Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8215
Revised: February 9, 2014
Accepted: April 2, 2014
Published online: July 7, 2014
Processing time: 191 Days and 11 Hours
AIM: To study the effects of mesenchymal stem cell (MSC) therapy on the prevention of acute rejection and graft vs host disease following small bowel transplantation.
METHODS: In our transplantation center, 6 isolated intestinal transplants have been performed with MSC therapy since 2009. The primary reasons for transplants were short gut syndrome caused by surgical intestine resection for superior mesenteric artery thrombosis (n = 4), Crohn’s disease (n = 1) and intestinal aganglionosis (n = 1). Two of the patients were children. At the time of reperfusion, the first dose of MSCs cultured from the patient’s bone marrow was passed into the transplanted intestinal artery at a dose of 1000000 cells/kg. The second and third doses of MSCs were given directly into the mesenteric artery through the arterial anastomosis using an angiography catheter on day 15 and 30 post-transplant.
RESULTS: The median follow-up for these patients was 10.6 mo (min: 2 mo-max: 30 mo). Three of the patients developed severe acute rejection. One of these patients did not respond to bolus steroid therapy. Although the other two patients did respond to anti-rejection treatment, they developed severe fungal and bacterial infections. All of these patients died in the 2nd and 3rd months post-transplant due to sepsis. The remaining patients who did not have acute rejection had good quality of life with no complications observed during the follow-up period. In addition, their intestinal grafts were functioning properly in the 13th, 25th and 30th month post-transplant. The patients who survived did not encounter any problems related to MSC transplantation.
CONCLUSION: Although this is a small case series and not a randomized study, it is our opinion that small bowel transplantation is an effective treatment for intestinal failure, and MSC therapy may help to prevent acute rejection and graft vs host disease following intestinal transplantation.
Core tip: Intestinal transplantation significantly improves prognosis and increases quality of life in patients with short gut syndrome. Transplantation of mesenchymal stem cells (MSCs) has been studied recently in animal models and in clinical trials of patients with hepatic failure, end-stage liver disease and inherited metabolic disorders. However, studies and data related to MSC therapy in small bowel transplantation are scarce. We think that MSC therapy may help to prevent acute rejection and graft vs host disease following intestinal transplantation.
- Citation: Doğan SM, Kılınç S, Kebapçı E, Tuğmen C, Gürkan A, Baran M, Kurtulmuş Y, Ölmez M, Karaca C. Mesenchymal stem cell therapy in patients with small bowel transplantation: Single center experience. World J Gastroenterol 2014; 20(25): 8215-8220
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8215
Intestinal transplantation significantly improves the prognosis of patients with short gut syndrome and increases their quality of life.
Canine models of small bowel and multivisceral abdominal transplantation were first described by Lillehei et al[1] and Starzl et al[2]. The first long-term survivor after combined liver and small bowel transplantation (SBTx) was reported by Grant et al[3]. Initial successful isolated small bowel transplant procedures were reported by Deltz et al[4], Goulet et al[5], and Starzl et al[6] during 1989-1991. The first SBTx in Turkey was performed in our clinic in 2003[7].
Clinical studies have reported the possible beneficial therapeutic effect of mesenchymal stem cells (MSCs) in supporting the engraftment of hematopoietic stem cells in patients with hematological malignancies[8]. The transplantation of MSCs has also recently been studied in animal models and in clinical trials of patients with hepatic failure, end-stage liver disease and inherited metabolic disorders. Modulatory cytokines produced by MSCs can inhibit immunocyte proliferation and migration to the liver, thereby attenuating inflammatory injury and reducing hepatocyte apoptosis. In addition, MSCs play an important role in liver fibrosis regression and in supporting the function, proliferation and differentiation of endogenous hepatocytes[9,10]. Recent studies have documented MSC synthesis and the release of several cytokines and growth factors such as, interleukin-11, hepatocyte growth factor, fibroblast growth factor-2 and insulin-like growth factor-I[11-13]. Each of these factors has previously been described as facilitating intestinal mucosa repair, either through enhancement of cell proliferation or inhibition of epithelial cell apoptosis, or by a combination of both[14-16].
As MSCs are able to inhibit T-cell proliferation in vitro and in vivo and exert similar inhibitory effects on B, dendritic, and natural killer cells as well as having the ability to enhance or maintain the re-epithelization process of small intestinal epithelium, we planned this study to determine the effects of MSC therapy on the prevention of acute rejection and graft vs host disease following small bowel transplantation.
In our transplantation center, 6 isolated intestinal transplants have been performed since 2009. All were deceased donor transplants. The primary reasons for these transplants were short gut syndrome caused by intestine resection for superior mesenteric artery thrombosis (n = 4), Crohn’s disease (n = 1) and intestinal aganglionosis (n = 1). Two of the patients were children. The demographic data of these patients are shown in Table 1.
| Age (yr) | Gender | Primary disease | Donor type | HLA miss-match |
| 12 | F | SMATR-TSBR | Deceased | 2 |
| 45 | M | SMAT-TSBR | Deceased | 5 |
| 31 | F | Crohn-TSBR | Deceased | 4 |
| 48 | M | SMAT-TSBR | Deceased | 6 |
| 50 | M | SMAT-TSBR | Deceased | 4 |
| 0.67 | M | Aganglionosis | Deceased | 6 |
The first step in the transplant procedure was to create space for the graft by removing any failed recipient organs. All adhesions were removed. The graft artery was anastomosed to the infrarenal aorta and the graft vein was drained to the infrarenal vena cava. Jejunojejunostomy was performed for oral-side reconstruction of the gastrointestinal tract and a feeding tube gastrostomy was carried out for postoperative enteral feeding. For anal-side reconstruction, a simple ileostomy was preferred.
Anti-thymocyte immunoglobulin 5 mg/kg was the standard induction immunosuppressive treatment initiated prior to transplantation. A steroid bolus was given at a dose of 10 mg/kg and tapered off gradually. Tacrolimus was given parenterally for maintenance immunosuppression with a target trough blood level of 15-20 ng/mL. At the time of reperfusion, the first dose of MSCs cultured from the patient’s bone marrow was infused into the transplanted intestinal artery at a dose of 1000000 cells/kg. Standard post-transplant antimicrobial and antiviral prophylaxis were given.
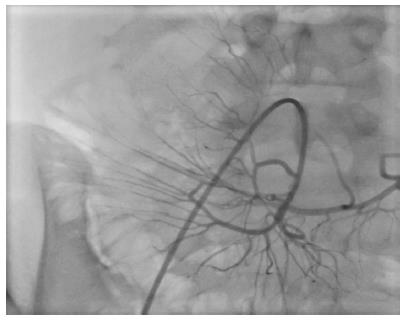
After enteral feeding was started, immunosuppression was continued with an mTOR inhibitor together with low-dose tacrolimus. Antibiotics were administered immediately after surgery, including agents to prevent cytomegalovirus, Pneumocystis carinii, and fungal infections. The second and third doses of MSCs were infused directly into the mesenteric artery through the arterial anastomosis using an angiography catheter, under local anesthesia, on day 15 and 30 post-transplant (Figure 1). Endoscopic evaluations were carried out bi-weekly for the first two weeks and then weekly in the following months. Biopsies were taken if rejection was suspected. Following the initiation of enteral feeding in the first week, TPN was tapered off gradually.
Bone marrow (BM; 10-20 mL) was aspirated from the iliac crest using a standard procedure, and transferred into an acid-citrate-dextrose filled transfusion bag. The samples obtained from each patient were packed in an insulated shipping container with cold packs, and delivered to the Aticell® laboratory (Trabzon, Turkey) of Atigen-Cell® Inc (Ankara, Turkey). All procedures for the generation of clinical-grade autologous MSCs were performed under good manufacturing practice (GMP) conditions in Aticell® laboratories. Mononuclear cells were separated from BM by a standard Ficoll density gradient separation procedure, washed with phosphate-buffered saline (PBS), re-suspended in GMP-qualified (Advanced Therapy Medicinal Product; ATMP-Ready) low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; PAA Laboratories GmbH, Pasching, Austria) containing 10% autologous (v/v) human serum (AHS), and seeded at a density of 2-3 × 105 cells/cm2 in 75-cm2 flasks. The cultures were maintained at 37 °C in a humidified 5% (v/v) CO2 atmosphere for 3 d, after which nonadherent cells were removed by replacing the medium, and adherent cells were further cultured for an additional 7-9 d. When the cultures approached confluence (70%-80%), adherent cells were detached by treatment with ATMP-Ready trypsin/EDTA solution (PAA Laboratories GmbH, Pasching, Austria) and placed in large culture flasks at a density of 4-5 × 103 cells/cm2. Cells for infusion were serially subcultured up to passage four (P4), and harvests (P3-P4) were cryopreserved in DMEM supplemented with 10% (v/v) dimethyl sulphoxide and AHS. On the day of injection, MSCs were defrosted, washed thrice with phosphate buffered saline (PBS; PAA Laboratories GmbH, Pasching, Austria), and then re-suspended in PBS containing hydroxyethyl starch and AHS solution at a final concentration of 1-2 × 107 cells/mL. Criteria for the clinical use of MSCs included a viability > 90%; an absence of microbial contamination (bacteria, fungus, or mycoplasma); the expression of CD73, CD90 and CD105 by > 90% of cells; and the absence of CD14, CD34, and CD45 (expression of each by < 3% of cells), as assessed by flow cytometry.
Autologous MSC therapy was used by permission of the Turkish Ministry of Health and informed consent was obtained from the patients.
The median follow-up period was 10.6 mo (min: 2 mo-max: 30 mo). Three of the patients developed severe acute rejection and were given bolus steroid therapy. One of these patients did not respond to the steroid treatment and died on the 4th day of rejection 3 mo post-transplant. The other two patients responded to bolus steroid treatment, but both developed bacterial and fungal infections which did not respond to antimicrobial and antifungal treatments. These patients died 2 and 3 mo post-transplant, respectively, due to sepsis.
In the patient operated on for intestinal ischemia and necrosis associated with post-traumatic mesenteric artery injury, mild acute rejection was determined during small bowel biopsy under endoscopic examination in the first month. However, no infection was detected at tissue biopsy and viral serologic analyses were negative during this period. This rejection episode was controlled by increasing steroid therapy. To prevent compromise of renal function, tacrolimus was stopped in the third month and sirolimus was added to the immunosuppressive therapy. In the third month, the patient was on total oral feeding and was monitored on an outpatient basis. Since the graft functioned normally in the first year post-transplant and weight gain and nutrition were satisfactory, ileocolonic anastomosis was performed. During the 14th month of monitoring, obstructions in the lymphatics were determined on lymphoscintigraphy performed due to lymphedema in the left lower extremity, below the right knee and in the left arm. All extremity and abdominal vascular Doppler USG examinations in this period were normal. Sirolimus therapy was stopped as it was thought that this agent may have been responsible for the patients’ condition. It was decided that monitoring should continue with low-dose tacrolimus. There were no further rejection episodes. The patient is now in the 30th month post-transplant and has achieved a weight of 65 kg (height: 175 cm), with lymphedema persisting, but decreasing in the extremities.
The patient with Crohn’s disease is now 25 mo post-transplant with a permanent ileostomy and has had no complications. No rejection episodes and no systemic infection were observed in this patient. Her endoscopic evaluations and biopsies have all been normal.
The child with type 4 Waardenburg syndrome is now 13 mo post-transplant. He developed gastrointestinal bleeding 5 mo post-transplant due to a gastric ulcer and medically-treated severe urosepsis. Diffuse CD 20(+) and CD 79a(+), compatible with EBV-positive PTLD, were determined at tissue biopsy. The patient was placed on valganciclovir prophylaxis and both tacrolimus and sirolimus dosages were reduced (5 ng/mL). As the presence of EBV DNA was detected in the serum (3200 copies/mL), intravenous immune globulin 2 g/kg was administered and a weekly dose of rituximab at 375 mg/m2 was started. Persistent gastrointestinal hemorrhage was seen after the third dose of rituximab. During endoscopic examination, several ulcerous areas were identified in the transplanted bowel together with an ulcer in the pre-anastomosis area of the duodenum. Bleeding was controlled with hemoclips. Histopathologic findings, from a graft biopsy performed during the same endoscopy session, revealed that, although reduced, lymphoproliferative disorders persisted. His ileostomy output was higher than expected and weight gain was only 1 kg. He still receives combined enteral and parenteral nutrition. He has had no acute rejection.
There is a significant need for small bowel transplantation in Turkey[17]. The key to postoperative management after intestinal transplantation is early detection of acute rejection, a serious complication. Observation of the intestinal mucosa by routine protocol endoscopy and pathological assessment with serial biopsies are important to detect early-stage rejection[18]. Twice-weekly endoscopies are carried out following transplantation in our clinic. Also, increased ileostomy output, a change in stool character, and fever indicates acute rejection and endoscopy is implemented in these situations.
Acute cellular rejection (ACR) is particularly persistent in the first 3 mo. The level, which is reported to be 63.5%, was shown to have declined following the introduction of treatment with rATG[19]. Graft-vs-host disease (GVHD) occurs when donor alloreactive T lymphocytes transferred within the transplanted organ mount a destructive cellular immune response against recipient tissue. SBTx is associated with the transfer of a large number of potentially alloreactive donor lymphocytes, which have the capacity to initiate GVHD[20-22]. The reported incidence of GVHD after SBTx is 6.5% in children and 4.7% in adults[23]. MSCs have the ability to inhibit T cell proliferation in vitro and in vivo and exert similar inhibitory effects on B, dendritic and natural killer cells. Thus, MSCs hold promise as a new stem cell therapy class for autoimmune disease, solid organ transplantation and the treatment of GVHD[24-26]. In recent studies it was shown that modulatory cytokines produced by MSCs could inhibit immunocyte proliferation and migration, thereby attenuating inflammatory injury and reducing cellular apoptosis[13,14].
Three of our patients had a biopsy proven severe ACR episode and one patient had a mild ACR episode within the first 3 mo after transplantation. Rejection attacks were controlled by pulse steroid therapy, with the exception of one patient who did not respond to steroid treatment and died on the 4th day of rejection in the 3rd month post-transplant. Our research suggests that MSCs therapies given with the transplant may contribute to the prevention of graft rejection and may help recovery of the intestinal mucosa after a rejection attack. In conclusion, we observed rejection in 4 (3 severe and 1 mild) of our 6 patients (66%) and three responded to pulse steroid treatment. These findings are very similar to those of Nayyar et al[19]. We did not administer another course of MSC injections to the surviving patients during their follow-up periods.
In the immune compromised state, bacterial translocation can trigger acute rejection and systemic infection with overlapping symptoms. The International Intestinal Transplant Registry reported sepsis as the major cause of death after SBTx in 202 of 439 (46%) deceased recipients[27]. Two of our patients responded to bolus steroid treatment, but developed bacterial and fungal infections which did not respond to antimicrobial and antifungal treatment. Sepsis was the cause of death in these two patients (33%).
The levels of kidney function disorder secondary to high immunosuppressive therapies in small bowel transplant patients, with high rejection levels, have been reported to be 46% in patients with SBTx[28]. Renal dysfunction occurred in our two pediatric cases, and tacrolimus was stopped in one patient. Renal dysfunction secondary to elevated ileal output and high immunosuppressive therapy was observed in our 9-mo post-transplant patient. Sirolimus is an immunosuppressive agent used after major organ transplantation. We started sirolimus instead of tacrolimus in one patient. However, sirolimus was suspected to be the cause of lymphedema seen in the first year of treatment, and a partial improvement in findings was observed when treatment was stopped. However, lymphedema persists even after 2 years. Sirolimus-related lymphedema associated with lymphangiogenesis has been reported in organ transplant patients[29].
In conclusion, this is a small series and not a randomized study and does not show the effectiveness of MSC treatment in SBTx. However, we believe that MSC therapy may prevent and limit ACR effects in the transplanted small intestine and facilitate intestinal mucosa repair. Randomized prospective studies are required to prove the effects of MSC therapy.
Small intestinal transplantation is now routinely considered for patients with irreversible intestinal failure and complications of parenteral nutrition. Although technically possible for some time, immunological rejection was an impenetrable barrier to success until the development of powerful immunosuppressive agents. There are still important immunology related problems. Recent studies have demonstrated that mesenchymal stem cells (MSCs) increase the synthesis and release of several factors facilitating intestinal mucosa repair and preventing immunologic reactions.
As MSCs are able to inhibit immunologic refractory cells attacking transplanted organs and have the ability to enhance or maintain the re-epithelization process of small intestinal epithelium, the authors planned this study to determine the effects of MSC therapy on the prevention of acute rejection in patients undergoing small bowel transplantation.
Small-intestine transplantation continues to evolve as a surgical procedure used in the management of intestinal failure in children and adults and offers the hope of increased longevity and improved quality of life in patients with intestinal failure. Over the last three decades, intestinal transplantation has evolved from an experimental to a standard therapeutic option for patients with intestinal failure, but is less successful in other solid organ transplantation due to high rejection rates. In order to overcome this problem we planned this study to determine the effects of MSC therapy on the prevention of acute rejection following small bowel transplantation.
This study suggests that MSC therapy may prevent and limit acute cellular rejection effects in the transplanted small intestine and facilitate intestinal mucosa repair.
Short bowel syndrome: A malabsorbtion disorder caused by surgical removal of the small intestine, or rarely due to the complete dysfunction of a large segment of bowel. Mesenchymal stem cells: Multipotent stromal cells that can differentiate into a variety of cell types. Acute rejection: Destruction of transplanted tissue by the recipient’s immunologic cells and factors.
The authors reported to use mesenchymal stem cells for intestinal transplantation. Although the cases of patients are low, their results are important for intestinal transplantation. Authors did an interesting work in experiments.
P- Reviewers: Minana MD, Yao CL, Zhang L S- Editor: Gou SX L- Editor: Webster JR E- Editor: Liu XM
| 1. | Lillehei RC, Goott B, Miller FA. Homografts of the small bowel. Surg Forum. 1960;10:197-201. [PubMed] |
| 2. | Starzl TE, Kaupp HA Jr. Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28-30. [PubMed] |
| 3. | Grant D, Wall W, Mimeault R, Zhong R, Ghent C, Garcia B, Stiller C, Duff J. Successful small-bowel/liver transplantation. Lancet. 1990;335:181-184. [PubMed] |
| 4. | Deltz E, Mengel W, Hamelmann H. Small bowel transplantation: report of a clinical case. Prog Pediatr Surg. 1990;25:90-96. [PubMed] |
| 5. | Goulet O, Révillon Y, Jan D, Brousse N, De Potter S, Cerf-Bensussan N, Rambaud C, Buisson C, Pellerin D, Mougenot JF. Small-bowel transplantation in children. Transplant Proc. 1990;22:2499-2500. [PubMed] |
| 6. | Starzl TE, Todo S, Tzakis A, Alessiani M, Casavilla A, Abu-Elmagd K, Fung JJ. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335-344. [PubMed] |
| 7. | Kaçar S, Gurkan A, Karaca C, Varıls㉨ha C, Karaoğlan M. Small bowel transplantation: The first. Turkish J Surg. 2004;20:18-20. |
| 8. | Christ B, Dollinger MM. The generation of hepatocytes from mesenchymal stem cells and engraftment into the liver. Curr Opin Organ Transplant. 2011;16:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Lin H, Xu R, Zhang Z, Chen L, Shi M, Wang FS. Implications of the immunoregulatory functions of mesenchymal stem cells in the treatment of human liver diseases. Cell Mol Immunol. 2011;8:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675-C682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, Yang YS, Im HJ, Kim KH, Jung HL. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31:119-126. [PubMed] |
| 12. | Schinköthe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res. 2007;67:9501-9506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol. 1999;276:G249-G258. [PubMed] |
| 15. | Kanayama M, Takahara T, Yata Y, Xue F, Shinno E, Nonome K, Kudo H, Kawai K, Kudo T, Tabuchi Y. Hepatocyte growth factor promotes colonic epithelial regeneration via Akt signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293:G230-G239. [PubMed] |
| 16. | Mylonas PG, Matsouka PT, Papandoniou EV, Vagianos C, Kalfarentzos F, Alexandrides TK. Growth hormone and insulin-like growth factor I protect intestinal cells from radiation induced apoptosis. Mol Cell Endocrinol. 2000;160:115-122. [PubMed] |
| 17. | Gurkan A. Where are we in small bowel transplantation? 1st National Transplant Immunology and Genetics Congress. Girne: Cyprus 2008; . |
| 18. | Lee RG, Nakamura K, Tsamandas AC, Abu-Elmagd K, Furukawa H, Hutson WR, Reyes J, Tabasco-Minguillan JS, Todo S, Demetris AJ. Pathology of human intestinal transplantation. Gastroenterology. 1996;110:1820-1834. [PubMed] |
| 19. | Nayyar N, Mazariegos G, Ranganathan S, Soltys K, Bond G, Jaffe R, Sun Q, Nucci A, Kosmach B, Squires R. Pediatric small bowel transplantation. Semin Pediatr Surg. 2010;19:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Triulzi DJ, Nalesnik MA. Microchimerism, GVHD, and tolerance in solid organ transplantation. Transfusion. 2001;41:419-426. [PubMed] |
| 21. | Nakamura K, Nalesnik M, Todo S, Takenaka T, Yagihashi A, Iwaki Y, Abu-Elmagd K, Reyes J, Tzakis A, Warty V. Lymphocyte trafficking using in situ hybridization and physioanatomy of the intestinal immune system after human small bowel transplantation. Transplant Proc. 1992;24:1197-1198. [PubMed] |
| 22. | Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579-1582. [PubMed] |
| 23. | Mazariegos GV, Abu-Elmagd K, Jaffe R, Bond G, Sindhi R, Martin L, Macedo C, Peters J, Girnita A, Reyes J. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004;4:1459-1465. [PubMed] |
| 24. | Zhang X, Jiao C, Zhao S. Role of mesenchymal stem cells in immunological rejection of organ transplantation. Stem Cell Rev. 2009;5:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 27. | Braun F, Broering D, Faendrich F. Small intestine transplantation today. Langenbecks Arch Surg. 2007;392:227-238. [PubMed] |
| 28. | Suzuki M, Mujtaba MA, Sharfuddin AA, Yaqub MS, Mishler DP, Faiz S, Vianna RM, Mangus RS, Tector JA, Taber TE. Risk factors for native kidney dysfunction in patients with abdominal multivisceral/small bowel transplantation. Clin Transplant. 2012;26:E351-E358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Desai N, Heenan S, Mortimer PS. Sirolimus-associated lymphoedema: eight new cases and a proposed mechanism. Br J Dermatol. 2009;160:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |