Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8244
Revised: February 10, 2014
Accepted: April 8, 2014
Published online: July 7, 2014
Processing time: 210 Days and 5.3 Hours
AIM: To elucidate the potential impact of the grade of complications on long-term survival of gastric cancer patients after curative surgery.
METHODS: A total of 751 gastric cancer patients who underwent curative gastrectomy between January 2002 and December 2006 in our center were enrolled in this study. Patients were divided into four groups: no complications, Grade I, Grade II and Grade III complications, according to the following classification systems: T92 (Toronto 1992 or Clavien), Accordion Classification, and Revised Accordion Classification. Clinicopathological features were compared among the four groups and potential prognostic factors were analyzed. The Log-rank test was used to assess statistical differences between the groups. Independent prognostic factors were identified using the Cox proportional hazards regression model. Stratified analysis was used to investigate the impact of complications of each grade on survival.
RESULTS: Significant differences were found among the four groups in age, sex, other diseases (including hypertension, diabetes and chronic obstructive pulmonary disease), body mass index (BMI), intraoperative blood loss, tumor location, extranodal metastasis, lymph node metastasis, tumor-node-metastasis (TNM) stage, and chemotherapy. Overall survival (OS) was significantly influenced by the complication grade. The 5-year OS rates were 43.0%, 42.5%, 25.5% and 9.6% for no complications, and Grade I, Grade II and Grade III complications, respectively (P < 0.001). Age, tumor size, intraoperative blood loss, lymph node metastasis, TNM stage and complication grade were independent prognostic factors in multivariate analysis. With stratified analysis, lymph node metastasis, tumor size, and intraoperative blood loss were independent prognostic factors for Grade I complications (P < 0.001, P = 0.031, P = 0.030). Age and lymph node metastasis were found to be independent prognostic factors for OS of gastric cancer patients with Grade II complications (P = 0.034, P = 0.001). Intraoperative blood loss, TNM stage, and chemotherapy were independent prognostic factors for OS of gastric cancer patients with Grade III complications (P = 0.003, P = 0.005, P < 0.001). There were significant differences among patients with Grade I, Grade II and Grade III complications in TNM stage II and III cancer (P < 0.001, P = 0.001).
CONCLUSION: Complication grade may be an independent prognostic factor for gastric cancer following curative resection. Treatment of complications can improve the long-term outcome of gastric cancer patients.
Core tip: Only a few studies have determined the potential impact of surgical complications, especially the grade of complications, on long-term survival of patients with gastric cancer. We found that complication grade might be an independent prognostic factor for patients with gastric cancer after gastrectomy. It can be used to stratify the risk for gastric cancer prognosis. Meticulous surgery is needed and new methods should be considered to decrease the amount of intraoperative blood loss.
- Citation: Jiang N, Deng JY, Ding XW, Zhang L, Liu HG, Liang YX, Liang H. Effect of complication grade on survival following curative gastrectomy for carcinoma. World J Gastroenterol 2014; 20(25): 8244-8252
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8244
Gastric cancer is the second most common cause of cancer-related death worldwide[1]. D2 lymphadenectomy has become the standard treatment for curable gastric cancer, however, it always brings a simultaneous increase in surgical complications[2]. Western countries have published complication rates ranging from 35% to 46%, and mortality rates from 4% to 16% after D2 lymph node dissection[3]. Many studies have indicated that, in gastric cancer, the presence or absence of complications is an important factor that could influence the prognosis of patients following curative gastrectomy[4]. Accurate grading of complications is essential to analyze surgical outcomes, but methods for classification of complications are not uniform and the traditional classification is too complicated. To date, few studies have determined the potential impact of surgical complications, especially the complication grade, on long-term survival of patients with gastric cancer. Hence, the aim of this study was to reclassify the complications and investigate whether the grade of complications in patients undergoing curative gastrectomy could provide a new survival prognostic factor.
A total of 1750 patients with gastric cancer underwent surgery at Tianjin Medical University Cancer Institute and Hospital between January 2002 and December 2006 and were entered into a prospectively maintained database. Eligibility criteria for inclusion in this study were as follows: (1) gastric adenocarcinoma identified by histopathological examination; (2) histologically confirmed R0 resection; (3) availability of complete follow-up data; (4) radical resection and D2 lymphadenectomy performed; and (5) patients with ≥ 15 lymph nodes retrieved. The exclusion criteria were: (1) patients who underwent palliative surgery; and (2) patients who had distant metastasis or peritoneal dissemination that was confirmed during the operation. Based on these criteria, 999 patients out of 1750 were excluded from this study. Among those excluded, 315 had < 15 lymph nodes harvested for pathological examination, 210 had undergone palliative gastrectomy, 237 had D0 and D1 lymph node resection, 27 had died within 1 mo after surgery, 70 had distant metastasis before gastrectomy, 30 had peritoneal dissemination before gastrectomy, and 110 were lost to follow-up. Ultimately, 751 patients were included in the analysis.
Three hundred and five patients had postoperative complications. Complications were defined as any deviation from the normal postoperative course. There were 214 men and 91 women aged 23-82 years (mean: 62.5 ± 11.8 years).
Complications were classified according to the following: T92, Accordion Classification, and Revised Accordion Classification[5-7]. The 751 patients were divided into four groups: no complications; Grade I (required only minor invasive procedures that could be done at the bedside); Grade II (required treatment with drugs other than those allowed for minor complications; no general anesthesia: required management by an endoscopic, interventional procedure or reoperation without general anesthesia); and Grade III (general anesthesia or single-organ failure; general anesthesia and single-organ failure or multisystem organ failure).
D2 lymphadenectomy was performed according to the guidelines for lymph node stations[8]. The choice of surgical procedure for reconstruction was made by the surgeon. Resection margin was pathologically confirmed as negative. Postoperative adjuvant chemotherapy and neoadjuvant chemotherapy were implemented according to the tumor stage, physical condition, and willingness of the patient. Chemotherapeutics consisted of 5-fluorouracil, leucovorin and oxaliplatin. Radiotherapy was not used in the present study.
The clinicopathological features included sex, age (≤ 65 and > 65 years), other diseases (including hypertension, diabetes and chronic obstructive pulmonary disease), BMI (normal and abnormal), laboratory findings (white blood cell count and serum albumin), tumor size (< 5 and ≥ 5 cm), intraoperative blood loss (< 200 and ≥ 200 mL), tumor location, histology, extranodal metastasis (positive and negative), serosal invasion, lymph node metastasis, TNM stage, postoperative chemotherapy, and type of gastrectomy. The tumors were staged according to the Union for International Cancer Control (UICC) Classification System, 7th edition, whereas lymphadenectomy and lymph node stations were defined according to the Japanese Classification of Gastric Carcinoma, 3rd English edition. Tumors were classified into two groups based on histology: differentiated type including papillary, well or moderately differentiated adenocarcinoma; and undifferentiated type including poorly differentiated or undifferentiated adenocarcinoma, signet ring cell carcinoma and mucinous carcinoma.
Clinicopathological features were compared among the four groups. We evaluated independent prognostic factors for patients with gastric cancer. Finally, we explored the possible independent prognostic factors associated with all grades of complications.
All patients were followed with a standardized protocol. The follow-up was conducted until November 2012 or death, and data were collected based on clinical review or telephone consultation after discharge. There were no patients lost to follow-up.
The analyses were performed with SPSS for Windows version 13.0. Actuarial survival rate was determined by the Kaplan-Meier method, with univariate comparisons between groups through the Log-rank test. Independent prognostic factors were identified using the Cox proportional hazards regression model. One-way analysis of variance or t-test was used in univariate analysis to identify possible factors associated with laboratory findings. P < 0.05 indicated significant differences.
The patients were divided into four groups according to the complication grade (Table 1). There were 105 patients with Grade I complications, 106 with Grade II and 94 with Grade III. There were no significant differences in laboratory findings, tumor size, histology, serosal invasion, and type of gastrectomy among the four groups. Age > 65 years was more frequent in patients with Grade II complications, while male patients had gastric complications more frequently than female patients. Patients who had other diseases including hypertension, diabetes and chronic obstructive pulmonary disease were more prone to postoperative complications. Intraoperative blood loss ≥ 200 mL was more frequent in patients with Grade II and III complications than in those with Grade I complications. Tumor location in the upper third was more frequent in patients with Grade I and III complications. In patients with Grade II complications, 37.7% of tumors were located in the lower third. As the grade of complications increased, so did extranodal metastasis, lymph node metastasis and TNM stage, while chemotherapy was more frequently performed in patients with Grade I than in those with other complications.
| Characteristic | Complication group | F/χ2 | P value | |||
| No (n = 446) | Grade I (n = 105) | Grade II (n = 106) | Grade III (n = 94) | |||
| Age (yr) | 24.064 | < 0.001 | ||||
| ≤ 65 | 239 (53.6) | 74 (70.5) | 39 (36.8) | 50 (53.2) | ||
| > 65 | 207 (46.4) | 31 (29.5) | 67 (63.2) | 44 (46.8) | ||
| Gender | 10.265 | 0.016 | ||||
| Male | 302 (67.7) | 62 (59.0) | 83 (78.3) | 69 (73.4) | ||
| Female | 144 (32.3) | 43 (41.0) | 23 (21.7) | 25 (26.6) | ||
| Other diseases | 34.383 | < 0.001 | ||||
| No | 358 (80.3) | 87 (82.9) | 70 (66.0) | 52 (55.3) | ||
| Yes | 88 (19.7) | 18 (17.1) | 36 (34.0) | 42 (44.7) | ||
| BMI (kg/m2) | 14.640 | 0.002 | ||||
| Normal | 348 (78.0) | 83 (79.0) | 72 (67.9) | 58 (61.7) | ||
| Abnormal | 98 (22.0) | 22 (21.0) | 34 (32.1) | 36 (38.3) | ||
| Laboratory findings | ||||||
| WBC (102/mm3) | 6.5 ± 2.1 | 6.4 ± 2.3 | 6.6 ± 2.3 | 6.4 ± 2.2 | 0.311 | 0.733 |
| Serum albumin (mg/dL) | 40.3 ± 5.7 | 40.1 ± 5.0 | 41.1 ± 5.6 | 40.2 ± 6.0 | 0.700 | 0.497 |
| Tumor size (mm) | 3.728 | 0.292 | ||||
| < 5 cm | 178 (39.9) | 46 (43.8) | 36 (34.0) | 31 (33.0) | ||
| ≥ 5 cm | 268 (60.1) | 59 (56.2) | 70 (66.0) | 63 (67.0) | ||
| Intraoperative blood loss (mL) | 9.875 | 0.020 | ||||
| < 200 | 211 (47.3) | 55 (52.4) | 39 (36.8) | 33 (35.1) | ||
| ≥ 200 | 235 (52.7) | 50 (47.6) | 67 (63.2) | 61 (64.9) | ||
| Tumor location | 23.550 | 0.005 | ||||
| Lower 1/3 | 204 (45.7) | 11 (10.5) | 40 (37.7) | 27 (28.7) | ||
| Middle 1/3 | 48 (10.8) | 11 (10.5) | 9 (8.5) | 10 (10.6) | ||
| Upper 1/3 | 111 (24.9) | 57 (54.3) | 39 (36.8) | 36 (38.3) | ||
| 2/3 or more | 83 (18.6) | 26 (24.8) | 18 (17.0) | 21 (22.3) | ||
| Histology | 5.596 | 0.133 | ||||
| Differentiated | 147 (33.0) | 27 (25.7) | 43 (40.6) | 34 (36.2) | ||
| Undifferentiated | 299 (67.0) | 78 (74.3) | 63 (59.4) | 60 (63.8) | ||
| Extranodal metastasis | 17.533 | 0.001 | ||||
| Positive | 70 (15.7) | 18 (17.1) | 19 (17.9) | 32 (34.0) | ||
| Negative | 376 (84.3) | 87 (82.9) | 87 (82.1) | 62 (66.0) | ||
| Serosal invasion | 3.734 | 0.292 | ||||
| No | 90 (20.2) | 25 (23.8) | 18 (17.0) | 13 (13.8) | ||
| Yes | 356 (79.8) | 80 (76.2) | 88 (83.0) | 81 (86.2) | ||
| Lymph node metastasis | 23.866 | 0.005 | ||||
| pN0 | 129 (28.9) | 32 (30.5) | 35 (33.0) | 14 (14.9) | ||
| pN1 | 57 (12.8) | 17 (16.2) | 14 (13.2) | 28 (29.8) | ||
| pN2 | 112 (25.1) | 20 (19.0) | 23 (21.7) | 20 (21.3) | ||
| pN3 | 148 (33.2) | 36 (34.4) | 34 (32.1) | 32 (34.0) | ||
| TNM stage | 39.915 | < 0.001 | ||||
| I | 35 (7.8) | 12 (11.4) | 7 (6.6) | 4 (4.3) | ||
| II | 101 (22.6) | 28 (26.7) | 23 (21.7) | 22 (23.4) | ||
| III | 291 (65.2) | 62 (59.0) | 61 (57.5) | 50 (53.2) | ||
| IV | 19 (4.3) | 3 (2.9) | 15 (14.2) | 18 (19.1) | ||
| Chemotherapy | 19.76 | < 0.001 | ||||
| Yes | 261 (58.5) | 72 (68.6) | 45 (42.5) | 43 (45.7) | ||
| No | 185 (41.5) | 33 (31.4) | 61 (57.5) | 51 (54.3) | ||
| Type of gastrectomy | 1.004 | 0.800 | ||||
| Subtotal | 315 (70.6) | 71 (67.6) | 74 (32.4) | 62 (66.0) | ||
| Total | 131 (29.4) | 34 (32.4) | 32 (30.2) | 32 (34.0) | ||
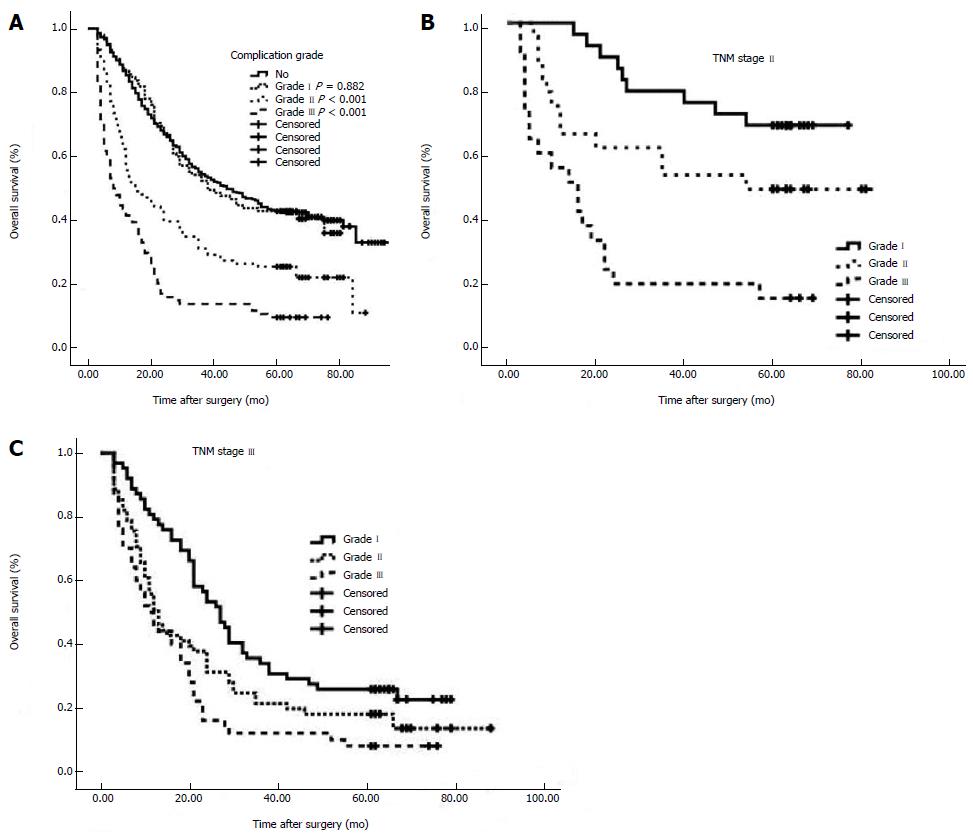
The 5-year overall survival (OS) rates were 43.0%, 42.5%, 25.5% and 9.6% for no complications, and Grade I, Grade II and Grade III complications, respectively (Figure 1A). There was no difference between Grade I and no complications (P = 0.882). A total of 10 factors evaluated in the univariate analysis had a significant effect on survival: age, tumor size, intraoperative blood loss, tumor location, extranodal metastasis, serosal invasion, lymph node metastasis, TNM stage, type of gastrectomy, and grade of complications. In multivariate analysis, grade of postoperative complications was found to be an independent prognostic factor for OS in gastric cancer (P < 0.001) (Table 2).
| Characteristic | S | 5-yr OS | Univariate analysis | Multivariate analysis | ||
| χ2 | P value | HR (95%CI) | P value | |||
| Age (yr) | 15.816 | < 0.001 | 1.378 (1.147-1.654) | 0.001 | ||
| ≤ 65 | 402 | 42.8 | ||||
| > 65 | 349 | 28.7 | ||||
| Gender | 1.842 | 0.175 | ||||
| Male | 516 | 34.3 | ||||
| Female | 235 | 40.9 | ||||
| Other diseases | 2.457 | 0.117 | ||||
| No | 567 | 37.2 | ||||
| Yes | 184 | 33.7 | ||||
| BMI | 1.675 | 0.196 | ||||
| Normal | 561 | 37.6 | ||||
| Abnormal | 190 | 32.6 | ||||
| Tumor size | 57.692 | < 0.001 | 1.296 (1.049-1.600) | 0.016 | ||
| < 5 cm | 291 | 52.9 | ||||
| ≥ 5 cm | 460 | 25.9 | ||||
| Intraoperative blood loss (mL) | 22.678 | < 0.001 | 1.259 (1.038-1.526) | 0.019 | ||
| < 200 | 338 | 45.3 | ||||
| ≥ 200 | 413 | 29.1 | ||||
| Tumor location | 29.940 | < 0.001 | 1.078 (0.99-01.173) | 0.086 | ||
| Upper 1/3 | 189 | 31.2 | ||||
| Middle 1/3 | 78 | 37.2 | ||||
| Lower 1/3 | 336 | 44.6 | ||||
| 2/3 or more | 148 | 23.0 | ||||
| Histology | 0.895 | 0.344 | ||||
| Differentiated | 251 | 37.8 | ||||
| Undifferentiated | 500 | 35.6 | ||||
| Extranodal metastasis | 42.214 | < 0.001 | 1.190 (0.956-1.482) | 0.120 | ||
| Positive | 139 | 18.7 | ||||
| Negative | 612 | 40.5 | ||||
| Serosal invasion | 44.226 | < 0.001 | 1.185 (0.878-1.600) | 0.266 | ||
| No | 146 | 61.0 | ||||
| Yes | 605 | 30.4 | ||||
| Lymph node metastasis | 159.593 | < 0.001 | 1.342 (1.211-1.487) | < 0.001 | ||
| pN0 | 210 | 66.2 | ||||
| pN1 | 116 | 38.8 | ||||
| pN2 | 175 | 28.0 | ||||
| pN3 | 250 | 15.6 | ||||
| TNM stage | 182.016 | < 0.001 | 1.525 (1.238-1.879) | < 0.001 | ||
| I | 58 | 79.3 | ||||
| II | 174 | 59.2 | ||||
| III | 464 | 25.9 | ||||
| IV | 55 | 5.6 | ||||
| Chemotherapy | 0.420 | 0.517 | ||||
| Yes | 421 | 35.2 | ||||
| No | 330 | 37.6 | ||||
| Type of gastrectomy | 13.241 | < 0.001 | 1.073 (0.881-1.307) | 0.485 | ||
| Subtotal | 522 | 40.2 | ||||
| Total | 229 | 27.5 | ||||
| Complication grade | 131.080 | < 0.001 | 1.456 (1.343-1.579) | < 0.001 | ||
| None | 446 | 43.0 | ||||
| GradeI | 105 | 42.5 | ||||
| GradeII | 106 | 25.5 | ||||
| GradeIII | 94 | 9.6 | ||||
Multivariate analysis of factors associated with all grades of complications was performed (Table 3). In multivariate analysis, lymph node metastasis, tumor size, and intraoperative blood loss were independent prognostic factors for Grade I complications (P < 0.001, P = 0.031, P = 0.030). Age and lymph node metastasis were found to be independent prognostic factors for OS of gastric cancer patients with Grade II complications (P = 0.034, P = 0.001). Intraoperative blood loss, TNM stage, and chemotherapy were found to be independent prognostic factors for OS in gastric cancer patients with Grade III complications (P = 0.003, P = 0.005, P < 0.001).
| Characteristic | Grade I | Grade II | Grade III | |||
| Multivariate analysis | Multivariate analysis | Multivariate analysis | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Lymph node metastasis | 1.947 (1.506-2.517) | < 0.001 | 1.577 (1.208-2.058) | 0.001 | ||
| Age | 1.751 (1.044-2.939) | 0.034 | ||||
| Tumor size | 1.913 (1.059-3.453) | 0.031 | ||||
| Intraoperative blood loss | 1.850 (1.063-3.222) | 0.030 | 2.099 (1.288-3.421) | 0.003 | ||
| TNM stage | 1.607 (1.158-2.228) | 0.005 | ||||
| Chemotherapy | 2.354 (1.451-3.819) | < 0.001 | ||||
The 5-year OS rates of the patients with Stage I disease were 83.3%, 57.1% and 50.0% for different grades of postoperative complications, respectively (P = 0.213). The 5-year OS rates of patients with Stage II disease were 67.9%, 47.8% and 13.6% for different grades of postoperative complications, respectively (P < 0.001, Figure 1B). The 5-year OS rates of patients with Stage III disease were 25.8%, 18.0% and 8.0% for different grades of postoperative complications, respectively (P = 0.001, Figure 1C). The 5-year OS rates of patients with Stage IV disease were 0%, 6.7% and 0% for different grades of postoperative complications, respectively (P = 0.303) (Table 4).
| Grade I | Grade II | Grade III | χ2 | P value | |
| 5-yr OS | 5-yr OS | 5-yr OS | |||
| TNM | |||||
| I | 83.3 | 57.1 | 50.0 | 3.094 | 0.213 |
| II | 67.9 | 47.8 | 13.6 | 24.908 | < 0.001 |
| III | 25.8 | 18.0 | 8.0 | 15.121 | 0.001 |
| IV | 0 | 5.3 | 0 | 2.387 | 0.303 |
D2 lymph node dissection has gradually become the standard surgery in gastric cancer to improve patient outcome[9]. Skillful tumor removal corresponds with long-term survival, because the vast majority of patients, even those who have negative margins, eventually die from recurrent disease[10,11], and postoperative complications caused by D2 lymph node dissection also increase. Postoperative complications are associated with the prognosis of many malignant tumors such as breast cancer, hepatic carcinoma, and colorectal cancer[12,13]. Kressner et al[13] have reported that postoperative complications influence long-term survival of colon cancer, and postoperative complications are a risk factor for overall mortality in both univariate and multivariate analyses. Cho et al[14] have suggested that postoperative complications affect prognosis and recurrence patterns in patients with periampullary cancer after pancreaticoduodenectomy.
As to gastric cancer, few studies have focused on the grade of postoperative complications[15]. In 1992, Clavien et al[7] proposed general principles for classifying surgical complications based on a therapy-oriented, four-level severity classification. Twelve years later, they published the modified Clavien-Dindo classification, which added detail to the more serious complications. This system has been validated in a large cohort of patients and has universal applicability[16]. Recently, however, Strasberg et al[17] analyzed 127 published surgical studies that used Clavien-Dindo classification or its modifications and found many inconsistencies. As a result, they introduced an extensive modification of the classification, called the Accordion Severity Grading System[5]. Specifically, the Accordion System added flexibility by introducing an expandable classification, and clarity was improved by introducing rigorously defined qualitative terms. It also provides a web-based method for compiling complication data in a standard tabular form. However, to date, the value of all complication severity grading systems, including the Accordion Modification, still need to be elucidated and the traditional classifications are too complicated. Hence, the aim of this study was to reclassify the complications and investigate whether the grade of complications in patients received curative gastrectomy could be a prognostic factor.
The prognosis of patients after surgical resection depends on various tumor-specific factors (primary tumor location, TNM stage classification, tumor differentiation, and the presence or absence of lymph node metastasis), surgery-related factors (status of resection margins and blood loss), and treatment-related factors (systemic disease treatment with either adjuvant or neoadjuvant therapy)[18,19]. Although there has been recent progress in the early detection of tumors, the development of effective adjuvant therapy continues to be unsatisfactory. Therefore, the ability to determine potentially controllable prognostic factors to improve the dismal long-term outcomes of gastric cancer is a goal of surgeons. Previous studies have reported that postoperative complications are potentially serious, life-threatening events that may prolong hospital stay and increase costs[20]. Li et al[21] have reported that the occurrence of in-hospital postoperative complications was an independent predictor of a worse 5-year OS rate after radical resection of gastric cancer. In the present study, we divided patients into four groups according to the complication grade, as defined by T92 (Toronto 1992 or Clavien), Accordion Classification and Revised Accordion Classification. We evaluated the potential prognostic factors and found that grade of postoperative complications was significantly associated with long-term survival of patients who underwent radical surgery. The 5-year OS rates were 42.5%, 25.5% and 9.6% for Grade I, Grade II and Grade III complications, respectively (P < 0.001). Our multivariate analysis demonstrated that, in addition to these conventional factors, the postoperative complication grade was an independent predictor for OS (HR = 1.456, 95%CI: 1.343-1.579; P < 0.001). The small number of patients with UICC Stage I cancer might explain why cancer stage is not correlated with OS, because only 12, seven and four UICC Stage I patients had Grade I, II and III complications, respectively. Postoperative complications included anastomotic leakage, gastric motility disorders, anastomotic block, wound infections, intra-abdominal abscesses, infectious diarrhea, bleeding, bowel obstructions, arrhythmia, angina pectoris, pneumonia, atelectasis, thrombosis, unexplained fever, delirium, ocular fungal infection, and multiple organ failure, which affected the prognosis of gastric cancer patients.
The classification of complications allowed us to study the risk factors related to different severities of morbidity. When we analyzed the risk factors for all complications, age, tumor size, intraoperative blood loss, lymph node metastasis, TNM stage, and chemotherapy were significant. With regard to age, Wydra et al[22] demonstrated that age itself is not a risk factor for postoperative complications in colorectal cancer in spite of the higher rate of accompanying diseases in elderly patients, whereas Yamada et al[23] reported that patients aged > 85 years are more likely to suffer postoperative pneumonia after gastrectomy than younger patients, and preoperative risk assessment is essential for the older patients. In our study, age was a risk factor for Grade II postoperative complications (P = 0.034). Patients aged > 65 years often have underlying diseases such as hypertension and chronic obstructive pulmonary disease. And in patients with the underlying diseases, the prognosis is correspondingly worse. In previous studies, increased intraoperative blood loss is associated with an elevated risk of complications[24]. Dhar et al[25] have reported that intraoperative blood loss > 500 mL was an independent prognostic factor. Kamei et al[26] have demonstrated that the cumulative survival rate was significantly lower in patients with intraoperative blood loss ≥ 475 mL compared with < 475 mL (P = 0.0038), and intraoperative blood loss was a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. In our study, intraoperative blood loss was a risk factor for Grade I and III postoperative complications. More blood loss can cause immunity, resistance and other aspects of decline, thus increasing the risk of complications.
Some studies have confirmed that chemotherapy increases the incidence of postoperative complications[27,28]. As a result of the toxicity of chemotherapeutic drugs, the prognosis of patients with severe postoperative complications will vary. Therefore, the choice of chemotherapeutic drugs is important, especially for patients with underlying diseases. In the present study, chemotherapy was an independent prognostic factor only for Grade III complications (HR = 2.354, 95%CI: 1.451-3.819; P < 0.001). As a result of its relatively rapid disease progression, surgery plus chemotherapy for patients with gastric cancer may have a good prognosis in patients with severe complications.
Lymph node metastasis and TNM stage are independent prognostic factors that have long been associated with gastric cancer[29,30]. According to the results of the present study, TNM stage was an independent prognostic factor for Grade III complications, and lymph node metastasis was an independent prognostic factor for Grade I and II complications. Intraoperative blood loss (HR = 2.099) and chemotherapy (HR = 2.354) had a significantly higher effect on Grade III complications than TNM stage (HR = 1.607). This may have been because chemotherapy and intraoperative blood loss had more recent effects than other factors, and were more suitable for identifying patients with higher grade complications.
The limitations of the present study were its retrospective nature, a relatively small sample population and the presence of several confounding factors. There was a lack of standardized postoperative chemotherapy regimens during that period, which may have affected patient survival. Despite these limitations, we believe that the grade of complications in gastric cancer is an important and adverse prognostic indicator. Further investigations should be performed with a larger, multicenter, randomized prospective cohort to evaluate the prognostic effect of complication grade and identify the underlying mechanism.
In conclusion, the complication grade may be an independent prognostic factor for gastric cancer patients after curative resection. It can be used to stratify the prognostic risk of gastric cancer. Meticulous surgery is needed and new methods should be considered to decrease the amount of intraoperative blood loss.
Many studies have indicated that, in gastric cancer, the presence or absence of complications is an important prognostic factor following curative gastrectomy. The traditional classification of complications was too complicated and the methods for classification were not uniform. Hence, the aim of this study was to reclassify the complications and investigate whether the grade of complications could provide a new prognostic factor in patients undergoing curative gastrectomy.
Gastrectomy with D2 lymph node dissection has been increasingly regarded as the standard surgical procedure for most patients with operable gastric cancer, but it also brings a simultaneous increase in surgical complications. In the present study, the authors demonstrated that the complication grade was an independent prognostic factor for gastric cancer patients after curative resection.
Few studies have reported the value of all complication severity grading systems, including the Accordion Modification, in assessing the long-term survival of gastric cancer following curative gastrectomy. In this study, the authors reclassified the grade of complications and demonstrated that the grade was an independent prognostic factor for gastric cancer patients after curative resection.
By understanding the characteristics and prognostic factors for gastric cancer patients with different complication grades, this study may provide evidence for treatment planning for gastric cancer patients with different complication grades in China.
Extranodal metastasis is defined as the presence of tumor cells in extramural soft tissues that is discontinuous with either the primary lesion or locoregional lymph nodes.
The authors revealed that the new classification may be an independent prognostic factor for patients with gastric cancer after gastrectomy. Lymph node metastasis, tumor size, and intraoperative blood loss were independent prognostic factors for Grade I complications; age and lymph node metastasis were independent prognostic factors for overall survival (OS) of patients with Grade II complications; and intraoperative blood loss, tumor-node-metastasis stage, and chemotherapy were independent prognostic factors for OS in patients with Grade III complications. The conclusions of the study are of potential significance for clinicians.
P- Reviewer: Meister T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Schmidt B, Yoon SS. D1 versus D2 lymphadenectomy for gastric cancer. J Surg Oncol. 2013;107:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 3. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1308] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 4. | Marrelli D, Pedrazzani C, Neri A, Corso G, DeStefano A, Pinto E, Roviello F. Complications after extended (D2) and superextended (D3) lymphadenectomy for gastric cancer: analysis of potential risk factors. Ann Surg Oncol. 2007;14:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8632] [Article Influence: 539.5] [Reference Citation Analysis (0)] |
| 6. | Rassweiler JJ, Rassweiler MC, Michel MS. Classification of complications: is the Clavien-Dindo classification the gold standard? Eur Urol. 2012;62:256-258; discussion 259-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518-526. [PubMed] |
| 8. | Sano T. [Evaluation of the gastric cancer treatment guidelines of the Japanese Gastric Cancer Association]. Gan To Kagaku Ryoho. 2010;37:582-586. [PubMed] |
| 9. | Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, Law C, Coburn N. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012;15 Suppl 1:S60-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394-400. [PubMed] |
| 11. | Catarci M, Montemurro LA, Ghinassi S, Di Cintio A, Leone L, Cosentino LM, Viarengo MA, Grassi GB. Long-term results of tailored D(2) lymph node dissection after R(0) surgery for gastric cancer. Updates Surg. 2011;63:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Murthy BL, Thomson CS, Dodwell D, Shenoy H, Mikeljevic JS, Forman D, Horgan K. Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer. 2007;97:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Kressner U, Graf W, Mahteme H, Påhlman L, Glimelius B. Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum. 2002;45:316-321. [PubMed] |
| 14. | Cho JY, Han HS, Yoon YS, Hwang DW, Jung K, Kim YK. Postoperative complications influence prognosis and recurrence patterns in periampullary cancer. World J Surg. 2013;37:2234-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Jung MR, Park YK, Seon JW, Kim KY, Cheong O, Ryu SY. Definition and classification of complications of gastrectomy for gastric cancer based on the accordion severity grading system. World J Surg. 2012;36:2400-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 17. | Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 511] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 18. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 997] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 19. | Takahashi I, Matsusaka T, Onohara T, Nishizaki T, Ishikawa T, Tashiro H, Wakasugi K, Kume K, Maehara Y, Sugimachi K. Clinicopathological features of long-term survivors of scirrhous gastric cancer. Hepatogastroenterology. 2000;47:1485-1488. [PubMed] |
| 20. | Sah BK, Zhu ZG, Chen MM, Yan M, Yin HR, Zhen LY. Gastric cancer surgery and its hazards: post operative infection is the most important complication. Hepatogastroenterology. 2008;55:2259-2263. [PubMed] |
| 21. | Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol. 2013;19:4060-4065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 22. | Wydra J, Kruszewski W, Jasiński W, Szajewski M, Ciesielski M, Szefel J, Walczak J. Is age a risk factor of postoperative complications in colorectal cancer? Pol Przegl Chir. 2013;85:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Yamada H, Shinohara T, Takeshita M, Umesaki T, Fujimori Y, Yamagishi K. Postoperative complications in the oldest old gastric cancer patients. Int J Surg. 2013;11:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Liang YX, Guo HH, Deng JY, Wang BG, Ding XW, Wang XN, Zhang L, Liang H. Impact of intraoperative blood loss on survival after curative resection for gastric cancer. World J Gastroenterol. 2013;19:5542-5550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Watanabe R, Kohno H, Nagasue N. Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors. World J Surg. 2000;24:588-593; discussion 593-594. [PubMed] |
| 26. | Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. 2009;33:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Valenti V, Hernandez-Lizoaín JL, Beorlegui MC, Diaz-Gozalez JA, Regueira FM, Rodriguez JJ, Viudez A, Sola I, Cienfuegos JA. Morbidity, mortality, and pathological response in patients with gastric cancer preoperatively treated with chemotherapy or chemoradiotherapy. J Surg Oncol. 2011;104:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann Surg Oncol. 2012;19:2452-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Kikuchi S, Sakakibara Y, Sakuramoto S, Kobayashi N, Shimao H, Mieno H, Kato Y, Kadowaki K, Hiki Y, Kakita A. Recent results in the surgical treatment of gastric cancer according to the Japanese and TNM classification. Anticancer Res. 2001;21:3589-3593. [PubMed] |
| 30. | Marrelli D, Pedrazzani C, Corso G, Neri A, Di Martino M, Pinto E, Roviello F. Different pathological features and prognosis in gastric cancer patients coming from high-risk and low-risk areas of Italy. Ann Surg. 2009;250:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |