INTRODUCTION
The liver shows a remarkable uniform anatomical structure composed of the regular arrangement of mainly hexagonal lobules in a honey comb-like pattern. At the periphery of these lobules blood from the portal venules and the hepatic arterioles enters the sinusoids, the smallest capillaries of the liver which form an anastomosing network that runs concentrically to the central vein where the blood drains into the hepatic venules[1-3]. The hepatocytes lined up in a sponge-like arrangement between the sinusoids along the porto-central axis show a remarkable heterogeneity with respect to the biochemical and physiological functions they perform. This dynamic structural and functional heterogeneity, known as metabolic zonation[4], seems to be the precondition for simultaneously performing the plethora of functions necessary to maintain metabolic homeostasis of the organisms under the various physiological conditions. Progress in metabolic zonation and its regulation was comprehensively reviewed from time to time[5-7], and culminated in the discovery of Wnt/β-catenin signalling as a master regulator of zonation[8,9]. The present review tries to give an update on the impressive development of research on zonation during the last decade. It will be conjectured that perturbation of the sophisticated regulation of zonation may presumably have an enormous impact on the development of various metabolic diseases. Therefore, a major aim of our review is to identify important open questions that need to be answered, in order to gain deeper insight into the mechanisms of liver zonation, its regulation and possible malfunction in disease.
NEW METHODS
A survey of traditionally used methods for studying liver zonation has recently be provided[10]. Therefore, we will focus here mainly on novel developments facilitating progress in elucidating hepatocyte heterogeneity and its consequences on a very broad scale, particularly at the “omics” level. Collection of intact tissue from periportal and pericentral zones by laser-capture microdissection (LCM)[11] certainly marks an improvement compared to digitonin-collagenase perfusion (DCP)[12], because it avoids tissue damage and extracted RNA can be directly used for microarray studies. In contrast to DCP which provides data for the most important cell-type in the liver, the hepatocyte, LCM cannot, however, discriminate between different contributions of hepatocytes and non-parenchymal cells[11].
Microscopic inspection of immunolabelled or otherwise stained sections is still one of the best and most reliable methods to reveal liver cell heterogeneity, because the antigen content of each cell, parenchymal or non-parenchymal, can separately be investigated and discerned, and gradients in expression across the periportal-pericentral axis can easily be visualised. In the case of proteins, detection of individual proteins is possible by specific antibodies and has been used efficiently since the early times of investigation in this field. Nowadays, whole slide scans can be prepared that favour inspection of the heterogeneity from the global level (liver lobe) down to the sub-microscopic level. Concerning human liver, the collection of various immunostainings in the Human Protein Atlas (http://www.proteinatlas.org) can actually be used for an initial orientation on whether a certain protein is heterogeneously expressed in liver or not. Although this possibility is very helpful, the reliability of these findings is often limited by the small number of liver donors, the small size of the sections, and the questionable quality of the antibodies despite the honourable effort invested into their evaluation.
With respect to other parameters, such as lipids or metabolites no comparable methods were available in the past. This has changed, because novel techniques based on mass spectrometry have been developed. For instance, in situ lipidomics analysis by cluster time-of-flight-secondary ion mass spectrometry (TOF-SIMS) imaging was used to map liver biopsies of patients with in non-alcoholic fatty liver disease (NAFLD)[13]. This technique allowed to map lipids on liver sections at the micrometer scale and to simultaneously characterize their molecular distribution. Briefly, accumulations of triacylglycerols, diacylglycerols, monoacylglycerols, fatty acids (with the apparition of myristic acid) and a selective macrovacuolar localization of cholesterol were observed in steatotic areas of fatty livers compared to control livers[13]. Remarkably, the pronounced zonation of lipid droplet accumulation NAFLD was underscored by the detection of very fine differences in lipid localizations depending on alkyl acid chain lengths and other molecular features. The drawback of this fascinating method is that TOF-SIMS is a hard ionization technology hampering the detection and discrimination of intact phospholipids.
In a more recent study, this drawback was circumvented by using matrix assisted laser desorption ionization-imaging mass spectrometry (MALDI-IMS), a more “soft” technology[14]. These workers investigated hepatic phospholipid abundance by quantitative lipidomic profiling and phospholipid localization on sections of patients with simple steatosis and non-alcoholic steatohepatitis (NASH). In essence, this pioneering study shows several new aspects relevant for normal and pathologic phosopholipid metabolism: (1) phospholipid metabolism is strongly zonated in normal liver of obese patients; (2) the marked zonation of several molecular species of phosphatidyl choline present in controls and patients with simple steatosis is lost in NASH specimens; and (3) phospholipid zonation is associated with the presence of an intrahepatic proinflammatory phenotype[14]. Although these studies strongly suggest that loss, shift or gain of zonation of phospholipid metabolism is characterisitic of the development of progressive phenotypes in NAFLD, it remains unclear whether they may be causative or merely consequences of this process.
Another, even broader methodological approach is in situ sequencing of RNA in tissue sections[15] which allows the association of sequencing results to histological structures. Unfortunately, this technique has not yet been used for studying liver zonation. Nonetheless, these developments may provide us with a first glimpse on the potential of future research for studying liver zonation.
REGULATION OF ZONATION
Obviously, all types of signals such as gradients of oxygen, nutrients, metabolites, hormones and cytokines do have an influence on zonation in so far as they modulate the activity of various enzymes involved in the metabolic pathways. This modulation can, but not necessarily has to, result in the shift of zonation, since the protein content or the enzyme activity within the cells can vary independently of the number of cells involved. Indeed, in contrast to many dynamic metabolic pathways several apparently stable functions have been identified over the years the localization of which is almost invariable under a large variety of physiological and even pathological conditions[6]. One of these, hepatic glutamine synthesis via glutamine synthetase shows a very peculiar and stable pericentral localization in less than 3 rows of hepatocytes surrounding the central veins[16]. It was this fascinating “all or none” expression which lead to the discovery of morphogens as master regulators of metabolic zonation (for review[9]).
Morphogen signalling
Morphogens are signalling molecules which play a decisive role in embryogenesis, morphogenesis, and organogenesis[17,18]. Characteristically, their pathway activity is high on average during these processes, but may fluctuate considerably in different phases of development. Usually, in the mature organism, their activity is strongly down-regulated. Reactivation, if any, occurs during tissue regeneration following damage or loss of tissue[19] and, in particular, during development of various types of cancer[20,21]. Under all conditions, one outstanding feature of morphogen signalling is gradient formation over short distances coupled with discrete interpretation of this gradient to regulate a finite number of events along the gradient[22-24]. Therefore, morphogen signalling pathways are involved in all kinds of tissue patterning.
In the last decade, accumulating evidence was collected demonstrating that morphogen signalling pathways are deeply involved in regulating metabolism in the mature liver. The first example was Wnt/β-catenin signalling[25,26] an inhibitory component of which, the adenomatous polyposis coli (APC) gene product, later became apparent as “zonation keeper”[8,27,28]. Further examples for regulation of liver metabolism by morphogens comprise signalling through various members of the fibroblast growth factor (FGF) family[29,30], the transforming growth factor (TGF)-beta family including the bone morphogenetic proteins (BMPs)[31,32], the notch receptor[33-35] and hepatocyte growth factor (HGF)[36,37]. Although it is very likely that many, if not all, morphogens which regulate different aspects of liver metabolism are likewise involved in shaping liver zonation, we will concentrate herein only on Wnt/β-catenin, HGF and Hedgehog signalling for which most evidence is available at present.
Wnt signalling
The dominant role of Wnt/β-catenin signalling in governing metabolic zonation in the liver is now well established and has been reviewed in many excellent reviews[9,28,38,39]. Its activity is highest around the central vein of the liver lobules. Therefore, it is particularly involved in the regulation of pathways limited to or predominating in the pericentral zone such as glutamine synthesis, drug metabolism, bile acid and heme synthesis[8,40,41]. However, the expression of certain periportal functions (e.g., gluconeogenesis, and ureogenesis) is also influenced by the Wnt/β-catenin signalling pathway[8]. Usually, these portal functions are down-regulated, if β-catenin signalling is induced[39,42]. Whether these diverse actions have anything to do with the puzzling and apparently mutually exclusive expression of E-cadherin and N-cadherin in the periportal and pericentral zone, respectively[43], remains an open question that needs to be addressed in the future. Because of the broad influence of the activity of this pathway on liver zonation, it seems appropriate to consider Wnt/β-catenin signalling as a master regulator of zonation. Nonetheless, there may be some exceptions from the rule. In several liver functions such as lipid and ethanol metabolism β-catenin signalling may be essential, but most probably is not sufficient for proper regulation. Thus, Wnt signalling does not seem to affect lipid metabolism per se, but rather in response to additional challenges such as ethanol or methionine and choline-deficient diet[44,45].
HGF and RAS
HGF was identified in 1991 as a morphogen able to induce 3-dimensional (3-D) morphogenesis[46], while other functions, e.g., as a growth factor, migration stimulus, and cell survival, were already known before[47-50]. With respect to liver, HGF is primarily seen as a mitogen for hepatocytes, transiently activated in the early phase of liver regeneration after partial hepatectomy[51-53]. Its proliferation stimulating activity seems to be higher in the periportal zone than in the perivenous zone[54].
HGF is usually synthesized in mesenchymal cells, while its receptor, Met, is expressed in the epithelia in close vicinity[48]. The contribution of HGF to the regulation of zonation was suggested on the basis of cell culture experiments using HGF as an inducer of rat sarcoma (RAS) signalling[55]. The idea that opposing signalling pathways triggered by Ha-ras- and β-catenin-dependent factors might determine zonation of (periportal) gene expression in murine liver was developed by the Schwarz group[55,56] based on comparisons of expression patterns of periportal and pericentral hepatocytes with those of liver tumors carrying activating mutations in either the Ha-ras or the ctnnb1 gene. Definitive prove of this hypothesis as well as of the contribution of HGF in this mechanism is still lacking. Perhaps, RAS signalling is kind of a nodal point integrating various signals that lead to activation or inhibition of ras signalling. Furthermore, the downstream effectors of RAS are also still unclear, although members of the mitogen-activated protein kinase (MAPK) cascade are potent candidates[57].
Concerning the influence on zonation, interactions between RAS signalling and WNT/β-catenin signalling as well as Hedgehog (Hh) signalling are of special interest. Such interactions have been recently reviewed mainly with respect to proliferation, differentiation and tumorigenesis[57-59]. It remains to be demonstrated whether they are relevant, at least partially, also for regulating zonation in adult liver especially as these interactions are highly context-dependent. Furthermore, the relative strength of each pathway activity compared to the others may be a major point distinguishing these interactions in cancer development from those in liver zonation (see discussion in[10]).
Hedgehog signalling
Components of Hh signalling have been found to be expressed at very low levels in hepatocytes[60], whereas much higher levels could be measured in hepatic stellate cells[61,62] and in cholangiocytes[63]. These findings have lead to the widespread opinion that Hedgehog signalling in hepatocytes is not functionally active under normal conditions, a view that has paralyzed research in this field for almost a decade[10]. Only in certain states of liver damage, for instance, in heavily steatotic (ballooned) hepatocytes[64,65] or in response to lipotoxic challenge[66] it was realized that Hh signalling was upregulated resulting in enhanced synthesis of sonic hedgehog (Shh). Another state in which Hh signalling is induced in apparently normal hepatocytes is partial hepatectomy (PH)[67]. After 70% PH, the mRNA level of Indian hedgehog (Ihh) increases up to 48 h and precedes that of Shh which mainly increases between 48 and 72 h. In all cases, increased Hh signalling seems to aid in regeneration and repair acting not only on the hepatocytes but also on other cell types including progenitor cell populations[68,69].
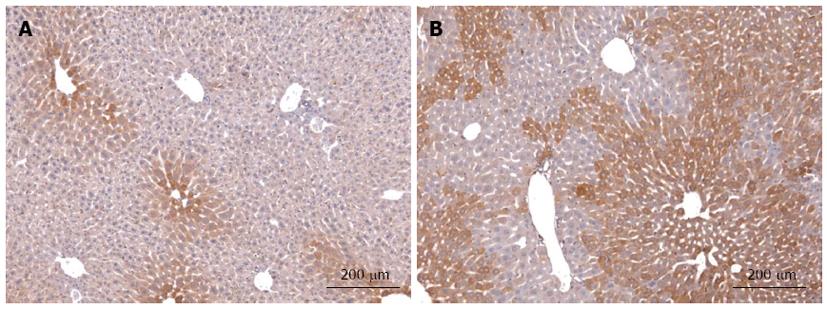
Despite the apparently low activity in hepatocytes we found many hints from literature, mainly derived from other organs that, by analogy, pointed to an involvement of Hh signalling in the regulation of metabolism in healthy mature hepatocytes. We have recently compiled these arguments in a hypothesis strongly suggesting that hepatocellular Hh signalling may contribute to liver zonation in spite of its low activity[10]. Indeed, using transgenic mice with conditional deletion of smoothened (Smo) we were able to demonstrate that even the very low activity of this pathway has a profound influence on mature liver function in particular on the expression of Igf1 in hepatocytes and, consequently, on the level of insulin-like growth factor I (IGF-I) protein in the serum[70]. Actually, these changes are due to the downregulation of the transcription factor GLI3 in response to the deletion of Smo in hepatocytes. Interestingly, the levels of all three GLI transcription factors are so low that their changes can be detected only by sensitive quantitative RT-PCR, but not by ordinary microarray gene expression analyses. Another fact is also very interesting: As we have hypothesized[10], Ihh is the main ligand involved and its expression is downregulated in the Smo-knockout mice[70]. On the other hand, immunohistochemistry showed that Ihh is expressed exclusively in the most distal pericentral area[70] (Figure 1). This is in line with the known fact that Ihh is a target of Wnt/β-catenin signalling[71]. The dependence of Ihh production on the activity of β-catenin signalling is further demonstrated by the extension of its pericentral distribution in transgenic mice with enhanced β-catenin signalling (Figure 1). Taken together, these findings indicate that expression of Ihh by (pericentral) hepatocytes is under the control of both, Hh and Wnt signalling. Though this fits nicely to another feature predicted by our hypothesis, namely, the tight crosstalk between Wnt/β-catenin and Hh signalling pathways[10], the specific contribution of Hh signalling to the regulation of zonation remains still to be established.
Figure 1 Immunohistochemical staining of Indian hedgehog in normal liver and after up-regulation of β-catenin signalling.
Ihh protein was detected in liver sections of wild type (WT) mice (A) and mice with down-regulated expression of APC protein after three month of age (B). Ihh protein in WT mice shows a clear gradient which is highest in hepatocytes surrounding the central veins. After up-regulation of β-catenin signalling the zone of Ihh-positive hepatocytes is considerably extended as are other known pericentral markers (e.g., glutamine synthetase; c.f.[8,42]). Sections were stained with Rabbit anti-mouse Ihh polyclonal antibody (Abcam) followed by the EnVision® + Dual Link System-horse radish peroxidase (DAKO) and counterstained with hemalum (Mayer solution).
Gradient formation
Morphogens usually act through gradients over short distances (less than 10-20 cells) that can be visualized by immunofluorescence in developing tissues[22,23]. In adult liver similar attempts failed, because they are too low and often only the highest activity is detectable[9,10,72]. Thus, the direction of the morphogen gradients (Wnt, central to portal; HGF, portal to central; Hedgehog, still unknown) can only be deduced from downstream effects and/or target activities. Likewise, the origin of these gradients is still unknown as is the possible (local) source of Wnt, HGF, and Hh signals. In the case of Hh signalling, the pericentral localization of Ihh in healthy mature hepatocytes has been reported[70], but it is unclear whether this is cause or consequence of pathway activity. Concerning Wnt factors, many different molecular species were found to be expressed in all liver cell types[73], but their function in determining zonation remains elusive. There are some hints that the crucial Wnt signal is derived from endothelial cells of the central veins in line with very early assumptions of the source of an inducing factor for distribution of GS[9,74], but direct experimental evidence is still lacking. This uncertainty about the morphogen gradients is a considerable drawback, because it not only hampers our deeper understanding of how zonation is controlled, but also our possibilities to explore the dominant role of the morphogens for therapeutic intervention. On the other hand, it may well be that we are asking the wrong question, if we ask for “the crucial source” of the morphogens. Perhaps, there are various sources, but the intensive molecular crosstalk between different morphogen pathways through direct and indirect feedback loops is able to produce stable gradients in pathway activity along the sinusoids. The fact that manipulating just one specific inhibitory component of Wnt signalling, the tumor suppressor gene product APC, is sufficient to eradicate the entire gradient[8], can be interpreted in favour of this assumption. If so, we need to learn more about the crosstalk of morphogen signalling pathways, in particular in the adult stage, in order to understand liver zonation. One recent observation from our laboratory may be key concerning the crosstalk between the Wnt- and the Hedgehog signalling pathways. As mentioned above, we observed that Ihh expression in hepatocytes shows a strong pericentral dominance in normal mice (c.f. Figure 1) that becomes less pronounced upon knockout of Smo[70]. In transgenic mice with a moderate increase in the Wnt signalling expression of Ihh was strongly enhanced and extended towards the portal tract (Figure 1) as does the expression pattern of other pericentral markers in these mice, e.g., glutamine synthetase (GS). Even under these conditions many periportal hepatocytes remain free of any staining indicating that Ihh is not expressed in these cells. Taken together these results demonstrate that the expression of Ihh in normal hepatocytes is positively coupled with both, Hh and Wnt signalling. It should be emphasized that under these conditions, at least in mice younger than 4 mo, hepatocellular production of Ihh is physiological[70] and does not indicate damaged or death hepatocytes as has been inferred from certain disease models[63,64].
CURRENT PICTURE OF METABOLIC ZONATION
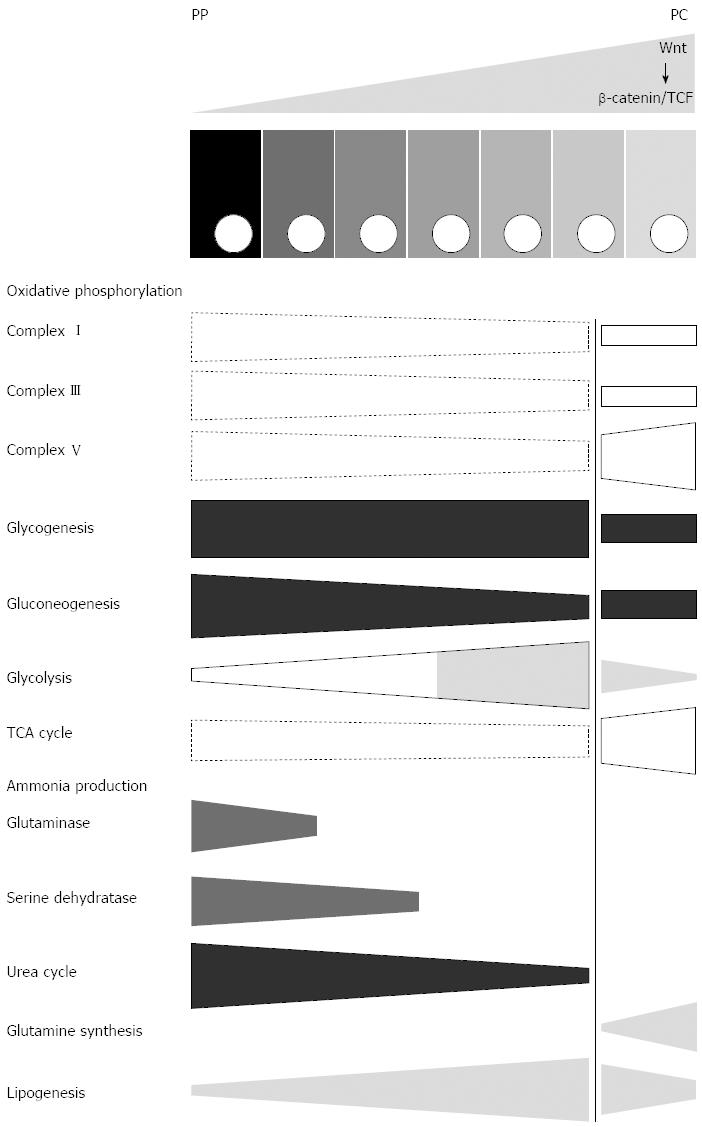
The plethora of metabolic functions of the liver and their complex underlying network of metabolic reactions make it impossible to provide a complete picture of the zonation of all individual pathways and molecular steps that have been investigated so far. Instead, we are aiming at providing a rough overview on novel progress and findings that were recently be achieved. Some information mainly based on a recent proteome study[42] is assembled in Figure 2 in a schematic way. Other important aspects are briefly summarized in the text.
Figure 2 Scheme illustrating aspects of zonation of central metabolism in normal mice.
Selected functions of central metabolism are depicted as conical boxes showing the direction of their porto-central gradients with specific emphasis of the strength of this gradient in the glutamine synthetase-positive zone (right of the vertical line). Dashed boxes indicate gradients that have been determined using a single method only (proteome analysis), instead of at least two independent methods (other boxes). Since the data is valid for normal mice, the presumably corresponding gradient of Wnt/β-catenin signalling is depicted at the top of the scheme, above the row of the hepatocytes. PP: Periportal; PC: Pericentral.
Central metabolism
The picture of zonation of carbohydrate metabolism has not seen much improvement/refinement over the last years, since most phenomena were already comprehensively studied and described in the past[75]. What has changed most, are some regulatory aspects and certain connections between carbohydrate, energy and lipid metabolism. Though the importance of hormonal signals (e.g., insulin and glucagon) or metabolic factors (e.g., oxygen tension) emphasized earlier[7] is beyond doubt, it became apparent that Wnt/β-catenin signalling unexpectedly plays a dominant role also in controlling zonation of many aspects of carbohydrate metabolism[8,42,76].
On the basis of enhanced lactate dehydrogenase protein content it was suggested that up-regulation of Wnt/β-catenin signalling may result in a Warburg-like rewiring of glycolysis[76]. However, this interpretation may be too simple, since a more careful proteomic study clearly showed that except for lactate dehydrogenase all other glycolytic enzymes were strongly down-regulated[42]. Since up-regulation of Wnt/β-catenin in hepatocytes simultaneously results in the up-regulation of GS expression[8], these findings strongly support the conclusion that GS-positive cells (including GS-positive HCC[77] generally run with a lower glycolysis rate than the rest of the hepatocytes, even the GS-negative pericentral ones (Figure 2).
Concerning amino acid metabolism, the picture of zonation that could be drawn in former reviews was still relatively coarse[6,78,79] but meanwhile could be considerably refined, at least for the mouse. On the basis of microarray gene expression studies on periportal and pericentral hepatocyte preparations, especially three genes encoding enzymes of histidine catabolism and of an enzyme of histamine metabolism were found to be preferentially expressed in periportal hepatocytes[80]. Likewise, several genes encoding enzymes of glycine and serine metabolism were also periportally expressed where they might support gluconeogenesis as well as urea synthesis from serine.
Most uncertainties with respect to a possible zonation were formerly reported for certain aspects of lipid metabolism, because of incomplete or controversial findings[6,79]. The reasons for the high incidence of conflicting results in lipid metabolism appear enigmatic, but may be due to relatively shallow gradients of pathway activites, on the one hand, and a greater variability in different physiological states, on the other. The last two decades have seen a considerable improvement of the situation with many novel results which have solved some of the ambiguities. However, we are still far from a satisfactory understanding and several new questions have emerged. In the framework of this review, it is beyond scope to provide a comprehensive overview on all the novel results concerning zonation of lipid metabolism. Recently, an excellent review on this subject was published by Hijmans et al[81], and additional aspects will be reviewed specifically elsewhere (Schleicher et al in preparation). Also in the case of lipid metabolism, the gene expression study[80] of provided novel information by showing the periportal expression of Apolipoprotein CII, of phosphatide phosphatase type 2c and of ATP citrate lyase in the mouse. Preferential localization of apolipoprotein (Apo)E was also found in rats[82], but gender-associated modifications/variations were observed (see below).
Energy metabolism
The heterogeneity of mitochondria of periportal and pericentral hepatocytes was already noted very early based on morphologic features of these organelles[83]. Apart from the description of the heterogeneity of some mitochondrial enzymes such as succinate dehydrogenase[84] very few functional characteristics are known (for work before 1992 see[6]). Importantly, a significantly higher cytosolic ATP/ADP ratio in the periportal compared to the pericentral hepatocytes was shown by Quistorff et al[85]. However, the situation seems rather variable and mitochondrial function, in particular mitochondrial redox state, responds dynamically to alterations of hormones and oxygen supply[86].
Recently, the proteome study performed on mice with conditional knockout of APC revealed interesting differences between hepatocytes with enhanced β-catenin signalling and different wild type mice[42]. Many enzymes of citric acid cycle were upregulated up to 3.8-fold with the exception of succinyl-CoA ligase which was down-regulated 1.5-fold. Proteins of complexes I to III were mostly downregulated between 1.7-fold and 3.6-fold. In contrast, some proteins of complex V were upregulated[42]. These findings seem to hold particularly for cells with (enhanced) expression of GS and suggest a very specific effect of Wnt/β-catenin signalling on the zonation of oxidative phosphorylation (OXPHOS) and energy production (Figure 2). Interestingly, deletion of β-catenin was found to induce inverse changes determined by using functional parameters[87]. Thus, they nicely fit to the proteome data and are in line with the periportalization of the hepatocytes indicated also by other functions in β-catenin-KO mice[40].
Drug metabolism
Zonation of drug metabolism is of considerable importance for liver function, particularly under conditions of liver diseases. Therefore, comprehensive reviews have been published in the past for fetal[88] and adult liver[80,89] as well as with special emphasis on enterohepatic circulation[90]. Here, we would like to focus on recent progress in understanding of the regulation of a large part of enzymes of phase I and phase II biotransformation. The dependence of the pericentral expression of several cytochrome P450 isozymes on the activity of the Wnt/β-catenin signalling was already noticed in 2006 by Hailfinger et al[56] and Sekine et al[40]. Obviously, the influence of β-catenin on cytochrome P450 expression is very complex including interactions with RAS-signalling[91] and signalling through the constitutive androstane receptor (CAR)[92]. Work on these subjects has excellently been reviewed by Braeuning et al[91,93]. Similarly, the zonal expression of certain phase II enzymes such as glutathione-S-transferases is affected by Wnt/β-catenin signalling[94]. It will be a considerable challenge for future work to elucidate the influence of other morphogen pathways on the heterogeneous expression of drug metabolizing enzymes and drug transporters in the liver.
Protein synthesis and autophagy
Recently, a novel Forkhead box O (FOXO)-dependent mechanism for inducing autophagy via up-regulation of glutamine synthetase was discovered[95]. Since a FOXO- and β-catenin-dependent regulation is found in liver only in a narrow zone of the lobules around the central veins[28], this mechanism is confined to about 7% of the hepatocytes indicating an extreme form of zonation. Though it is not known at present how autophagy is regulated in the remaining part of liver parenchyma, we have recently forwarded a hypothesis about a possible alternative mechanism based on characteristics derived from studies of the regulation of autophagy in whole liver[96]. The intriguing idea of this mechanism is that the extent of autophagy in liver might be determined by the extracellular concentration of glutamine (in the periportal and the proximal pericentral zone), while it is determined by the intracellular concentration of glutamine in the distal pericentral zone. Moreover, we have hypothesized that the assumed mechanism for the regulation of autophagy in the periportal zone may be controlled by Hh signalling which has just been found to play a role in regulating zonation[10]. Indeed, there is strong evidence from other organs for a close crosstalk of the mammalian target of rapamycin (mTOR) and hedgehog pathways[97,98] that may be relevant also for the liver. Given the novel influence of liver hedgehog activity on the IGF-I regulatory network[70] and the known interrelationship between IGF signalling, FOXO activity and longevity[99,100], the question arises whether the extent of liver autophagy in total is harmonized through such a circuit with body size and the life-span of the organism.
Besides these hints on zonation of autophagy, there is preliminary information from new proteome data in the Smo-KO mice[70] suggesting a prominent zonation of total protein biosynthesis (Gebhardt R, Matz-Soja M, and Shevchenco, unpublished observation). These findings generalize the long known fact that the synthesis of the main plasma protein, albumin, is zonated with a periportal predominance[6].
This dual scenario, zonation of both, autophagy and protein biosynthesis, leads to a number of important questions: (1) what happens in the liver after a long-lasting fasting period? How is the extraorbitant loss of liver protein reported under such conditions by the Lamers group[101] distributed among the different liver zones? (2) is the dominance of urea cycle, tricarboxylic-acid cycle, oxidative phosphorylation and, later on, of gluconeogenesis after fasting not only a results of the switch from insulin to glucagon signalling, but also the result of loss of bulk protein in pericentral hepatocytes? or (3) which other (pericentral) liver functions (e.g., heme or bile acid synthesis) may be affected by such changes and what is their impact on the entire organism? So far, it is obvious that our knowledge base for answering these questions with the necessary precision is not sufficient yet.
Antioxidative mechanisms
Mitochondria as well as peroxisomes are major sources of reactive oxygen species (ROS)[102] far exceeding that produced by the cytochrome P450 system of biotransformation. Protection of these organelles from damaging effects of ROS, especially of superoxide, is provided by superoxide dismutase 2 which generates H2O2, a potent oxidant. The removal of H2O2 is performed by the antioxidant enzymes catalase, GSH peroxidase 1 and peroxiredoxin-III[103]. Zonation of ROS production and removal is determined by the zonal differences in mitochondrial structure and function, the pericentral predominance of peroxisomes, and zonal differences in GSH production and content (for review[6]).
The role of MnSOD in zonal gene expression is exemplified in the case of GS expression in MnSOD-KO mice, where the ordinary pericentral localization of this enzyme is lost and replaced by a scattered expression within the parenchyma[104].
Importantly in this context, the β-catenin pathway has been suggested to act as a switch between various transcription programs sensitive to hypoxia and oxidative stress[105]. Since most of these effects are mediated via hypoxia-inducing factor (HIF) and FOXO transcription factors, these interactions could affect the influence of the oxygen gradient and other stress factors across the porto-central axis on various zonated metabolic pathways described to be oxygen-dependent[73]. The fact that H2O2 is considered a key regulatory component in this concept appears in a new light given the possible zonation of the antioxidative mechanisms via peroxiredoxin which were not yet known at that time. The zonation of peroxiredoxins, thioredoxins, and glutaredoxins has not yet been studied, but it can be assumed that they predominate in the pericentral zone due to their association with both, mitochondria and peroxisomes. These mechanisms may be of critical importance in NAFLD, particularly in the development of NASH[106,107] and, because of their presumable zonation, may influence the precipitation of the first signs of this disease in the pericentral zone.
Specific functions
An entire synthetic pathway the zonation of which was described only recently is represented by heme biosynthesis[55,108]. An interesting characteristic of this pathway is that although it is present in all hepatocytes with a definitive pericentral dominance, the distribution of the individual enzyme proteins is not directly comparable. While it can be very broad for one enzyme (e.g., δ-aminolevulinate synthase), it may be much more restricted to the pericentral zone for another one (e.g., coproporphyrinogen oxidase). These variations suggest that this pathway is not simply a linear sequence of reactions taking place in each cell (although with different speed), but rather may frequently be diverted for exchanging certain metabolites with other organs in the body. This may result in various feedback mechanisms ensuring the proper adaptation of heme synthesis to whole body requirements. This exchange may be controlled by Wnt/β-catenin signalling and ras signalling, since both pathways reciprocally influence heme biosynthesis[108].
In a recent study focussing on sexual dimorphism in the heterogeneity of gene expression in mouse liver several new zonated functions for either males or females were described by using LCM technology[11]. Among certain canonical pathways known to be predominantly localized to the pericentral zone, retinoic acid biosynthesis was newly described[11]. Most probably, however, this function which is related to vitamin A storage and metabolism must be assigned to the heterogeneity of hepatic stellate cells[109] rather than to that of hepatocytes.
FUNCTIONAL SIGNIFICANCE OF ZONATION
About 50 years ago when more and more examples of heterogeneous distributions of enzymes in liver parenchyma were discovered this phenomenon was still considered a curiosity by most biochemists and physiologists. Soon, however, it became apparent that such examples were not exemptions but the rule and are characteristic for many metabolic pathways. This was the time when the term “metabolic zonation” was coined by Jungermann and Sasse[4]. In this concept mainly based on findings concerning carbohydrate metabolism the spatial separation of anabolic and catabolic reactions was central, and the avoidance of futile cycles of ATP hydrolysis between key gluconeogenic and glycolytic reactions provided an easy explanation for this concept/separation. Another reason was that the simultaneous performance of opposite metabolic pathways in an organ might aid in quickly adapting to different metabolic needs by simply switching from one function to the opposite one without time- and energy-consuming biosynthesis of respective enzymes (for review see[6]). Although metabolic zonation was realized to play a certain role in maintenance of body homeostasis, this connection received less attention.
In the light of the recent discoveries mentioned above, two functional characteristics seem most important for understanding the power of metabolic zonation: (1) maintenance of metabolic homeostasis; and (2) optimization of liver function. Appropriate maintenance of metabolic homeostasis in the body probably implicates that every adaptation to exogenous nutritional challenge or any specific endogenous demand is reflected by a corresponding alteration of liver zonation, irrespective of whether it is almost negligible or very intensive. Of course, profound changes are immediately obvious and may be brought about by hormones and other signals required for regulating daily metabolism. The corresponding metabolic pathways appear “dynamic”.
In contrast, the minor ones are probably due to slight changes in the signalling activity of the morphogen signalling network. The corresponding pathways appear “static” and need much longer time until visible changes can be detected. However, their consequences in the long run may be likewise dramatic, since under these conditions body homeostasis may gradually get lost. It is tempting to speculate that certain changes in liver zonation exerted by altered activities of one or more morphogen pathway(s) play a causative role in metabolic diseases. Examples for such a connection may be found in the association of certain polymorphisms in Wnt signalling with type 2 diabetes[110-112].
The fundamental role of the optimization principle became obvious during a study on model-based optimization[113] within the framework of the “Virtual Liver” project (http://www.virtual-liver.de). The aims of this study were (1) to extend an existing 2-compartment model of ammonia metabolism (2-CM; representing the periportal and pericentral zones) to a 16-compartent model (16-CM; where each compartment represents an individual hepatocyte in a mouse liver lobule); and (2) to determine optimal enzyme activity distributions alongside the 16 compartments using a non-linear programming algorithm. The optimization problem is formulated based on biologically motivated enzyme constraints (limited capacity for protein synthesis) and objective functions that represent different physiological strategies of the liver.
Using this approach, several characteristics of zonation of ammonia metabolism could be predicted that were in surprising accord with experimental findings[113]. These include a small periportal compartment for glutamine breakdown, a larger “periportal” down-stream compartment dedicated to ureogenesis without glutaminase support, and a very small pericentral compartment dedicated exclusively to glutamine synthesis. Evidence for the existence of a “periportal” down-stream region with a glutaminase-independent ureogenesis was provided by Comar et al[114] using a sophisticated liver perfusion system with cannulation of both, the portal vein and the hepatic artery.
By generalizing these findings, zonation appears as a perfect means for optimizing the metabolic capacity of the liver. The regulatory systems determining zonated expression can be viewed as mere parts of sophisticated mechanisms transforming complex metabolic cues into the proper zonation. Future research using systems biology approaches should aim at identifying further examples of the optimization principle.
EMERGING SUBJECTS AND MISSING RESEARCH AREAS
As pointed out above, the fundamental role of zonation in the control of body homeostasis at the metabolic level which is emerging from novel regulatory mechanisms dominated by morphogen pathways is still far from being understood. However, it may bear a considerable potential for explaining metabolic adaptation to different nutritional states and requirements as well as the development of metabolic diseases. Adaptive processes may profit from the fact that metabolic zonation is associated with different gradients of transcription factors along the porto-central axis. Since combinatorial interactions among transcription factors are critical for directing tissue-, cell-type- and cell position-specific gene expression[115,116], independent regulation of the transcription factors by several signalling pathways creates the possibility to shift the expression of individual enzymes or transporters as well as that of whole pathways or of subgroups of them. How such combinatorial interactions may contribute to liver zonation was illustrated in a recent review for β-catenin and several other TFs co-regulating pericentral GS expression[28]. Further interactions with β-catenin were described for the androgen receptor[117], many members of the steroid receptors[118], and for several other nuclear receptors such as hepatocyte nuclear factor 4 (HNF4)[119]. Even though HNF4 has been shown to be almost homogeneously expressed throughout the liver lobules[120], the large diversity of HNF4 variants at the protein level (Gaunitz, Gebhardt, unpublished observation) calls for careful interpretation of these results. Interestingly, conditional knockout of HNF4 in liver did not affect pericentrally expressed genes, but resulted in the additional expression of some of these genes in the periportal zone, albeit at a lower level[121].
Such shifts could result in the dynamic overlap of opposing metabolic pathways, e.g., of gluconeogenesis and glycolysis, resulting in more or less regulatory influence of metabolic intermediates and other metabolic (feedback) regulators on the activity of these pathways. But the contrary could also take place, as suggested by the almost static expression of GS: despite varying or shifting profiles of individual transcription factors, the invariability of one of them, here of β-catenin, may guarantee the exclusive expression of GS in some few hepatocytes around the central vein[9,28]. Under certain, possibly rare conditions, however, a third scenario may occur. The combinatorial game of transcription factors in zonation could lead to aberrant events of gene expression resulting in either gain of functions that are not characteristic of mature liver (e.g., selective gain of embryonic features) or loss of definitively liver-specific functions (e.g., partial dedifferentiation). All these events could contribute to the development of metabolic diseases.
The reflections outlined above illustrate how important it will be to profoundly understand the regulatory network underlying metabolic zonation. In this context it needs to be pointed out that research on zonation with respect to two important features of metabolism, namely circadian rhythm and gender dimorphism is largely missing.
It is known for a long time that liver metabolism underlies profound rhythmic changes, daily and seasonally[122-125]. Because of lack of research in this field, these changes are not yet adequately mirrored at the level of metabolic zonation. Only occasionally respective data was reported. Long ago, for instance, daily alterations in glycogen metabolism were investigated[126] and a remarkable rhythmicity in time and zonal localization was found. Recently, we have observed that liver glutaminase protein shows a strong circadian rhythm being highest in the morning and lowest at night (Gebhardt, Kalkhoff, von Bergen, unpublished observation). This finding points to corresponding changes in the periportal expression zone of this enzyme and challenges the currently prevailing view that urea cycle activity is continuously fuelled (and mainly driven) by the prior breakdown of glutamine entering the liver via portal vein and hepatic artery[127]. Perhaps, there are periods where such fuelling is neither necessary, nor helpful for ensuring a balanced nitrogen metabolism.
Likewise, gender dimorphism of liver metabolism is well studied globally[124,128-131], but not sufficiently comprehensive on the level of lobular heterogeneity. Some examples should be mentioned. It has long been discovered that the very small pericentral zone where glutamine synthetase is expressed is larger in males than in females of many mouse strains[132]. However, whether and how this difference impacts on amino acid and ammonia metabolism is completely unknown. Furthermore, the difference in the size of the expression zone of this enzyme seems to hint - according to the current molecular explanation for the limited range of the expression of this gene - at a higher pericentral activity of Wnt/β-catenin signalling in males than in females. Although it was frequently emphasized that this may be explained by the molecular interaction between the androgen receptor and β-catenin, final prove of this obvious guess is still missing. Similar phenomena with sex differences were occasionally reported such as zonation of ApoE synthesis[82] or gender-specific interplay through Wnt/β-catenin signalling and constitutive androgen receptor in drug metabolism[92], but remained isolated. In case of Apo E synthesis, a clear periportal to pericentral gradient of mRNA levels was observed in male rats, whereas a bowl-like distribution with lowest expression in the midzonal area was found in female rats by in situ hybridization[82]. A considerable step forward was achieved by Saito et al[11], who used LCM combined with micro-arrays in order to compare gender effects on zonal gene expression. Interestingly, they found that sexual dimorphism seems more pronounced in zone 1 than in zone 3.
CONCLUSION
Applications of “omics” technologies, novel approaches for refined spatial localization, and the use of transgenic mice with liver-specific knockout of different morphogens have strongly improved our understanding of liver zonation. In particular, the emerging network of several morphogens that work as master regulators of zonation has provided new insight into the basic mechanisms that govern zonation. Instead of Wnt/β-catenin signalling acting alone, it is now certain that this pathway acts in close cooperation with Hedgehog and RAS signalling. Of course, many important questions remain unanswered so far and, because of the complexity of the crosstalk, a clear picture will require analysis by a thorough systems biology approach based on space- and time-dependent data. These should incorporate data on alterations of zonation during the daily rhythm of metabolism and should also include gender dimorphism.
However, even with the first rough idea of this emerging regulatory network, it can be concluded that disturbance of liver zonation will considerably impact on body homeostasis, particularly in diseased states. How far the causative role of altered liver zonation in the development of metabolic diseases may reach is hard to predict on the basis of current fragmentary knowledge, but it certainly will be worth studying.
P- Reviewers: Kim JS, Takahashi T, Takahashi JG, Zhang XL S- Editor: Qi Y L- Editor: A E- Editor: Liu XM