Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8320
Revised: February 9, 2014
Accepted: April 8, 2014
Published online: July 7, 2014
Processing time: 194 Days and 17.2 Hours
Lymphangioma is an uncommon benign tumor that develops in the lymphatic system. Abdominal lymphangiomatosis is extremely rare in adult patients, and the clinical symptoms of this condition are complicated and atypical. We report a case of abdominal lymphangiomatosis in a 38-year-old female who presented with intestinal bleeding and protein-losing enteropathy, as well as lesions in the lung and bones. A computed tomography scan revealed multiple small cystic lesions without enhancement. Histological examination revealed microscopic cysts were submucosal, with walls composed of thin fibrous tissue, and D2-40 stained highlight the lining of the lymphatic channels by immunohistochemical method. We make a comparison with the cases reported before, and also discuss the diagnose of diffuse pulmonary lymphangiomatosis and Gorham’s disease.
Core tip: Abdominal lymphangiomatosis is extremely rare in adult patients, and the clinical symptoms of this condition are complicated and atypical. We report a case of abdominal lymphangiomatosis in a 38-year-old female who presented with intestinal bleeding and protein-losing enteropathy, as well as lesions in the lung and bones. From this case and releated the literature review, we can achieve a better understanding of this disease.
- Citation: Lin RY, Zou H, Chen TZ, Wu W, Wang JH, Chen XL, Han QX. Abdominal lymphangiomatosis in a 38-year-old female: Case report and literature review. World J Gastroenterol 2014; 20(25): 8320-8324
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8320.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8320
Lymphangioma is a rare benign tumor that develops in the lymphatic system. Lymphangiomas occasionally occur diffusely, and this process is referred to as “generalized lymphangiomatosis”[1]. This disease usually occurs in children and involves the skin. Mesentery lymphangiomatosis is extremely rare in adults. We reported one case of abdominal lymphangiomatosis with lung and bone lesions in a 38-year-old female, which had not previously been reported.
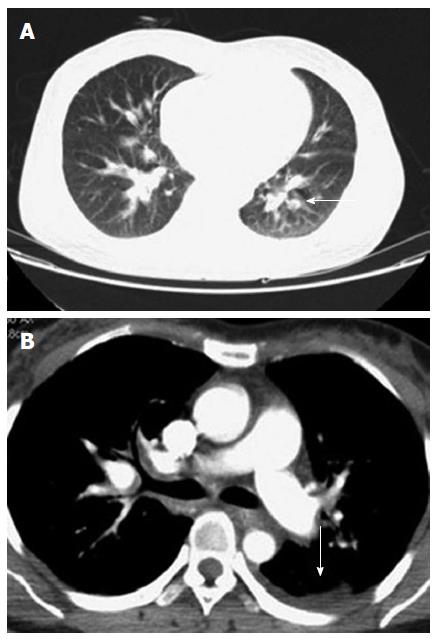
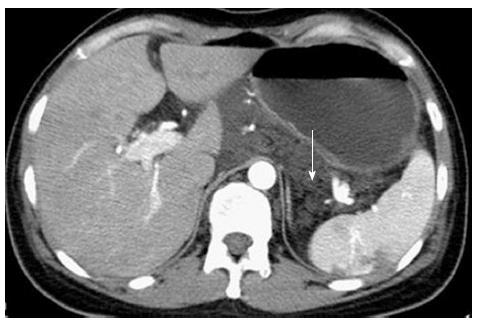
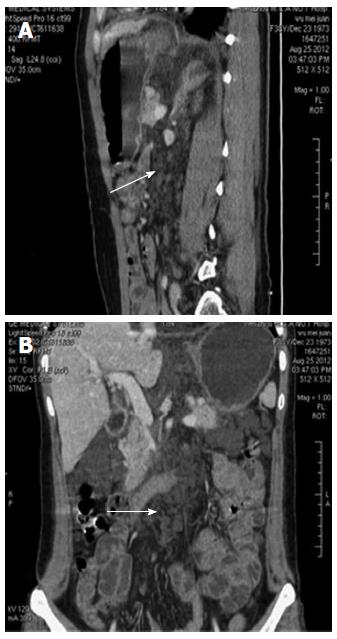
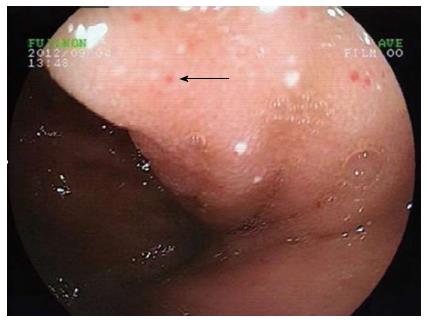
The patient was a 38-year-old female who had complained of melena for 3 mo and weakness for 10 d. In addition, she had a history of 4 mo of hemoptysis, and she had received antibiotic therapy at a local hospital for pneumonia. She had no history of trauma, no history of surgery, and no other meaningful history. Upon physical examination, she presented an anemic appearance but no other positive signs. Laboratory values upon admission indicated microcytic hypochromic anemia, with hemoglobin value of 74 g/L and albumin value of 20.9 g/L. The tumor markers (CEA, CA-125, and CA-199) were within normal limits. A laboratory examination for tuberculosis (T-SPOT TB test) was negative. Other blood laboratory tests were normal. Chest computed tomography (CT) enhancement scan revealed a low-density lesion along the pulmonary vessels and a small amount of effusion in the left pleural cavity (Figure 1). There were local high density regions on both sides of the clavicle and the sternum and nodular increased densities in the head of the right humerus. The right scapula had a local high-density appearance. Abdominal CT revealed multiple small cystic lesions without enhancement distributed in the fundus of the stomach, the peripancreatic area, the mesenteric area, the retroperitoneal space and the spleen. The biggest lesion reached 62 mm (Figures 2 and 3). Abdominal ultrasonography revealed multiple cystic dark areas in the abdomen cavity that featured communicative branches, and a slow blood flow signal was detected. We punctured the abdominal cystic mass using B ultrasonic positioning, and the cytology of the puncture fluid revealed the following characteristics: scattered with lymphocytes of mainly inflammatory cells, no tumor cells, and a negative qualitative analysis for chyle. The pathology of the humerus nodules after puncture indicated coagulation necrotic bone tissue. Gastroscopy and double-balloon enteroscopy revealed chronic non-atrophic gastritis and duodenal lymphangiectasia with hemorrhage (Figure 4). The patient was cured by decreasing gastric acid, along with hemostatic and fluid infusion initially, but she continued to present melena. She then underwent an exploratory laparotomy, which revealed multiple cysts in the right upper quadrant of the greater omentum and retroperitoneal space. The cysts had a thin wall and contained clear cyst fluid, with tortuous, dilated sponge-like blood vessels next to the cysts. Consequently, the surgeon used cavity mirrors to perform surgically assisted distal gastric resection and bi II-type anastomosis. Pathology after the surgery indicated abdominal lymphangioma (Figures 5, 6 and 7). However, she continued to present melena iron-deficiency anemia and hypoproteinemia after the surgery.
Lymphangiomas consist of endothelium-lined spaces, which are supported by connective tissue stromata of variable thickness that contain lymphoid tissue, round cells, and smooth muscle[1]. A total of 90% of lymphangiomas occur in children under 2 years of age and involve the head and neck. These lesions are rarely observed in adult patients[2]. These benign malformations are classified into four categories[3]: capillary lymphangioma, cavernous lymphangioma, cystic lymphangioma (hygroma) and hemolymphangioma (a combination of hemangioma and lymphangioma). Lymphangiomatosis is one subtype of lymphatic disease, which is a much rarer condition. It can be limited to a particular organ or structure (e.g., spleen, liver, or thoracic cavity) or involve a more generalized process[4].
A PubMed search with the words “lymphangiomatosis” found a total of articles of 359 articles. There are about 56 articles on adult patients with abdominal lymphangiomatosis in English. The most frequent symptom is abdominal pain. A subset of the patients are asymptomatic. Only 3 articles[5-7] described adult patients of lymphangiomatosis with gastrointestinal bleeding and hypoproteinemia. Compared to these cases, this patient presented concomitant lung and bone lesions, which will be discussed in the following section. Some abdominal lymphangiomatosis are accompanied by other lymphatic abnormalities such as lymphangiectasia. Protein-losing enteropathy is the complication of a variety of intestinal disorders characterized by an excessive loss of proteins into the gastrointestinal tract due to impaired integrity of the mucosa[8]. Intestinal lymphangiectasia is one of the reasons of protein-losing enteropathy. We administered a small intestinal fiberscopy, which clearly revealed the pathological changes of the intestine that could explained why this patient had hypoproteinemia. Aggressive surgery should be avoided in these symptomless cases because it is now known that these lesions are benign[9]. Kochman et al[10] suggested that asymptomatic lymphangiomas should most likely be left alone. However, when they cause significant mortality because of their large size, their critical locations and the possibility of becoming secondarily infected, almost all lesions require surgical treatment. Incomplete removal can lead to recurrence, even after many years[11]. It is difficult to perform a complete resection when multiple lesions are found, as our patient continued to present melena and hypoproteinemia. Other treatments reported include endoscopic snare polypectomy, interferon-alpha, radiation therapy, propranolol, bevacizumab, sirolimus, percutaneous drainage and sclerotherapy, among others[4,12-19]. The initiation of a low-fat diet in this patient was able to effectively raise the hemoglobin level and the total serum protein level[7].
The clinical symptoms of lymphangiomatosis are complicated and atypical. A plain radiograph may indicate a diagnosis of these diseases. CT and nuclear magnetic resonance imaging are the current techniques used to evaluate lymphangiomatosis, which appear as multicystic fluid-filled masses. They are particularly important because the multiorgan involvement suggests a diagnosis of generalized lymphangiomatosis[1]. The lesions showed as sharply defined, non-enhanced cystic lesion. In the skeletal system, multiple geographic osteolytic lesions with predominantly sclerotic borders were observed[20]. Bipedal lymphangiography with post-lymphangiographic computed tomography may confirm the diagnosis by revealing a bony accumulation of contrast medium[21]. Richard reported a case of retroperitoneal cystic abdominal lymphangiomatosis diagnosed using fine-needle aspiration[22].
Lymphangiomatosis have been observed in all organs such as kidney, lung, liver, but except the brain, which is devoid of lymphatic channels. Diffuse pulmonary lymphangiomatosis (DPL) is one type of lymphangiomatosis. Two of the most universal characteristics can be observed in the CT scan of a DPL patient: diffuse fluid infiltration of the mediastinal soft tissue and pleural effusions[23]. High resolution CT can reveal the other characteristic features of DPL: bilateral diffuse peribronchovascular thickening, interlobular septal thickening and ground-glass opacities[24]. The chest CT of our patient was in concordance with the disease performance. According to monism, her pulmonary pathological change was caused by systemic lymphangiomatosis. However, after active mobilization, she continued to refuse lung pathological biopsy and lymphangiography. So the results are insufficient to make a diagnosis in the lungs. Patients with bone involvement in lymphangiomatosis may be given an additional diagnosis of Gorham’s disease (GD). The progressive and massive destruction of bone without signs of repair are distinctive, although not specific, to GD. Progressive destruction of the bone ensues, featuring dissolution, fragmentation, and sometimes fracture[25]. The pathological osteochondral lesions of this patient are the common diseased regions of GD, but their imaging appearances are not as the literature reported. Consequently, a diagnosis of GD cannot be given.
Lymphangiomatosis is a rare disease that usually has no specific clinical performance, so the doctor can easily succumb to misdiagnosis and missed diagnosis. Making a definitive diagnosis requires a complete medical history, careful physical examination and thorough assistant examination; sometimes, even an invasive method is necessary. Clinical doctors must improve their understanding of this disease.
The authors thank Rong-Rong Wang for proofreading the pathological materials.
The patient had complained of melena for 3 mo and weakness for 10 d.
This case was diagnosed with lymphangiomatosis.
The abdominal lympangiomatosis could be diagnosed by pathological method, but only according to the image data, the authors couldn’t make a definite diagnose to the lung and bone lesion.
Laboratory values upon admission indicated microcytic hypochromic anemia, with hemoglobin value of 74 g/L and albumin value of 20.9 g/L.
Abdominal computed tomography revealed multiple small cystic lesions without enhancement distributed in the fundus of the stomach, the peripancreatic area, the mesenteric area, the retroperitoneal space and the spleen.
Histology of the omentum majus showed microscopic cysts were submucosal, with walls composed of thin fibrous tissue, and D2-40 stained highlight the lining of the lymphatic channels by immunohistochemical method.
Distal gastric resection and bi II-type anastomosis were performed in this case.
Only 4 articles describing adult patients of lymphangiomatosis with gastrointestinal bleeding including our case have been reported in the English literature.
Diffuse pulmonary lymphangiomatosis is a rare disease characterized by infiltration of the lung, pleura and mediastinum with thin-walled lymphangiomas. Gorham’s disease is a rare condition characterized by progressive osteolysis of bone.
Lymphangiomatosis is a rare disease that usually has no specific clinical performance, improving the understanding of this disease can avoid misdiagnosis and missed diagnosis.
This paper reported a very unusual and interesting case of abdominal lymphangiomatosis. A thorough revision of the literature and a good description of the clinical case were made. A short comment about protein losing enteropathy is necessary in the text. Also, it is advisable to mention that lymphngiomatosis can also be observed in the kidneys.
P- Reviewer: Vega J S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Marom EM, Moran CA, Munden RF. Generalized lymphangiomatosis. AJR Am J Roentgenol. 2004;182:1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Fang YF, Qiu LF, Du Y, Jiang ZN, Gao M. Small intestinal hemolymphangioma with bleeding: a case report. World J Gastroenterol. 2012;18:2145-2146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Kosmidis I, Vlachou M, Koutroufinis A, Filiopoulos K. Hemolymphangioma of the lower extremities in children: two case reports. J Orthop Surg Res. 2010;5:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Blei F. Lymphangiomatosis: clinical overview. Lymphat Res Biol. 2011;9:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Amadori G, Micciolo R, Poletti A. A case of intra-abdominal multiple lymphangiomas in an adult in whom the immunological evaluation supported the diagnosis. Eur J Gastroenterol Hepatol. 1999;11:347-351. [PubMed] |
| 6. | Iwabuchi A, Otaka M, Okuyama A, Jin M, Otani S, Itoh S, Sasahara H, Odashima M, Kotanagi H, Satoh M. Disseminated intra-abdominal cystic lymphangiomatosis with severe intestinal bleeding. A case report. J Clin Gastroenterol. 1997;25:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Takami A, Nakao S, Sugimori N, Ishida F, Yamazaki M, Nakatsumi Y, Saito M, Otake S, Nakamura S, Matsuda T. Management of disseminated intra-abdominal lymphangiomatosis with protein-losing enteropathy and intestinal bleeding. South Med J. 1995;88:1156-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (32)] |
| 9. | Jung SW, Cha JM, Lee JI, Joo KR, Choe JW, Shin HP, Kim KY. A case report with lymphangiomatosis of the colon. J Korean Med Sci. 2010;25:155-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kochman ML, Wiersema MJ, Hawes RH, Canal D, Wiersema L. Preoperative diagnosis of cystic lymphangioma of the colon by endoscopic ultrasound. Gastrointest Endosc. 1997;45:204-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Rieker RJ, Quentmeier A, Weiss C, Kretzschmar U, Amann K, Mechtersheimer G, Bläker H, Herwart OF. Cystic lymphangioma of the small-bowel mesentery: case report and a review of the literature. Pathol Oncol Res. 2000;6:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chung WC, Kim HK, Yoo JY, Lee JR, Lee KM, Paik CN, Jang UI, Yang JM. Colonic lymphangiomatosis associated with anemia. World J Gastroenterol. 2008;14:5760-5762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Rostom AY. Treatment of thoracic lymphangiomatosis. Arch Dis Child. 2000;83:138-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Grunewald TG, Damke L, Maschan M, Petrova U, Surianinova O, Esipenko A, Konovalov D, Behrends U, Schiessl J, Wörtler K. First report of effective and feasible treatment of multifocal lymphangiomatosis (Gorham-Stout) with bevacizumab in a child. Ann Oncol. 2010;21:1733-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Aman J, Thunnissen E, Paul MA, van Nieuw Amerongen GP, Vonk-Noordegraaf A. Successful treatment of diffuse pulmonary lymphangiomatosis with bevacizumab. Ann Intern Med. 2012;156:839-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Valerio M, Meuwly JY, Tawadros C, Jichlinski P. Percutaneous drainage and sclerotherapy as definitive treatment of renal lymphangiomatosis. Can Urol Assoc J. 2012;6:E3-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Güvenç BH, Ekingen G, Tuzlaci A, Senel U. Diffuse neonatal abdominal lymphangiomatosis: management by limited surgical excision and sclerotherapy. Pediatr Surg Int. 2005;21:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ozeki M, Fukao T, Kondo N. Propranolol for intractable diffuse lymphangiomatosis. N Engl J Med. 2011;364:1380-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Gordon KD, Mortimer PS. Progressive lymphangiomatosis and Gorham’s disease: case report and clinical implications. Lymphat Res Biol. 2011;9:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Yang DH, Goo HW. Generalized lymphangiomatosis: radiologic findings in three pediatric patients. Korean J Radiol. 2006;7:287-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Griffin GK, Tatu WF, Fisher LM, Keats TE, Tegtmeyer CJ, Fechner RE. Systemic lymphangiomatosis: a combined diagnostic approach of lymphangiography and computed tomography. J Comput Tomogr. 1986;10:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Siderits R, Ouattara O, Abud A, Moubarak I, Mcintosh N, Godyn J. Retroperitoneal cystic abdominal lymphangiomatosis diagnosed by fine needle aspiration: a case report. Acta Cytol. 2009;53:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Satria MN, Pacheco-Rodriguez G, Moss J. Pulmonary lymphangiomatosis. Lymphat Res Biol. 2011;9:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | DU MH, Ye RJ, Sun KK, Li JF, Shen DH, Wang J, Gao ZC. Diffuse pulmonary lymphangiomatosis: a case report with literature review. Chin Med J (Engl). 2011;124:797-800. [PubMed] |
| 25. | Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |