Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8612
Revised: February 14, 2014
Accepted: April 5, 2014
Published online: July 14, 2014
Processing time: 230 Days and 4.9 Hours
AIM: To follow up patients with pseudotumoral chronic pancreatitis (PCP) to assess their outcome and identify an optimal surveillance interval.
METHODS: Data obtained prospectively were analyzed in a retrospective manner. Patients with clinical evidence of chronic pancreatitis (abdominal pain in the epigastrium, steatorrhea, and diabetes mellitus), endoscopic ultrasound (EUS) criteria > 4, and EUS-fine needle aspiration (FNA) were included. A pseudotumor was defined as a non-neoplastic space-occupying lesion, a cause of chronic pancreatitis that may mimic changes typical of pancreatic cancer on CT or endoscopic ultrasound but without histological evidence. A real tumor was defined as a neoplastic space-occupying lesion because of pancreatic cancer confirmed by histology.
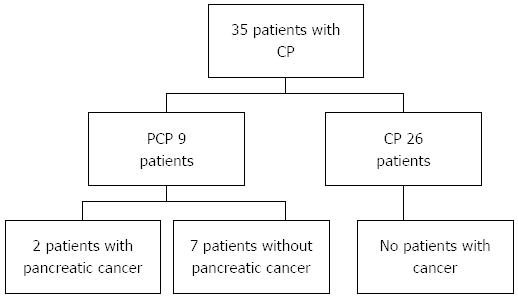
RESULTS: Thirty-five patients with chronic pancreatitis were included, 26 (74.2%) of whom were men. Nine (25.7%) patients were diagnosed with pseudotumoral chronic pancreatitis and two (2/35; 5.7%) patients with pseudotumoral chronic pancreatitis were diagnosed with pancreatic cancer on follow-up. The time between the diagnosis of pseudotumoral chronic pancreatitis and pancreatic adenocarcinoma was 35 and 30 d in the two patients. Definitive diagnosis of pancreatic adenocarcinoma was made by surgery. In the remaining six patients with pseudotumoral chronic pancreatitis, the median of follow-up was 11 mo (range 1-22 mo) and they showed no evidence of malignancy on surveillance. In the follow-up of patients without pseudotumoral chronic pancreatitis but with chronic pancreatitis, none were diagnosed with pancreatic cancer. According to our data, older patients with chronic pancreatitis are at risk of pseudotumoral chronic pancreatitis.
CONCLUSION: According to characteristics of patient, detection of PCP should lead a surveillance program for pancreatic cancer with EUS-FNA in < 1 mo or directly to surgical resection.
Core tip: Actually, there are no clear recommendations for follow-up of patients with chronic pancreatitis and solid pancreatic mass lesions. We followed-up patients with chronic pancreatitis and solid pancreatic mass lesions and we assessed the final outcome and identified an optimal surveillance interval. We found that almost one-third of patients with chronic pancreatitis had pseudotumoral chronic pancreatitis, and 22.2% had unresectable pancreatic adenocarcinoma less than 2 mo after the initial diagnosis. Endoscopic ultrasound fine needle aspiration can miss malignancy in nearly 25% of patients with pseudotumoral chronic pancreatitis.
- Citation: Téllez-Ávila FI, Villalobos-Garita &, Giovannini M, Chan C, Hernández-Calleros J, Uscanga L, Ramírez-Luna M&. Follow-up of patients with pseudotumoral chronic pancreatitis: Outcome and surveillance. World J Gastroenterol 2014; 20(26): 8612-8616
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8612.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8612
Pancreatic cancer (PC) at diagnosis is unresectable in 70% of cases[1]. The risk of developing PC is increased in patients with chronic pancreatitis. Surveillance in patients with chronic pancreatitis may represent an opportunity for early detection of PC[2]. The increase in risk for PC in patients with chronic pancreatitis ranges from 14.4-26.7 times in 10-year follow-up[1,3]. It is difficult to differentiate by images between pancreatic carcinoma and pseudotumor in the context of chronic pancreatitis[4,5]. In case of endoscopic ultrasound (EUS) some criteria have been proposed, but even using the fine needle aspiration (FNA) biopsy, the results have not been satisfactory[6,7]. Actually, there are no clear recommendations for follow-up of patients with chronic pancreatitis and solid pancreatic mass lesions[8]. The aim of this study was to follow up patients with chronic pancreatitis and solid pancreatic mass lesions to assess the final outcome and identify an optimal surveillance interval.
Data obtained prospectively were analyzed in a retrospective manner. Electronic and paper records of consecutive patients evaluated from March 2005 to December 2012 were evaluated. Patients with clinical evidence of chronic pancreatitis, EUS criteria > 4, and EUS FNA were included[9]. According to the local Ethics Committee, all patients signed an informed consent document.
Before the procedure, all patients had laboratory tests including prothrombin time and full blood count. The patients were placed in a left decubitus position and sedated using a combination of midazolam, propofol, and fentanyl by an anesthetist. Patients were continually monitored with an automated noninvasive blood pressure device, electrocardiogram, and pulse oximetry throughout the procedure. EUS was performed with a linear array echoendoscope, GFUCT-140 (Olympus America Inc; Center Valley, PA), by two echoendoscopists. All patients were hospitalized, and after the procedure they were observed with an automatic monitor for at least 4 h for surveillance of possible complications.
At first, the transducer was brought into a stable position in front of the targeted lesion. The metal spiral was then introduced into the biopsy channel, observing carefully that the needle piston was securely locked and the needle was completely retracted. The spiral was inserted entirely and the handle with the Luer-lock firmly screwed onto the biopsy channel. To ensure that the sheath was protecting the entire length of the working channel, we used the optic of the endoscope. With the stylet retracted but still inside the needle, the biopsy needle was moved forward into the lesion under full real-time ultrasound control. After penetration into the middle of a lesion, the stylet was completely removed. Upon reaching the optimal needle position in the middle of the lesion, a 10 mL syringe with a locking device was firmly screwed on the needle, and the syringe piston was pulled to create a low pressure. The syringe piston was locked in this position for permanent suction. The needle was moved to and fro 5-10 times inside the lesion under complete ultrasonic control. With the needle tip still in the lesion, suction was released and the needle was safely retracted inside the needle sheath and locked in a secure position.
All patients had a CT with a 64-slice multidetector CT (Somatom, Sensation 64; Siemens München Germany) and images were obtained with a section thickness of 3 mm with a reconstruction interval of 2-2.5 mm. All cases were analyzed on a workstation with the capability to produce coronal reformatted images. Patients received intravenous (IV) contrast; 120 mL of Conray (Mallinckrodt Baker Inc., St Louis Missouri, United States) was given 45 s prior to the CT examination. Forty milliliters of ioditrast M60 (Justesa Imagen Mexicana) was diluted in 1000 mL of water and given to all patients orally 1 h prior to CT. All patients received IV and oral contrast. All CT images were analyzed by at least two certified radiologists and discussed with the endoscopic team before the procedure (EUS-FNA). All CT and endoscopic studies were performed in the same center.
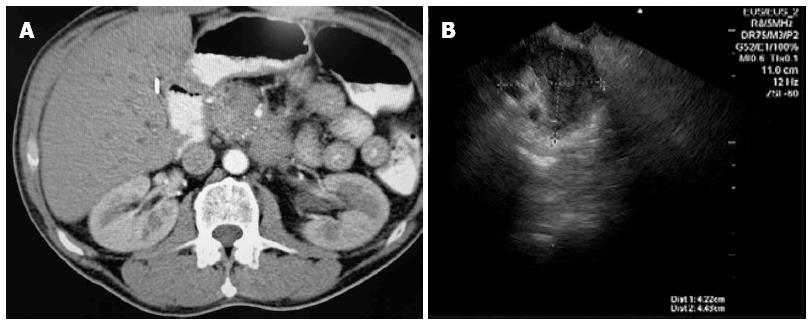
A pseudotumor (Figure 1) was defined as a non-neoplastic space-occupying lesion, a cause of chronic pancreatitis that may mimic changes typical of pancreatic cancer on CT or endoscopic ultrasound but without histological evidence. It should be recognized, however, that even this definition of “pseudotumor” is highly subjective since it relies on the quality of the preoperative diagnostic evaluation as well as the skills of the interpreters of the tests performed[10]. A real tumor was defined as a neoplastic space-occupying lesion because of pancreatic cancer confirmed by histology. Clinical characteristics considered associated with chronic pancreatitis were: abdominal pain in the epigastrium, often with radiation to the back; steatorrhea; and diabetes mellitus[11].
Medians, ranges, and proportions were used to summarize the demographics and clinical variables. Using the χ2 test or Mann-Whitney U test, according variables, differences between groups were tested. A two-tailed P value < 0.05 was considered significant. All analyses were performed by SPSS V.20 for Mac.
A total of 200 pancreatic EUS were performed because of clinical suspicion of chronic pancreatitis (abdominal pain in the epigastrium with radiation to the back, or exocrine pancreatic insufficiency with chronic diarrhea and/or steatorrhea). Thirty-five patients with diagnosis of chronic pancreatitis were included. Twenty-six (74.2%) patients were men and 9 (25.8%) patients were women. The median age was 38 years (range 18-75 years). All patients had clinical and EUS criteria. Twenty-two (62.8%) patients had 4 EUS criteria, 6 (17%) patients had five criteria, and 7 (20%) patients had ≥ 6 criteria. Nine (25.7%) patients were diagnosed with pseudotumoral chronic pancreatitis. Clinical and demographic characteristics of included patients classified by the presence/absence of pseudotumoral chronic pancreatitis are shown in Table 1. In Tables 2 and 3, clinical data, demographics, and imaging characteristics of included patients with pseudotumor and chronic pancreatitis are shown.
| Patient | Age, yr | Gender | Number of diagnostic criteria for chronic pancreatitis by EUS | Evidence of pseudotumor on CT | Interval (“time between”) or follow-up |
| 1 | 64 | F | 4 | No | 1 mo |
| 2 | 48 | F | 4 | No | 13 mo |
| 3 | 53 | M | 4 | No | 21 mo |
| 4 | 44 | F | 4 | Yes | 5 mo |
| 5 | 75 | F | 4 | Yes | 22 mo |
| 6 | 69 | M | 4 | Yes | 13 mo |
| 7 | 18 | M | 6 | Yes | 30 d |
| 8 | 56 | M | 5 | Yes | 30 d |
| 9 | 52 | M | 4 | Yes | 30 d |
| Pseudotumor/patient | Maximum diameter (mm) | Localization | Vascular involvement | Presence of lymphadenopathy | EUS FNA/adequate sample | Surgery | Final diagnosis |
| 1 | 35 | Body | Yes | No | Normal/yes | No | CP |
| 2 | 30 | Head | No | Yes | CP/yes | No | CP |
| 3 | 20 | Head | No | No | Normal/yes | No | CP |
| 4 | 35 | Head | Yes | Yes | Inflammation/yes | Yes | CP |
| 5 | 35 | Neck | No | No | CP/yes | No | CP |
| 6 | 28 | Body | No | No | CP/yes | No | CP |
| 7 | 30 | Neck | No | Yes | Non-neoplastic cells/yes | Yes | Myofibroblastic tumor |
| 8 | 40 | Head | Yes | Yes | Normal/yes | Yes | Pancreatic cancer |
| 9 | 40 | Head | Yes | Yes | CP/yes | Yes | Pancreatic cancer |
Two of nine (22.2%) patients with pseudotumoral chronic pancreatitis were diagnosed with pancreatic cancer on follow-up, although basal EUS FNA did not reveal malignant cells. One (11.1%) patient was diagnosed on follow-up with myofibroblastic tumor of the pancreas. The time between the diagnosis of pseudotumoral chronic pancreatitis and pancreatic adenocarcinoma was 35 and 30 d. The diagnosis of myofibroblastic tumor was 30 d after the pseudotumoral chronic pancreatitis diagnosis. The two patients with pancreatic adenocarcinoma had an unresectable pancreatic adenocarcinoma at the moment of final diagnosis. Definitive diagnosis of pancreatic adenocarcinoma was made by surgery. In the remaining six patients with pseudotumoral chronic pancreatitis, the median of follow-up was 11 mo (range 1-22 mo) and they showed no evidence of malignancy on surveillance.
In the follow-up of patients with chronic pancreatitis but without pseudotumoral chronic pancreatitis, none were diagnosed with pancreatic cancer. The median follow-up was 22 mo (range 1-67 mo) (Figure 2).
Almost one-third of patients with chronic pancreatitis had pseudotumoral chronic pancreatitis, and two of them (2/9; 22.2%) had unresectable pancreatic adenocarcinoma less than 2 mo after the initial diagnosis. The frequency of pseudotumoral chronic pancreatitis is not well known and little data exist. In one study with 85 patients with chronic pancreatitis, 6% (n = 5) of these patients had pseudotumoral chronic pancreatitis and 3.5% (n = 3) of them were diagnosed with pancreatic cancer[12]. In a more recent study, Burski et al[8] found that 29% (125/436) of patients with chronic pancreatitis had pseudotumoral chronic pancreatitis and 13% (16/125) of them were diagnosed with pancreatic adenocarcinoma on follow-up. In Table 4, published data about patients with chronic pancreatitis and pseudotumoral chronic pancreatitis are shown.
Regarding follow-up of patients with pseudotumoral chronic pancreatitis, there are not clear recommendations regarding the ideal imaging study and time for subsequent imaging relative to the initial diagnosis. Therefore, the surveillance for pancreatic cancer in patients with pseudotumoral chronic pancreatitis is not well established and it has a negative impact in this population[1,13-16]. In this study, in patients with pseudotumoral chronic pancreatitis for whom pancreatic cancer was diagnosed on follow-up, pancreatic cancer was confirmed at an advanced stage in a period of less than 2 mo after the initial detection. This data suggest a misdiagnosis rather than new onset of the neoplasm during follow-up. Because of that, surveillance programs for pancreatic cancer with intervals greater than 6 mo seem to be insufficient in patients with pseudotumoral chronic pancreatitis. In the study by Burski et al[8], it was concluded that an interval of 3-6 mo for surveillance for pancreatic cancer in patients with pseudotumoral chronic pancreatitis was not optimal due to rapid disease progression. Several studies have attempted to establish EUS imaging criteria (without tissue sampling) for the discrimination of benign inflammatory pseudotumors and tumors. Despite the high resolution of EUS, it does not provide reliable differentiation of benign and malignant lesions of the pancreas[17]. New technologies, such as EUS elastography and contrast-enhanced EUS (CE-EUS) could be important tools for differential diagnosis. In a multicenter study, 30 cases with benign nodule of chronic pancreatitis were studied with EUS elastography[18]. All nodules of chronic pancreatitis presented benign aspects (mixed green and low intensity of blue) and elastography showed malignant aspects (intense blue coloration) for all pancreatic adenocarcinomas, endocrine tumors, pancreatic metastases, and pancreatic sarcomas. In the study of Hocke et al[19], adenocarcinoma developed on chronic pancreatitis was non-enhanced after contrast injection. Conversely, pseudotumoral chronic pancreatitis was hypervascularized (91%) after SonoVue® injection. According to our data, older patients with chronic pancreatitis are at risk of pseudotumoral chronic pancreatitis, and could be candidates for closer follow-up (Table 1).
The limitations of our work are the small sample size and retrospective analysis. The nature of disease makes it difficult for a single center to have a bigger sample size. Multicenter studies must be considered for future designs. Our data are useful for future systematic reviews and meta-analyses.
In conclusion, we suggest that according to specific characteristics of patient, detection of pseudotumoral chronic pancreatitis should lead a close surveillance program for pancreatic cancer with EUS in less than 1 mo or directly to surgical resection. EUS FNA can miss malignancy in nearly 25% of patients with pseudotumoral chronic pancreatitis.
Pancreatic cancer at diagnosis is unresectable in 70% of cases. It is difficult to differentiate by images between pancreatic carcinoma and pseudotumor in the context of chronic pancreatitis. The authors followed-up patients with chronic pancreatitis and solid pancreatic mass lesions and we assessed the final outcome and identified an optimal surveillance interval.
According to characteristics of patient, detection of pseudotumoral chronic pancreatitis should lead a surveillance program for pancreatic cancer with endoscopic ultrasound fine needle aspiration in < 1 mo or directly to surgical resection.
This is an interesting retrospective analysis of patients with pseudotumoral lesions in the context of chronic pancreatitis. The results are alarming.
P- Reviewers: Chao CT, El-Sayed M, Keck T, Luo HS, Zhou GX S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Queneau PE, Adessi GL, Thibault P, Cléau D, Heyd B, Mantion G, Carayon P. Early detection of pancreatic cancer in patients with chronic pancreatitis: diagnostic utility of a K-ras point mutation in the pancreatic juice. Am J Gastroenterol. 2001;96:700-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1138] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 4. | Farrell JJ. Diagnosing pancreatic malignancy in the setting of chronic pancreatitis: is there room for improvement? Gastrointest Endosc. 2005;62:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Pery C, Meurette G, Ansquer C, Frampas E, Regenet N. Role and limitations of 18F-FDG positron emission tomography (PET) in the management of patients with pancreatic lesions. Gastroenterol Clin Biol. 2010;34:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Brand B, Pfaff T, Binmoeller KF, Sriram PV, Fritscher-Ravens A, Knöfel WT, Jäckle S, Soehendra N. Endoscopic ultrasound for differential diagnosis of focal pancreatic lesions, confirmed by surgery. Scand J Gastroenterol. 2000;35:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Brimienė V, Brimas G, Strupas K. Differential diagnosis between chronic pancreatitis and pancreatic cancer: a prospective study of 156 patients. Medicina (Kaunas). 2011;47:154-162. [PubMed] |
| 8. | Burski C, Varadarajulu S, Trevino J. Diagnosing cancer in chronic pancreatitis: The struggle persists. Gastrointest Endosc. 2012;75:AB193. [DOI] [Full Text] |
| 9. | Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Adsay NV, Basturk O, Klimstra DS, Klöppel G. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2004;21:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Feldman M, Friedman L, Brand L. Sleissenger and Fordtran’s Gastrointestinal and Liver disease. 9th ed. Philadelphia: Saunders Elsevier 2010; 985-1014. |
| 12. | Barthet M, Portal I, Boujaoude J, Bernard JP, Sahel J. Endoscopic ultrasonographic diagnosis of pancreatic cancer complicating chronic pancreatitis. Endoscopy. 1996;28:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Balthazar EJ. Pancreatitis associated with pancreatic carcinoma. Preoperative diagnosis: role of CT imaging in detection and evaluation. Pancreatology. 2005;5:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Vitone LJ, Greenhalf W, McFaul CD, Ghaneh P, Neoptolemos JP. The inherited genetics of pancreatic cancer and prospects for secondary screening. Best Pract Res Clin Gastroenterol. 2006;20:253-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Howes N, Neoptolemos JP. Risk of pancreatic ductal adenocarcinoma in chronic pancreatitis. Gut. 2002;51:765-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Gemmel C, Eickhoff A, Helmstädter L, Riemann JF. Pancreatic cancer screening: state of the art. Expert Rev Gastroenterol Hepatol. 2009;3:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246-250. [PubMed] |