient gel electrophoresis, targeted methods such as fluorescence in situ hybridization and quantitative PCR, new high-throughput sequencing, and 16S rRNA-based microarraying[20].
The microbiota in the gut can be altered by brain function, and microbial alteration can, in turn, influence brain function. It is evidenced by the finding that patients with IBS frequently have accompanying psychological disorders, such as anxiety or depression, and those with psychological stress are more likely to develop post-infectious (PI)-IBS. This connection between the microbiota, the gut, and the brain in IBS postulates the existence of a bidirectional, homeostatic network, and it is an exciting area of ongoing research.
Animal studies have demonstrated the influence of the intestinal microbiota on brain development. Brain dysfunction in Germ-free (GF) mice was reported, including an exaggerated hypothalamic-pituitary response to mild stress[21], more exploratory and risk-taking behavior[22], and altered brain chemistry and memory, indicative of impaired hippocampal development[23]. Brain chemistry and behavior were also influenced by altered microbiota; a study showed that transient alteration of the microbial composition by diet provoked exploratory behavior, accompanied by changes of in the levels of brain-derived neurotrophic factor in the specific regions of the brain such as hippocampus and amygdala[24]. The gut microbiota and the brain may be communicated by neural, metabolic (bacterial and host), immunologic, or endocrine pathways[25]. The neural pathways was first suggested in animal models; anxiety-related behavior was reduced after probiotic treatment, provided vagus nerve integrity was maintained[26,27]. Metabolic pathways were revealed in a study that brain function and behavioral changes were closely associated with bacterial metabolites such as SCFAs (which comprise most of the circulating organic acids) and tryptophan metabolites[28,29]. A role of immunologic pathways was demonstrated in animal and human studies showing that certain psychological disorders were associated with pro-inflammatory cytokines, whose levels had been altered by manipulating the composition of the microbiota[30-32]. Endocrine pathways in microbiota-gut-brain axis were suggested in a study showing that the endocrine structure and function of the GI tract which secrets a variety of hormones such as cholecystokinin and serotonin [5-hydroxytryptamine (5-HT)] were reduced in GF rats[33].
Likewise, the intestinal microbiota can be affected by signals from the central nervous system produced in response to stress or psychological disturbances. Stress can change GI motility and secretions, which alter the microbial habitat. The microbial habitat may also be altered by changes in gene expression of some microbial species. Conversely, the intestinal microbiota can influence neurotransmitters like norepinephrine, dopamine, and serotonin in the brain, and activation of the hypothalamic-pituitary-adrenal axis is also thought to be involved in the microbiota-gut-brain axis.
As a result of alteration of the microbiota in this axis, mucosal immunity may be activate and thereby epithelial barrier function can be disrupted, which could contribute to the visceral hypersensitivity and dysmotility in IBS. Furthermore, the intestinal microbiota may not only release metabolites but also induce the formation of host-derived immune mediators, thereby affecting the enteric nervous system both directly and indirectly. However, much about the role of the microbiota-gut-brain axis in IBS remains poorly understood.
Altered composition of the intestinal microbiota: Intestinal microbiota can be grouped into luminal and mucosal microbiota. It is generally accepted that the composition of the luminal and mucosal microbiota differs between patients with IBS and healthy controls, and the composition may also vary according to the subtype of IBS[34], although studies of the intestinal microbiota have been as diverse and complex as the microbiota itself, with inconsistent and conflicting results[29,35-44] (Table 1). According to both the early culture-based and the more recent advanced molecular studies, it was found in IBS that the proportions of specific bacterial groups were altered, the diversity of microbial populations was reduced, and the degree of variability in the microbiota composition was different. The findings included decreased levels of fecal lactobacilli and bifidobacteria, increased levels of facultative anaerobic bacteria dominated by streptococci and Escherichia coli (E. coli), increased ratios of Firmicutes:Bacteroidetes and higher counts of anaerobic organisms (such as clostridium)[44,45]. In addition, the microbiota of IBS patients reportedly belonged to entirely different enterotypes than those of healthy controls[34,46]. These inconsistent and sometimes conflicting results are thought to be due to the use of a single fecal sample irrespective of the fluctuating symptoms of IBS.
| Ref. | Subject (n) | Method | Finding |
| Si et al[35] | IBS (25) | Culture | Decreased Bifidobacterium |
| Control (25) | Increased Enterobacteriaceae | ||
| Malinen et al[36] | IBS (27) | qPCR | Decreased Lactobacillus in IBS-D |
| Control (22) | Increased Veillonella in IBS-C | ||
| Mättö et al[37] | IBS (26) | Culture | Increased coliform and aerob to anaerob ratio |
| Control (25) | PCR-DGGE | Temporal instability | |
| Codling et al[38] | IBS (41) | PCR-DGGE | No difference in fecal/mucosal |
| Control (33) | Temporal instability | ||
| Ponnusamy et al[39] | IBS (11) | DGGE | Increased diversity in Bacteroidetes, Lactobacillus |
| Control (8) | qPCR-16sRNA | ||
| Tana et al[29] | IBS (26) | Culture | Increased Lactobacillus and Veillonella |
| Control (26) | q-PCR | ||
| Lyra et al[40] | IBS (20) | qPCR | Increased Ruminococcus torques and decreased |
| Control (15) | Clostridium thermosuccinogenes in IBS-D | ||
| Krogius-Kurikka et al[41] | IBS (10) | 16S rRNA | Increased Proteobacteria and Firmicutes |
| Control (23) | sequencing | Decreased Actinobacteria and Bacteroidetes | |
| Kerckhoffs et al[42] | IBS (41) | FISH | Decreased Bifidobacterium |
| Control (26) | qPCR | ||
| Kassinen et al[43] | IBS (24) | 16S rRNA | Decreased Collinsella aerofaciens, Clostridium cocleatum, and Coprococcus eutactus |
| Control (23) | sequencing | ||
| Maukonen et al[44] | IBS (24) | PCR-DGGE | Decreased Clostridium coccoides |
| Control (16) | Temporal instability | ||
| Jeffery et al[46] | IBS (37) | 16S rRNA | Increased ratio of Firmicutes to Bacteroidetes |
| Control (20) | pyrosequencing | Clustering in IBS |
Altered metabolic activity of the intestinal microbiota: Intestinal microbiota may produce excessive amounts of gas by fermenting poorly absorbable carbohydrates (e.g., the so-called FODMAPs, fermentable oligosaccharides, disaccharides, monosaccharides and polyols), which may cause abdominal pain, bloating, flatulence, and distension in IBS. Additionally, altered fermentation of poorly absorbable carbohydrates could increase the production of SCFAs, which would then lead to release of 5-HT from the intestinal mucosa[47]. In fact, increased numbers of acetic and propionic acid-producing bacteria (Veionella and Lactobacillus spp) were reported in patients with IBS[29]. It has been demonstrated that the release of 5-HT initiated high-amplitude, propagated colonic contractions, accelerated intestinal transit, and increased gut motility[47,48], all of which may contribute to IBS symptoms, suggesting that fermentation products play a potential role of in contributing IBS symptoms.
However, considering the large variability due to different methodologies of microbiota studies, and individual differences in relation to dietary, genetic and geographical factors, as well as heterogeneity of the disease, these results should be cautiously interpreted. Research on the luminal and mucosal microbiota is still in infancy, and further studies using advanced techniques such as 16s rRNA and DNA sequencing are needed to improve our understanding of the microbiota changes in IBS.
The altered composition and metabolic activity of the intestinal microbiota found in IBS may be associated with activation of mucosal immunity and inflammation. Changes in the intestinal microbiota were observed after an episode of infective gastroenteritis with subsequent antibiotic use. In fact, some patients start to report IBS symptoms following such episodes[49], which suggests an association between IBS and activation of mucosal immunity and inflammation caused by altered microbiota. Chronic low-grade mucosal inflammation has been frequently observed in many studies of IBS patients and in animal models of IBS[50-56].
The intestinal microbiota plays an essential role in the development, functioning, and regulation of both intestinal and systemic immunities. By interacting with the microbiota, the intestinal (or enteric) immune system, composed of innate and adaptive immunity, helps to maintain normal GI function[57]. In IBS patients, however, the interactions between enteric immunity and commensal and/or pathogenic microbes were found to be dys-regulated. Under normal conditions, intestinal microbes are recognized via their ligands, identified by toll-like receptors (TLRs) on intestinal immune cells. Expression of TLRs in the colonic mucosa of IBS patients was found to be increased[58], as was the level of circulating antibodies such as antiflagellin antibodies[59]. Together, these findings suggest that in IBS, bacterial components such as lipopolysaccharides (LPS) and flagellin are recognized more frequently due to the increased TLRs and circulating antibodies. In addition, one of the anti-bacterial proteins, β-defensin-2, was found to be elevated in IBS[60]. These increased interactions of immunologic components with the microbiota could eventually lead to the mucosal inflammation in IBS.
Mucosal inflammation provoked by dysregulated innate and adaptive enteric immunities has been observed in many studies of IBS[61,62]. The numbers of activated mast cells were shown to be increased in the colon of IBS patients, and also to be in close proximity to enteric nerves, which correlated well with IBS symptoms[63], although this increase was specific to diarrhea predominant IBS (IBS-D)[52], and varied according to the region of the intestine[64]. In addition to mast cells, lymphocytes (CD4+ and CD8+ T cells) were also found to be elevated, suggesting that they may play a role in IBS, although there are some inconsistencies[50,55,56,65]. Immune alterations associated with IBS were also found in IgA-producing B cells[66], IgG+ B cells[67], and in the levels of pro- and anti-inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-10, IL-6, and interferon-γ in the intestinal mucosa of IBS patients[68]. Similarly, in the peripheral blood, levels of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α were higher in patients with IBS than in controls, but the levels varied according to IBS subtype[69]. It is thought that mucosal inflammation and activated immunity in IBS may lead to increased permeability of the intestinal mucosa, and may thus induce abnormal sensory and motor function, which could contribute to the symptoms of IBS. However, the association between activated immunity and the intestinal microbiota is not clearly established, and further studies in this area are warranted.
The activation of mucosal immunity and inflammation driven by the altered microbiota in IBS may increase mucosal permeability and impair epithelial barrier function. The intestinal epithelium functions not only as an exchanger, absorbing fluid and nutrients, but also as a protective barrier against pathogens. It is covered with a thick layer of mucus, composed of a complex mixture of glycoproteins, mucins, bactericidal enzymes, and secretory immunoglobulin A (IgA). Alterations to the epithelial barrier observed in IBS have included increased mucosal permeability, increased expression of specific proteins, e.g. MUC20 (gene involved in the production of mucin) and PARM1, and increased fecal excretion of the antibacterial protein β-defensin-2[60]. Increased mucosal permeability in the small intestine was observed in patients with IBS-D[70], and it was associated with the expression and distribution of tight junction proteins; lower levels of the protein zonula occludens (ZO)-1 were found in IBS patients than controls[71,72]. Elsewhere, increased permeability along with mast cell infiltration into the colon was found to be associated with the severity of IBS symptoms[73]. Both the increased permeability and symptoms of IBS were improved by lactic acid bacteria, suggesting that there may be an association between an altered epithelial barrier and IBS symptoms[74]. It is worth noting that one study of gut permeability found that the increase was limited to the colon[75], whereas another IBS study reported that the expression and distribution of ZO-1 was altered in the jejunum[76]. Generally, this increase in gut permeability was found to be associated with bacteria-related protease activity and its receptors in the intestinal epithelium[77]. It is also thought that a single-nucleotide polymorphism in the gene encoding the tight junction protein, E-cadherin, may increase the risk of developing PI-IBS[78]. On the other hand, some bacterial metabolites produced by the intestinal microbiota were found to improve epithelial barrier function[79]. It has also been suggested that the barrier dysfunction with increased mucosal permeability in IBS may also be associated with visceral hypersensitivity[80].
In addition to the mucosal inflammation of the gut that may affect sensory-motor and secretory functions, neuronal structure, and neurotransmitter release in the gut[81], the intestinal microbiota can directly affect intestinal sensory-motor functions[82]. Alterations in the microbiota induced by antibiotic treatment were found to precipitate visceral hypersensitivity, which was restored by probiotic treatment[83]. Probiotic treatment was also found to reduce sensation of pain via the enteric nerve in a model of visceral pain induced by colorectal distension[84]. A similar level of pain modulation was also achieved by inducing the expression of opioid and cannabinoid receptors[85]. With respect to motor disturbances, it has been reported that colonic motor function was enhanced by supernatants from the E. coli strain Nissle 1917, and that this was mediated by stimulation of smooth muscle cells[86]. Also, probiotic treatment was found to increase small-intestinal motor function in rats[87]. Furthermore, transplantation of healthy human fecal microbiota into GF mice increased their colonic motility and shortened GI transit, which was closely associated with the type and amount of carbohydrates in the diet[88]. The beneficial effects of the microbiota on motility were shown to be region-specific with migrating motor complex velocity increased in the jejunum but decreased in the colon[89]. These interactions between intestinal microbiota and GI sensory-motor function may be related to IBS, although the exact mechanism of the interactions is not well understood.
It seems that normal GI motility relies on TLR4 signaling stimulated by the microbiota. It was demonstrated that mice lacking TLR4, which is frequently stimulated by bacterial LPS, exhibited longer GI transit times and reduced abundance of colonic nitrergic neurons[90]. In addition to the microbiota itself, the metabolites from bacterial fermentation may also exert an effect on GI motility. One of the colonic metabolites, CH4, was shown to delay intestinal transit[91], H2S was shown to inhibit the contraction of intestinal smooth muscle[92], SCFA, to stimulate colonic transit by triggering 5-HT release[47], and tryptamine from tryptophan, to increase intestinal contractions[93]. Other bacterial metabolites that may be related to GI motility include bile acid metabolites[94] and ligands of GABA receptors with a suppressive effect on GI motility[95]. While the microbiota may affect gut sensory-motor function, the reverse may also be true: the microbial ecosystem in the gut may be disturbed by accelerated or decelerated GI transit[88]. It is thought that the changes in GI transit may alter the flow rate of intestinal contents and thereby affect the environment for resident bacteria, which then impinges on both the organizational structure and the gene expressed in the microbiota.
An association between diet and symptom development in IBS is reported frequently but its mechanisms are not clearly defined. Some of the proposed causative factors include hypersensitivity and/or allergic reaction to specific foods, and alterations of the habitat and metabolic activity of the intestinal microbiota. Diet is thought to be a powerful factor influencing the composition and metabolic activity of the microbiota in an individual. The composition of the microbiota in babies change after weaning, and in adults it varies according to geographic regions due to differences in the food consumed, the type of meat consumed, and cooking methods (whether the food is fried, baked or boiled). Therefore, any dietary strategy aimed at modifying the microbiota should be matched to the individual because different microbial species are responsive to different kinds of dietary components.
However, whether a change in the diet can directly affect the microbiota in IBS is not clear. This is partly due to the lack of well-designed, controlled trials that investigate the effects of diet on IBS. Although specific diets, e.g., the FODMAPs diet, have been shown to provoke IBS symptoms in some patients, not all studies regarding the effects of exclusion diets on the symptoms of IBS are completely reliable due to a variety of confounding factors, including a high placebo effect. Nevertheless, it can be speculated that in some IBS patients, intake of certain foods may provoke abnormal fermentation due to aspects of the composition of their intestinal microbiota and that the composition of the microbiota in those patients could be changed to normal by excluding the symptom-provoking foods.
Dietary fiber stimulates the production of SCFAs by mixing with microbes and enzymes. In a healthy gut, these by-products can improve the function and homeostasis of the GI tract. Although it has been suggested that some patients with IBS may benefit from dietary fiber, many patients report an increase in abdominal distension and bloating as a result of fermentation of the fiber. It may be that water holding properties of fiber and its ability to accelerate intestinal transit may alter the habitat for the microbiota and therefore indirectly affect its composition and metabolic activity.
It seems that individualized advice on dietary consumption of non-digestible carbohydrates in the management of IBS, as the inter-individual differences in the response of the microbiota lead to different responses to changes in diet[96].
Antibiotic treatment in IBS assumes that small intestinal bacterial overgrowth (SIBO) plays an important role in the development of IBS. Despite the limited validity and lack of standardization of the methods used to evaluate SIBO, treatment with non-absorbable antibiotics such as rifaximin has yielded a therapeutic benefit. Double-blind, placebo-controlled trials of rifaximin in IBS yielded an improvement in IBS symptoms, which correlated well with the reduced excretion of hydrogen in the breath[97,98]. These findings together with the positive effects of other antibiotic treatments, suggest that a short course of poorly absorbable antibiotics may be of some use in the management of IBS symptoms in some patients. However, data on the long-term effects of antibiotics in IBS are limited. Furthermore, information on the optimal dose of antibiotics, and predictors of treatment success and failure are needed to confirm the benefit of this type of treatment[99].
Effects of probiotics: By adhering to intestinal epithelial cells and competing for nutrients and space, probiotics can protect against pathogens. This protective effect of probiotics has been demonstrated in vitro using intestinal cell lines with lactobacilli, bifidobacteria and E. coli subspecies[100-102]. In addition, probiotics can improve mucosal barrier function and thereby prevent pathogens from increasing intestinal permeability[103,104]. Intestinal permeability can also be increased by stress, which may facilitate the subsequent translocation of pathogenic bacteria. However, it was observed that the increase in intestinal permeability caused by stress was inhibited by lactobacilli[105-107]. In addition, lactobacilli increased levels of bacterial fermentation products such as SCFAs (acetic, propionic and butyric acids) and thereby acidifying the colon, which subsequently increased the numbers of Bifidobacterium and Lactobacillus species and decreased clostridia[108]. In addition to these roles, probiotics were also shown to modulate immunity in animals with experimentally-induced colitis[109,110]. Furthermore, they were shown to reduce visceral hypersensitivity by increasing the expression of opioid and cannabinoid receptors in the intestinal mucosa[85].
However, regarding the effect of probiotics on IBS symptoms, the mechanism is not clearly defined. It is possible that probiotics may not only modulate gut dysmotility and hypersensitivity but also have anti-inflammatory properties. It was found that probiotic treatment attenuated intestinal dysmotility in a mouse model, induced intestinal cell mediators related to reduced hypersensitivity such as cannabinoid and opioid receptors, and normalized the ratio of cytokines IL-10/IL-12 in the systemic circulation.
Probiotic studies in IBS: A majority of studies of probiotics in IBS have been performed to evaluate their effect on either overall or specific IBS symptoms. Although most of them have used Lactobacillus or Bifidobacterium species, single strains or combinations of multiple strains have also been used with multiple doses (from 106/mL to 1010/mL) and for variable durations. Similarly, primary and secondary outcomes in those studies were evaluated using variable factors such as abdominal pain, symptom severity, quality of life, and global IBS symptoms. On balance, these studies found a therapeutic benefit, i.e., improvement in symptoms of bloating, flatulence, bowel frequency, and in global symptoms, although there are some inconsistencies between specific studies. In particular, beneficial effects of probiotics were reported in a well-designed study using bifidobacteria such as Bifidobacterium infantis 35624[30,111], B. lactis, B. animalis DN173010, and B. bifidum MIMBb75[112]. Symptom improvement was also reported in studies using probiotic mixtures such as Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440)[113], and Lactobacillus rhamnosus GG, L. rhamnosus LC705, Bifidobacterium breve Bb99 and Propionibacterium freudenreichii ssp. shermanii JS[114,115]. By contrast, negative results were reported in studies using other probiotic combinations[116], such as Lactobacillus paracasei spp. paracasei F19, L. acidophilus La5 and Bifidobacterium lactis Bb12[117,118], and Lactobacillus plantarum MF1298[119].
In recent studies, it was found that 4-wk treatment with probiotics improved IBS symptoms and altered composition of the microbiota as well[120], and that probiotic treatment in IBS patients reduced the genus Bacteroides to the levels of healthy controls and also improved global IBS symptoms[121]. However, as indicated in several meta-analyses, the previous studies of probiotics in IBS fail to report whether symptom improvement was accompanied by a change in the microbiota or not. Furthermore, many systematic reviews pointed out several study limitations including heterogeneity, inadequate statistical methods, and possible publication bias. Examples of heterogeneity include differences in types, doses, and delivery of probiotics[122-125], which may have produced different outcomes. Therefore, despite the reported benefits of probiotics in IBS, there are many aspects of potential treatment regimens that are yet to be established, such as adequate dosage, treatment duration, choice of species for each individual or symptom of IBS, target symptoms for probiotics, and probiotic formulation. Future studies should aim to identify which species, strains, and doses of probiotics provide the optimal therapeutic benefit to individual patients with IBS, and which specific symptoms of IBS should be the target of probiotic treatment.
Intestinal microbiota can play a substantial role in IBS. Although the microbiota may contribute directly to the symptoms of IBS, it is more likely that altered composition and metabolic activity of the microbiota caused by stress or other psychological disturbances indirectly activate mucosal immunity and inflammation, increase epithelial permeability, and reduce barrier function, thereby activating the sensory-motor dysfunction responsible for a variety of IBS symptoms. Therefore, our knowledge of the link between the microbiota and IBS may enable us to treat focusing on the possible mechanism of this disorder; Dysbiosis may be restored by probiotic or antibiotic treatment and also by diet modification. Activation of mucosal immunity and inflammation can be treated by immune-modulating agents. Increased intestinal permeability and barrier dysfunction can be a potential therapeutic target of probiotics. However, the microbial pathophysiology of IBS is not clearly understood, as microbiota alterations in IBS might be either a cause of IBS or a consequence of intestinal secretion and motility changed by IBS. Furthermore, due to the heterogeneity of IBS studies as well as IBS itself, there has been variability in the results of studies. Therefore, objective diagnostic modalities in IBS are warranted, and further studies using advanced molecular techniques are needed.
P- Reviewers: Camara NOS, Jin B, Pehl C, Riccardi C S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1476] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 2. | Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 950] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 3. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 4. | Parkes GC, Brostoff J, Whelan K, Sanderson JD. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol. 2008;103:1557-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Young VB, Schmidt TM. Overview of the gastrointestinal microbiota. Adv Exp Med Biol. 2008;635:29-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2501] [Cited by in RCA: 2746] [Article Influence: 183.1] [Reference Citation Analysis (1)] |
| 7. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7836] [Article Influence: 522.4] [Reference Citation Analysis (4)] |
| 8. | Swidsinski A, Loening-Baucke V, Lochs H, Hale LP. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol. 2005;11:1131-1140. [PubMed] |
| 9. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5822] [Cited by in RCA: 5032] [Article Influence: 359.4] [Reference Citation Analysis (2)] |
| 10. | Jeffery IB, Claesson MJ, O’Toole PW, Shanahan F. Categorization of the gut microbiota: enterotypes or gradients? Nat Rev Microbiol. 2012;10:591-592. [PubMed] |
| 11. | Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 12. | O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1785] [Article Influence: 93.9] [Reference Citation Analysis (2)] |
| 13. | Power SE, O’Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr. 2014;111:387-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Lupp C, Finlay BB. Intestinal microbiota. Curr Biol. 2005;15:R235-R236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. 2011;17:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 16. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3584] [Cited by in RCA: 4027] [Article Influence: 268.5] [Reference Citation Analysis (0)] |
| 17. | Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 670] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 18. | Jiang X, Locke GR, Choung RS, Zinsmeister AR, Schleck CD, Talley NJ. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57:756-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 482] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 20. | Fraher MH, O’Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol. 2012;9:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2097] [Cited by in RCA: 1863] [Article Influence: 88.7] [Reference Citation Analysis (1)] |
| 22. | Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255-264, e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 935] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 23. | Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 661] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 24. | Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599-609, 609.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 25. | Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1125] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 26. | Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 719] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 27. | Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050-16055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2500] [Cited by in RCA: 2633] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 28. | Ledochowski M, Widner B, Sperner-Unterweger B, Propst T, Vogel W, Fuchs D. Carbohydrate malabsorption syndromes and early signs of mental depression in females. Dig Dis Sci. 2000;45:1255-1259. [PubMed] |
| 29. | Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512-519, e114-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 30. | O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 959] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 31. | Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol Suppl. 2011;88:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 675] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 33. | Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology. 1994;107:1259-1269. [PubMed] |
| 34. | Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 773] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 35. | Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802-1805. [PubMed] |
| 36. | Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 498] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 37. | Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213-222. [PubMed] |
| 38. | Codling C, O’Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci. 2010;55:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 39. | Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol. 2011;60:817-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Lyra A, Rinttilä T, Nikkilä J, Krogius-Kurikka L, Kajander K, Malinen E, Mättö J, Mäkelä L, Palva A. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15:5936-5945. [PubMed] |
| 41. | Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 42. | Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15:2887-2892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 206] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 43. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 44. | Maukonen J, Satokari R, Mättö J, Söderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol. 2006;55:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185-194. [PubMed] |
| 46. | Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 635] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 47. | Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269-R1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 48. | Kamath PS, Hoepfner MT, Phillips SF. Short-chain fatty acids stimulate motility of the canine ileum. Am J Physiol. 1987;253:G427-G433. [PubMed] |
| 49. | Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006;101:1894-1899; quiz 1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 50. | Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 51. | Gwee KA, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, McKendrick MW, Moochhala SM. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523-526. [PubMed] |
| 52. | Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 490] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 54. | Macsharry J, O’Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 817] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 56. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 57. | Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 875] [Cited by in RCA: 767] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 58. | Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 59. | Schoepfer AM, Schaffer T, Seibold-Schmid B, Müller S, Seibold F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil. 2008;20:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Langhorst J, Junge A, Rueffer A, Wehkamp J, Foell D, Michalsen A, Musial F, Dobos GJ. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 62. | Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, De Giorgio R, Corinaldesi R, Stanghellini V. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 63. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] |
| 64. | O’Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O’Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 340] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 65. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. [PubMed] |
| 66. | Forshammar J, Isaksson S, Strid H, Stotzer PO, Sjövall H, Simrén M, Ohman L. A pilot study of colonic B cell pattern in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1461-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Ohman L, Lindmark AC, Isaksson S, Posserud I, Strid H, Sjövall H, Simrén M. B-cell activation in patients with irritable bowel syndrome (IBS). Neurogastroenterol Motil. 2009;21:644-650, e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, Cooney J, Keeling PW. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 455] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 70. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 71. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 72. | Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 73. | Vivinus-Nébot M, Dainese R, Anty R, Saint-Paul MC, Nano JL, Gonthier N, Marjoux S, Frin-Mathy G, Bernard G, Hébuterne X. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol. 2012;107:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 74. | Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Gecse K, Róka R, Séra T, Rosztóczy A, Annaházi A, Izbéki F, Nagy F, Molnár T, Szepes Z, Pávics L. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2012;85:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Martínez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, Sánchez A, Guilarte M, Antolín M, de Torres I. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 77. | Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 78. | Villani AC, Lemire M, Thabane M, Belisle A, Geneau G, Garg AX, Clark WF, Moayyedi P, Collins SM, Franchimont D. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 79. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1690] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 80. | Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 284] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 81. | Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006;126-127:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 82. | Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 83. | Verdú EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 84. | Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, Bienenstock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 541] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 86. | Bär F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Böttner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil. 2009;21:559-566, e16-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Lesniewska V, Rowland I, Laerke HN, Grant G, Naughton PJ. Relationship between dietary-induced changes in intestinal commensal microflora and duodenojejunal myoelectric activity monitored by radiotelemetry in the rat in vivo. Exp Physiol. 2006;91:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology. 2013;144:967-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 89. | Wu RY, Pasyk M, Wang B, Forsythe P, Bienenstock J, Mao YK, Sharma P, Stanisz AM, Kunze WA. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol Motil. 2013;25:e205-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 90. | Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annu Rev Physiol. 2012;74:177-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089-G1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 92. | Dhaese I, Van Colen I, Lefebvre RA. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol. 2010;628:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience. 1985;16:223-240. [PubMed] |
| 94. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1678] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 95. | Yurdaydin C, Walsh TJ, Engler HD, Ha JH, Li Y, Jones EA, Basile AS. Gut bacteria provide precursors of benzodiazepine receptor ligands in a rat model of hepatic encephalopathy. Brain Res. 1995;679:42-48. [PubMed] |
| 96. | Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1347] [Cited by in RCA: 1183] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 97. | Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 99. | Tack J. Antibiotic therapy for the irritable bowel syndrome. N Engl J Med. 2011;364:81-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Gopal PK, Prasad J, Smart J, Gill HS. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int J Food Microbiol. 2001;67:207-216. [PubMed] |
| 101. | Fujiwara S, Hashiba H, Hirota T, Forstner JF. Purification and characterization of a novel protein produced by Bifidobacterium longum SBT2928 that inhibits the binding of enterotoxigenic Escherichia coli Pb176 (CFA/II) to gangliotetraosylceramide. J Appl Microbiol. 1999;86:615-621. [PubMed] |
| 102. | Boudeau J, Glasser AL, Julien S, Colombel JF, Darfeuille-Michaud A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn’s disease. Aliment Pharmacol Ther. 2003;18:45-56. [PubMed] |
| 103. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. [PubMed] |
| 104. | Mangell P, Nejdfors P, Wang M, Ahrné S, Weström B, Thorlacius H, Jeppsson B. Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci. 2002;47:511-516. [PubMed] |
| 105. | Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthésy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901-1907. [PubMed] |
| 106. | Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 107. | Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 108. | Johansson ML, Nobaek S, Berggren A, Nyman M, Björck I, Ahrné S, Jeppsson B, Molin G. Survival of Lactobacillus plantarum DSM 9843 (299v), and effect on the short-chain fatty acid content of faeces after ingestion of a rose-hip drink with fermented oats. Int J Food Microbiol. 1998;42:29-38. [PubMed] |
| 109. | McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975-980. [PubMed] |
| 110. | Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520-528. [PubMed] |
| 111. | Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 517] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 112. | Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 113. | Enck P, Zimmermann K, Menke G, Müller-Lissner S, Martens U, Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome--a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 115. | Kajander K, Krogius-Kurikka L, Rinttilä T, Karjalainen H, Palva A, Korpela R. Effects of multispecies probiotic supplementation on intestinal microbiota in irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 117. | Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 119. | Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 120. | Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 121. | Ng SC, Lam EF, Lam TT, Chan Y, Law W, Tse PC, Kamm MA, Sung JJ, Chan FK, Wu JC. Effect of probiotic bacteria on the intestinal microbiota in irritable bowel syndrome. J Gastroenterol Hepatol. 2013;28:1624-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 122. | Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033-149; quiz 1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 123. | Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 124. | McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650-2661. [PubMed] |
| 125. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |