Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8722
Revised: March 10, 2014
Accepted: April 5, 2014
Published online: July 14, 2014
Processing time: 175 Days and 14.7 Hours
The ideal endpoint of hepatitis B virus (HBV) antiviral therapy is HBsAg loss, a difficult goal to obtain, especially in HBeAg negative patients. Herein, we report the results obtained by the addition of peg-interferon α-2a to a long-lasting nucleos(t)ide analogue therapy in a HBeAg negative, genotype D patient with steadily HBV-DNA negative/HBsAg positive values. In 2002, our Caucasian 44-year-old male patient received lamivudine and, 4 years later, added adefovir because of a virological breakthrough. In 2011, considering his young age, liver stiffness (4.3 kPa) and HBsAg levels (3533 IU/mL), we added Peg-interferon α-2a for six months (3 in combination with nucleos(t)ide analogues followed by 3 mo of Peg-interferon α-2a monotherapy). A decrease of HBsAg levels was observed after 1 mo (1.21 log) of Peg-interferon and 3 mo (1.88 log) after the discontinuation of all drugs. Later, a complete clearance of HBsAg was obtained with steadily undetectable HBV-DNA serum levels (< 9 IU/mL). HBsAg clearance by the addition of a short course of Peg-interferon α-2a represents an important result with clinical and pharmaco-economic implications, considering that nucleos(t)ide analogues therapy in HBeAg negative chronic hepatitis B patients is considered a long-lasting/life-long treatment.
Core tip: The ideal endpoint of antiviral therapy is HBsAg loss, a difficult goal to obtain, especially in HBeAg negative patients. A Caucasian 44-year-old male patient, HBeAg negative, genotype D, received lamivudine and, 4 years later, added adefovir because of a virological breakthrough. Five years later, considering his age, liver stiffness (4.3 kPa) and HBsAg levels (3533 IU/mL), we added Peg-interferon α-2a for six months (3 in combination with nucleos(t)ide analogues followed by 3 of Peg-interferon monotherapy), obtaining a complete HBsAg clearance. This result has important clinical and pharmaco-economic implications, since nucleos(t)ide analogues therapy in HBeAg negative patients is considered a long-lasting/life-long treatment.
- Citation: Barone M, Iannone A, Leo AD. HBsAg clearance by Peg-interferon addition to a long-term nucleos(t)ide analogue therapy. World J Gastroenterol 2014; 20(26): 8722-8725
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8722.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8722
Hepatitis B virus infection is a worldwide health problem. World Health Organization estimates that about 2 billion people have been infected by the virus (1/3 of the entire world population) and about 350 million (6%) are carriers of a chronic infection[1]. Italy ranks among the countries with low endemicity[2].
Currently, the treatment of chronic hepatitis B (CHB) is based on either a 12 mo-period of peg-interferon α-2a (PEG-IFN α-2a) or a long-lasting nucleos(t)ide analogues (NAs) administration[1-3]. The ideal endpoint of antiviral therapy, even if uncommon, is the loss of HBsAg ± seroconversion to anti-HBs[1-3]. HBe/anti-HBe seroconversion in HBeAg positive patients and disappearance of hepatitis B virus (HBV)-DNA from serum, however, represent significant end-points since they are correlated with a reduced risk of liver disease progression[4].
In HBeAg negative patients, HBsAg loss is exceptionally observed during the first 4-5 years of NAs treatment[1]. In fact, in patients undergoing lamivudine monotherapy HBsAg clearance rates are 1.9% and 11.7% at 5 and 7 years, respectively[5,6]. Also patients treated with adefovir show low percentages of HBsAg loss, ranging from 0% to 5% at 1 and 5 years, respectively[1,7]. Moreover, the combination treatment with lamivudine + adefovir does not increase their efficacy compared to the monotherapies (2.4% at 4 years)[8].
On the other hand, after 12 mo of PEG-IFN α-2a therapy, HBsAg clearance in HBeAg negative patients is 9% and 12% at 3 and 5 years of follow-up, respectively[1,9,10]. In these subjects, a rapid decrease in serum HBsAg is an important predictor of response to PEG-IFN α-2a treatment[11]. The combination therapy with PEG-IFN α-2a + NAs does not seem to increase the HBsAg loss rate as compared to the PEG-IFN α-2a monotherapy. In fact, 3 years after the administration of PEG-IFN α-2a (180 µg/wk) + lamivudine for 12 mo a 8% HBsAg loss was observed[9]. However, a combination therapy with PEG-IFN α-2a (180 µg/wk) + adefovir for 48 wk determined a 17% HBsAg loss after 2 years of follow-up[12].
Finally, only a few cases in the literature describe the effect of PEG-IFN α-2a as add-on therapy in patients undergoing long-lasting NAs treatment with steadily HBV-DNA negative/HBsAg positive values. Mangano et al[13] report a HBsAg/anti-HBs seroconversion obtained by the addition of PEG-IFN α-2a for 12 mo in a young CHB patient undergoing lamivudine therapy. However, they did not assess HBsAg titre before the add-on therapy, neither report HBV genotype, two well-known predictors of response to PEG-IFN α-2a. Kittner et al[14] after a 12-mo add-on therapy with PEG-IFN α-2a in 12 CHB patients undergoing NAs treatment report 2 cases of HBsAg/anti-HBs seroconversion. However, the first patient was a HBV genotype A (known to respond better to PEG-IFN α-2a[15]), while the second had a very low HBsAg titre (16 IU/mL) before the PEG-IFN α-2a administration.
A 44-year-old Caucasian male with CHB came to our attention in November 2002. His mother and one brother were affected by HBV-related cirrhosis and CHB, respectively, suggesting a mother-to-child transmission of the virus. The medical history of our patient was characterized by first determination of elevated alanine aminotransferase (ALT) levels in 1996 and diagnosis of HBV infection in June 2002.
In November 2002, he was admitted to our Gastroenterology Unit and blood examinations were performed that demonstrated ALT × 1.2 the upper limit of normal (ULN), negative HBeAg, positive anti-HBe, positive HBV-DNA (1.23 × 105 copies/mL, determined by the Versant HBV-DNA 3.0 assay), HBV genotype D and negative anti-HCV and anti-HDV IgG. Thus, he underwent liver biopsy, that showed a grade 2 stage 2 CHB (by METAVIR score system), and transient elastography, that resulted 8.5 kPa (IQR 0.7 kPa, SR 100%). According to these results and the guidelines in force at that time and because of the patient refusal to undergo interferon therapy, we started an antiviral treatment with lamivudine in December 2002 obtaining ALT normalization and a HBV DNA value < 2 × 103 cp/mL. In January 2006, a virological breakthrough to lamivudine was observed, with increased ALT levels (× 2.3 ULN) and HBV-DNA serum levels of 2.1 × 106 cp/mL (by the COBAS Amplicor HBV Monitor assay). Thus, adefovir (10 mg/day), the only NA rescue therapy available for lamivudine resistance at that time, was added, reaching undetectable HBV-DNA (< 2 × 102 copies/mL, by the COBAS Amplicor HBV Monitor assay) and normal ALT levels after 6 mo of combined therapy.
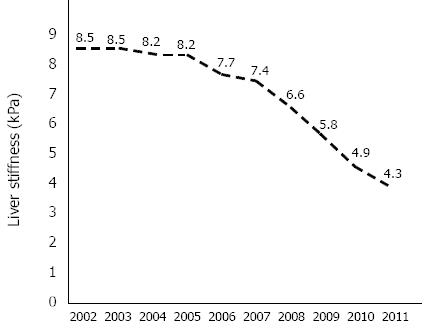
Liver stiffness, assessed every year by transient elastography (Figure 1), progressively decreased, reaching a value of 4.3 kPa (IQR 0.6 kPa, SR 100%) in February 2011.
From 2002 to 2005 HBV-DNA was tested by the Versant HBV-DNA 3.0 assay (Bayer Corporation, Tarrytown, NY) with a lower limit of quantification (LLQ) of 2 × 103 copies/mL, while from 2006 to 2010 it was determined by the COBAS Amplicor HBV Monitor assay (Roche Diagnostics, Indiannapolis, IN) with a LLQ of 2 × 102 copies/mL. Finally, from 2011, HBV-DNA serum levels were determined by the Roche TaqMan PCR Real Time assay (Roche Diagnostics, Indiannapolis, IN) with a LLQ of 9 IU/mL.
HBsAg has been tested since 2010 by the Roche Elecsys HBsAg II quantitative assay (Roche Diagnostics, Indiannapolis, IN). Serological HBV markers (HBsAg, anti-HBsAg, HBeAg, anti-HBe and anti-HBc IgG and IgM) and anti-HDV IgG were all detected using standard laboratory techniques. Anti-HCV was determined by commercial EIA (HCV 3.0 Ortho Clinical Diagnostics, Amersham, Bucks, United Kingdom).
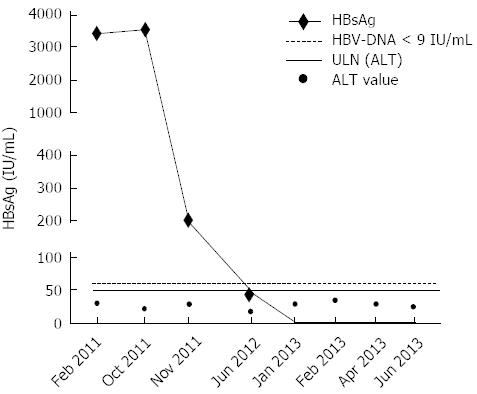
In February 2011, HBV-DNA was < 9 IU/mL, HBsAg titre was high (3,441 IU/mL)[16] and ALT was lower than ULN (× 0.6) (Figure 2). In October 2011, on the basis of these results and taking in consideration the young age of the patient (42 years), we decided to add PEG-IFN α-2a (180 µg/wk) for 6 mo with the intent of promoting HBsAg loss, also considering that the patient never underwent this kind of treatment. At this time HBV-DNA was < 9 IU/mL, HBsAg value 3533 IU/mL and ALT × 0.4 ULN (Figure 2). PEG-IFN α-2a (180 μg/wk) was added to NAs for 3 mo, then NAs treatment was interrupted while PEG-IFN α-2a was continued for additional 3 mo.
In November 2011, one month after the beginning of PEG-IFN α-2a, a decrease of HBsAg titre higher than one log was obtained (HBsAg 218 IU/mL, 1.21 log decrease). Moreover, three months after the discontinuation of the PEG-IFN α-2a therapy, a further decrease of HBsAg serum levels was observed (HBsAg 47 IU/mL, 1.88 log decrease), in presence of undetectable HBV-DNA (< 9 IU/mL) and normal ALT levels. Seven months later, the quantitative determination of HBsAg resulted negative. Later on, we performed 4 other HBsAg determinations (the last on June 2013), and all were negative, with HBV-DNA serum levels steadily undetectable (< 9 UI/mL) and normal ALT (Figure 2).
The advent of HBsAg titre determination has revived the possibility to start a course of Peg-interferon α-2a in HBV patients who never received interferon, considering that this prognostic parameter makes interferon treatment cost/effective and minimizes the possibility of adverse effects.
The rates of HBsAg clearance reported in the literature refer to either NAs[1,5-8] or PEG-IFN α-2a[1,9,10] monotherapies or to the combination of these drugs[9,12]. On the other hand, only few data are available about the overlap of PEG-IFN α-2a in steadily HBV-DNA negative/HBsAg positive patients, undergoing long-lasting NAs therapy, with the exception of the findings reported by Mangano et al[13] and Kittner et al[14], that present some limitations (lack of HBsAg titre and HBV genotype determination before the add-on therapy, or treatment of HBV genotype A, which is known to respond better to PEG-IFN α-2a[15], or treatment of a patient with a HBsAg titre as low as 16 IU/mL).
In the present study, we propose a therapeutical approach that takes in consideration a new combination of nucleos(t)ide analogues therapy and PEG-IFN α-2a. In fact, we used for 3 mo nucleos(t)ide analogues in combination with Peg-interferon α-2a followed by 3 mo of Peg-interferon α-2a monotherapy. Moreover, even if our original intent was to promote HBsAg loss, this schedule gave us the possibility to evaluate if the treatment with Peg-interferon alone prevents sudden HBV reactivation with hepatitis flares, after interruption of nucleos(t)ide analogues therapy.
Our results do not exclude the possibility that HBsAg clearance could be due to the beneficial effects of 5 years of therapy with analogues. However, a “casual” striking decrease of HBsAg after one month of PEG-IFN α-2a therapy seems unlikely.
Finally, our findings need to be verified by a prospective trial on patients under long-lasting NAs therapy with clinical (young age, low stage of fibrosis) and virological (HBeAg negative, steadily undetectable HBV-DNA, genotype D and high HBsAg titre) characteristics similar to those observed in our patient, in order to verify the efficacy of PEG-IFN α-2a add-on treatment on HBsAg clearance. This therapeutic strategy would produce obvious clinical and pharmaco-economic advantages, especially if a HBsAg-guided PEG-IFN α-2a therapy is adopted.
Patient was a 44-year-old Caucasian male with chronic hepatitis B.
According to the liver stiffness value assessed in February 2011 (4.3 kPa), his chronic hepatitis was characterized by mild fibrosis and not by moderate or severe fibrosis.
In October 2011, HBV-DNA was < 9 IU/mL (Roche TaqMan PCR Real Time assay), HBsAg value 3533 IU/mL (Roche Elecsys HBsAg II quantitative assay) and alanine aminotransferase × 0.4 ULN.
Liver stiffness, determined by transient elastography in February 2011, was 4.3 kPa (IQR 0.6 kPa, SR 100%).
Liver biopsy was performed only in 2002 and showed a grade 2 stage 2 chronic hepatitis B (by METAVIR score system).
Patient started lamivudine in December 2002, added adefovir in January 2006, because of a virological breakthrough to lamivudine, and, in October 2011, added Peg-interferon α-2a to nucleos(t)ide analogues for 3 mo, then nucleos(t)ide analogues were interrupted while Peg-interferon α-2a was continued for additional 3 mo.
Only few data are available about the overlap of Peg-interferon α-2a to nucleos(t)ide analogues in steadily HBV-DNA negative/HBsAg positive patients with the exception of the findings reported by Mangano et al and Kittner et al, that present some limitations (lack of HBsAg titre and HBV genotype determination before the add-on therapy, or treatment of HBV genotype A, which is known to respond better to Peg-interferon α-2a, or treatment of a patient with a HBsAg titre as low as 16 IU/mL).
In the present case, the authors propose a new therapeutical approach to promote HBsAg clearance, based on the overlap of Peg-interferon α-2a in steadily HBV-DNA negative/HBsAg positive patients undergoing long-lasting NAs therapy.
This case report offers a new approach to promote HBsAg clearance in patients with CHB treated with NUCs for long-time. This interesting observation must be checked in a clinical trial.
P- Reviewers: Guo XZ, Karatapanis S S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2400] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 2. | Stroffolini T, Gaeta GB, Mele A. AASLD Practice Guidelines on chronic hepatitis B and HBV infection in Italy. Hepatology. 2007;46:608-69; author reply 609. [PubMed] |
| 3. | Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, Dauvergne A, Cardoso AC, Asselah T, Nicolas-Chanoine MH. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J Hepatol. 2009;50:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [PubMed] |
| 5. | Idilman R, Cinar K, Seven G, Bozkus Y, Elhan A, Bozdayi M, Yurdaydin C, Bahar K. Hepatitis B surface antigen seroconversion is associated with favourable long-term clinical outcomes during lamivudine treatment in HBeAg-negative chronic hepatitis B patients. J Viral Hepat. 2012;19:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Fasano M, Lampertico P, Marzano A, Di Marco V, Niro GA, Brancaccio G, Marengo A, Scotto G, Brunetto MR, Gaeta GB. HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years. J Hepatol. 2012;56:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [PubMed] |
| 8. | Ghany MG, Feld JJ, Zhao X, Heller T, Doo E, Rotman Y, Nagabhyru P, Koh C, Kleiner DE, Wright EC. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Marcellin P, Piratvisuth T, Brunetto M. Increasing rates of HBsAg clearance and seroconversion in patients with HBeAg-negative disease treated with peginterferon alfa-2a ± lamivudine: results of 5-year post-treatment follow up. J Hepatol. 2009;50:S336. |
| 11. | Moucari R, Mackiewicz V, Lada O, Ripault MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer N, Bedossa P. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Takkenberg RB, Jansen L, de Niet A, Zaaijer HL, Weegink CJ, Terpstra V, Dijkgraaf MG, Molenkamp R, Jansen PL, Koot M. Baseline hepatitis B surface antigen (HBsAg) as predictor of sustained HBsAg loss in chronic hepatitis B patients treated with pegylated interferon-α2a and adefovir. Antivir Ther. 2013;18:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Mangano C, Squadrito G, Cacciola I, Carpentieri M, Foti G, Raimondo G. Effectiveness of add-on pegylated interferon alfa-2a therapy in a lamivudine-treated patient with chronic hepatitis B. Ann Hepatol. 2011;10:84-87. [PubMed] |
| 14. | Kittner JM, Sprinzl MF, Grambihler A, Weinmann A, Schattenberg JM, Galle PR, Schuchmann M. Adding pegylated interferon to a current nucleos(t)ide therapy leads to HBsAg seroconversion in a subgroup of patients with chronic hepatitis B. J Clin Virol. 2012;54:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. 2009;137:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Brunetto MR, Oliveri F, Colombatto P, Moriconi F, Ciccorossi P, Coco B, Romagnoli V, Cherubini B, Moscato G, Maina AM. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010;139:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 306] [Article Influence: 20.4] [Reference Citation Analysis (0)] |