Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8173
Revised: February 19, 2014
Accepted: April 5, 2014
Published online: July 7, 2014
Processing time: 225 Days and 13.2 Hours
AIM: To retrospective review the laparoscopic management of Meckel Diverticulum (MD) in two Italian Pediatric Surgery Centers.
METHODS: Between January 2002 and December 2012, 19 trans-umbilical laparoscopic-assisted (TULA) procedures were performed for suspected MD. The children were hospitalized for gastrointestinal bleeding and/or recurrent abdominal pain. Median age at diagnosis was 5.4 years (range 6 mo-15 years). The study included 15 boys and 4 girls. All patients underwent clinical examination, routine laboratory tests, abdominal ultrasound and technetium-99m pertechnetate scan, and patients with bleeding underwent gastrointestinal endoscopy. The abdominal exploration was performed with a 10 mm operative laparoscope. Pneumoperitoneum was established based on the body weight. Systematic overview of the peritoneal cavity allowed the ileum to be grasped with an atraumatic instrument. The complete exploration and surgical treatment of MD were performed extracorporeally, after intestinal exteriorization through the umbilicus. All patients’ demographics, main clinical features, diagnostic investigations, operative time, histopathology reports, conversion rate, hospital stay and complications were registered and analyzed.
RESULTS: MD was identified in 17 patients, while 1 had an ileal duplication and 1 a jejunal hemangioma. Fifteen patients had painless intestinal bleeding, while 4 had recurrent abdominal pain and exhibited cyst like structures in an ultrasound study. Eleven patients had a positive technetium-99m pertechnetate scan. In the patients with bleeding, gastrointestinal endoscopy did not name the source of hemorrhage. All patients were subjected to a TULA surgical procedure. An intestinal resection/anastomosis was performed in 14 patients, while 4 had a wedge resection of the diverticulum and 1 underwent stapling diverticulectomy. All surgical procedures were performed without conversion to open laparotomy. Mean operative time was 75 min (range 40-115 min). No major surgical complications were recorded. The median hospital stay was 5-7 d (range 4-13 d). All patients are asymptomatic at a median follow up of 4, 5 years (range 10 mo-10 years).
CONCLUSION: Trans-umbilical laparoscopic-assisted Meckel’s diverticulectomy is safe and effective in the treatment of MD, with excellent results.
Core tip: The Authors report a retrospective review on laparoscopic management of Meckel’s diverticulum (MD). The children were hospitalized for gastrointestinal bleeding and/or recurrent abdominal pain. The abdominal exploration was performed with a 10 mm operative laparoscope and the complete exploration and surgical treatment of MD were performed extracorporeally, after intestinal exteriorization through the umbilicus. Patients’ demographics, main clinical features, diagnostic investigations etc. were registered and analyzed. Meckel’s diverticulum was identified in 17 patients, while 1 had an ileal duplication and 1 a jejunal hemangioma. Our study confirms the value of laparoscopy in the management of MD for providing a definitive diagnosis and directing the subsequent surgical approach.
- Citation: Papparella A, Nino F, Noviello C, Marte A, Parmeggiani P, Martino A, Cobellis G. Laparoscopic approach to Meckel's diverticulum. World J Gastroenterol 2014; 20(25): 8173-8178
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8173.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8173
Meckel’s diverticulum (MD) is one of the most common malformations of the small intestine (2%-4%)[1-5]. In most cases it is clinically silent and found incidentally during abdominal exploration for other pathologies. Its presence is related to an obliteration defect of the omphalomesenteric duct, and for this reason it is localized on the antimesenteric border of the ileum[2,6], up to 100 cm from the ileocecal valve. When the MD is symptomatic, it may be responsible for severe episodes of intestinal bleeding[7,8], intussusception, bowel obstruction, or recurrent abdominal pain with repeated vomiting and/or nausea[1-5]. The presence of heterotopic mucosa, mostly gastric or pancreatic but also colic, is found in 20%-30% of cases[2]. In some cases, the diverticulum, including all three layers of the intestinal wall and associated vasculature, may be affected by an appendicitis like inflammation and may be responsible for acute or sub-acute intestinal obstruction[1]. In the pre-laparoscopic era, due to symptom variability, the diagnosis and treatment of MD was a challenge because in difficult cases, traditional diagnostic methods, such as X-ray and ultrasound or scintigraphy with Tc-99m pertechnetate (MS)[2,7] had high false negative or positive rates. The surgical approach to MD may vary depending on its morphology and anatomical variations, such as short diverticulum with a wide base or fibrous band to the umbilicus, long diverticulum with a narrow base, a patent vitellointestinal duct or a periumbilical sinus. Conventional surgical management has been laparotomy and simple diverticulectomy or wedge excision of the adjacent ileum or segmental ileal resection and anastomosis, which seems to be the most popular surgical procedure for MD to date[1]. The advances in laparoscopy have significantly aided the diagnosis and surgical treatment[3,5,6,9-12] of this disease, with excellent cosmetic results, shorter hospitalization and less post-operative pain. Currently, there are two laparoscopic procedures that are performed for MD. The first is called “trans-umbilical laparoscopic-assisted (TULA) Meckel’s diverticulectomy”, which allows the exteriorization of the diverticulum through the navel and the performance of the diverticulectomy outside of the abdomen with its repair in relationship to the enteric defect and morphology[13,14]. The second technique is a three port laparoscopic procedure that requires the use of an endoscopic linear stapler-cutting device. The TULA approach has gained significant support because it is safe and effective in the management of MD, and it offers low cost and excellent results[3,4,5,12]. Therefore, we report a retrospective review of the laparoscopic management of Meckel’s diverticulum in two Italian pediatric surgery centers.
Between January 2002 and December 2012, a retrospective review was performed on case records of 19 patients with suspected Meckel’s diverticulum. The children were hospitalized for gastrointestinal bleeding and/or recurrent abdominal pain.
Patients’ demographics, clinical features, diagnostic tests performed, histopathology reports, operative time, conversion to laparotomy, hospital stay and complications were analyzed. All patients underwent clinical examination, routine laboratory tests, abdominal ultrasound and MS. Patients with intestinal bleeding were subjected to upper and lower gastrointestinal endoscopy.
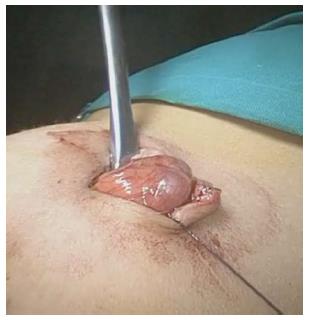
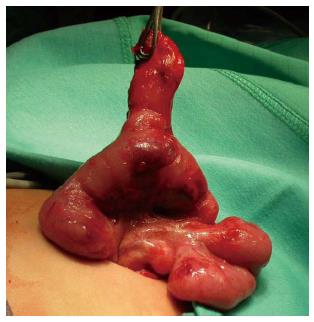
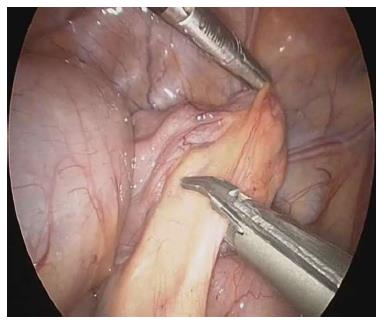
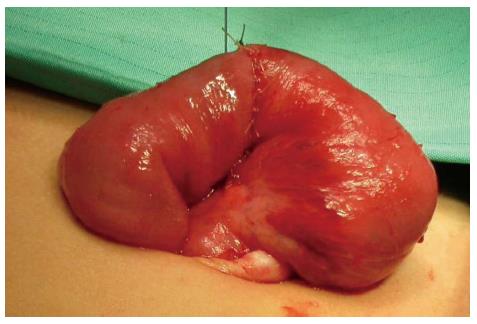
The procedure was performed under general anesthesia with an indwelling nasogastric tube and bladder catheter. All patients were submitted to diagnostic laparoscopy after obtaining informed consent. In all patients, a 10 mm Hasson trocar was inserted using the open technique. Pneumoperitoneum was established based on the body weight, with an insufflation of 0.5/L/min to a maximum pressure of 12/mmHg. After systematic overview of the peritoneal cavity with a 10 mm operative telescope, the MD was directly identified, grasped and delivered through the umbilical port (Figure 1), even if it was a giant diverticulum (Figure 2). When it was not possible to identify the MD, the terminal ileum was grasped, and the ileal exploration was systematically performed extra-corporeally or intra-corporeally by inserting two 3/5 mm working ports in the left and right iliac fossa (Figure 3). In the patients with MD, the diverticulectomy was performed extracorporeally with wedge resection or intestinal resection/anastomosis, performed along with a sleeve of the adjacent ileum (Figure 4), including the patients with ileal duplication and jejunal hemangioma. In one case, the resection was performed with a linear stapling device (Ethicon Linear cutter stapler) because the diverticulum was long. Prior to closing, the resected diverticulum was opened and carefully inspected to confirm the complete removal of ectopic mucosa. The intestine was relocated in the abdominal cavity, and the umbilical incision was closed with interrupted sutures, using 2-0 polyglactin to approximate the fascia.
The median age of the patients at the time of diagnosis was 5,4 years (range 6 mo-15 year). The male to female ratio was 15:4. All patients were subjected to a TULA surgical procedure. Seventeen patients underwent Meckel’s diverticulectomy. Of these, 4 had a wedge resection, 12 an intestinal resection/anastomosis with end-to-end hand sewn anastomosis and 4-0 polyglactin sutures and, in 1 patient, the diverticular resection was performed with a linear stapler device. In 2 patients, the search for an MD was unsuccessful, and an ileal duplication and jejunal hemangioma were found. In these patients, an intestinal resection with end-to-end hand sewn anastomosis was executed. In one patient, an appendectomy was performed during the same procedure because suppurative appendicitis with reactive lymphadenitis was found.
Fifteen patients had painless intestinal bleeding, while 4 had recurrent abdominal pain, and the ultrasound study showed a cyst-like structure. Five patients required blood transfusions pre-operatively. Eleven patients had a positive MS, including the patient with jejunal hemangioma. Upper and lower gastrointestinal endoscopy did not show the source of bleeding. The mean operative time was 75 min (range 40-115 min). No intra-operative or post-operative complications were recorded, except for one patient, who experienced fever 7 d after surgery that resolved with antibiotics. Histological analysis of the resected specimens confirmed heterotopic gastric mucosa (HGM) in 17 patients, which was associated with focal ulceration in four of them. In one patient, ileal duplication was confirmed, and in one case, a jejunal hemangioma was found. The patients were discharged with a medium stay of 5, 7 d (range 4-13 d). At a median follow up of 4, 5 years (range 10 mo-10 years), all patients were asymptomatic, with excellent cosmetic results and no late complications.
In recent years, advances in the field of pediatric surgery have included the development of endoscopy and minimally invasive surgery, which have changed the surgical approach to many diseases. MD is the most common congenital malformation of the intestinal tract, and most MDs are asymptomatic and found incidentally during abdominal exploration. Usually, MD has an incidence of 2%-4% and is symptomatic within the first 2 years of life. In our series, the median age at the time of diagnosis was 5, 4 years (range 6 mo-15 year). In his series, Palanivelu[11] reported an age range from 6 to 43 years. Likewise Rho et al[7] reported a range from seven days to 19 years, emphasizing that there is a wide age range because MD may be clinically silent for a long time. Therefore, a high index of suspicion is necessary for its diagnosis and treatment. Recently, a survey on the trends in the surgical management of MD in the United States has demonstrated that the laparoscopic approach has been underused and that it may improve results and cost[1]. Laparoscopy has been claimed as the first line procedure for diagnosing cases of complications due to MDs painless large intestinal bleeding[3,15]. In these cases, Tc99 scan has a reported sensitivity of 25%-92%[2]. This diagnostic technique is the best non-invasive method for pre-operative diagnosis of MD[7]. Menezes et al[2] conducted a retrospective review on the various presentations of symptomatic MD and assessed the sensitivity of the Meckel’s scan as a diagnostic tool in patients with gastrointestinal bleeding. They showed a sensitivity of 66.6% with a false negative rate of 33, 3%. In our series, MD was found during abdominal exploration in 17/19 patients, and MS was positive in 11 patients (57.8%). Interestingly, one patient with a jejunal hemangioma had a positive Tc99 scan as well. In their series of patients with positive MS, Rho et al[7] showed significant differences in complete blood count profiles compared to patients in whom the diagnosis had not been performed using MS. Therefore, they emphasize the clinical and hematological evaluation of the patient and recommend performing a Meckel’s scan in patients admitted to the hospital with lower GI bleeding, abdominal distension and/or pain and severe anemia[7]. False negative diagnosis may be due to ulcers and hemorrhage without ectopic gastric mucosa, cases with insufficient gastric mucosa to capture Tc99, and cases with a “wash out“phenomenon due to the accentuated intestinal transit[7]. The incidence of heterotopic mucosa in cases with MD varies between 15%-50%[9]. Shalaby et al[9] reported that the incidence of diverticular HGM in symptomatic patients was 77, 8%. It is reported that heterotopic mucosa in symptomatic patients is much higher than in patients with asymptomatic MD. In the symptomatic patients, ectopic mucosa is present in 61%-80% of cases and in only 13%-30% of incidental cases[9]. In our series, the incidence at histopathologic examination was 95.9%. This result could also be due to the high incidence of bleeding MD in which the HGM varies from 80% to 100%[7,9]. MS has false negative and positive rates; therefore, laparoscopy may be of great benefit for the diagnosis and planning of the surgical technique, especially in patients in whom the clinical findings are not clear. Some authors suggest a change in the diagnostic algorithm for massive and painless lower gastrointestinal bleeding in children[12,15], which is generally assumed to be the most important clinical sign[3,6]. Furthermore, gastrointestinal endoscopy cannot diagnose a MD, but we believe that it is still essential in identifying other possible sources of bleeding. In our experience, the MD can be found directly through the operative laparoscope, which is introduced through the first umbilical trocar, or a complete ileal examination may be performed extracorporeally or through the introduction of two accessory trocars.
We believe that the TULA extracorporeal small bowel exploration is easy and fast, and it allows accurate diagnosis in all cases. In patients in whom the ultrasound showed a cyst-like structure, laparoscopy and the ileal exploration showed an MD in two cases and ileal duplication in one. In the patient with jejunal hemangioma, the child presented with gastrointestinal bleeding, and the MS was positive. TULA small bowel exploration outside the abdominal cavity was performed without evidence of a diverticulum. The hemangioma was identified as a small soft palpable lesion of the bowel approximately 40 cm from the ligament of Treitz. In our opinion, intracorporeal laparoscopic small bowel exploration would not have been effective in this case, and only palpation of the bowel allowed proper diagnosis and treatment.
In all patients, the diagnosis was reached without complications or wasted time. This approach has also become our strategy when looking for a MD during an appendectomy and recurrent abdominal pain of uncertain nature[5].
Intracorporeal laparoscopy or TULA has been considered a valuable tool not only in the diagnosis but also in the treatment of MD. Laparoscopic treatment of MD was originally described by Attwood[16], who applied a staple transversally at the base of diverticulum to perform an assisted extracorporeal diverticulectomy. This procedure carries the risk of leaving heterotopic mucosal tissue, and the resection of the adjacent bowel is considered the treatment of choice today. In our series, all cases except one were treated by wedge or intestinal resection/anastomosis. In one case, an extracorporeal stapling diverticulectomy was performed.
Some authors[4] have studied the relationship between the location of gastric heterotopia and the external appearance of diverticulum for the proper choice of the surgical treatment. Their results support the data that, in long diverticula with a height-diameter ratio of more than 1, 6, the heterotopic mucosa is only located in the distal area; therefore, in these cases, simple transverse resection with a stapling device has been recommended. The contraindication for stapler excision is a very broad base or insufficient length of the diverticulum, which makes it impossible to apply the staple[11] because of the risk of leaving heterotopic tissue. Furthermore, the direction of the excision must be perpendicular to the ileum and not into the longitudinal axis to avoid any impairment of the ileal lumen. Prasad[3] suggested evaluating the HD ratio and resecting the neck of the diverticulum transversely. In addition, a frozen section of the specimen should be obtained to ensure that the stamp is free from ectopic epithelium. In their series, all patients underwent resection of the MD with a sleeve of ileum and intestinal anastomosis. This technique avoids the need for a frozen section and ensures the complete resection of the ectopic epithelium[2,3]. Furthermore, the diverticulum inspection after the resection in addition to palpation is useful in determining the complete surgical resection. Laparoscopic intraabdominal Meckel diverticulectomy has been performed with the application of Roeder’s loop on the base of the diverticulum, an endo-stapler, or intestinal resection with intra-corporeal suturing[5,6,9,11]. Therefore, after the segmental resection of MD, a side-to-side anastomosis can be performed laparoscopically but results in a longer operation, even when performed by skilled laparoscopic surgeons. We believe that extracorporeal resection and anastomosis is safe, simple and inexpensive. Moreover, it avoids the potential risk of intra-abdominal spillage. The TULA approach could be defined as the synthesis between an excellent modern diagnostic technique, laparoscopy, and the traditional surgical approach. In fact, it is possible to exteriorize the intestinal loop through the navel to palpate for the presence of ectopic mucosa at the base of the diverticulum[3] and to reconstruct the intestinal continuity outside of the abdomen with minimal risk for the patient during laparoscopy. Furthermore, the direct observation of the diverticulum and its measurement expressed in the HD Ratio could assist the choice of the surgical technique[4]. However, recommendations for the surgical treatment of Meckel’s diverticulum and its clinical sequelae come from case reports and single-institution case series. Therefore, we cannot confirm that the trans-umbilical laparoscopic-treatment of Meckel’s diverticulum is better than the conventional approach because we performed a retrospective study. However, the laparoscopic approach has the advantage of combining diagnosis and treatment, making it effective and suitable for the patient. This question would best be answered using a prospective, multi-institutional study.
An important issue of this technique is the creation of an adequate fascial opening during the open approach that allows easy exteriorization of the intestine for diagnosis and possible excision of the MD, facilitates the replacement of intestine into the abdomen, and prevents serious bleeding, congestion and edema of the intestine.
The use of an operative laparoscope could be difficult because of the axial movement of the telescope[5], which makes the intracorporeal manipulation and exploration of the intestine challenging in some patients. This difficulty can be avoided through the extracorporeal small bowel exploration or the introduction of an accessory trocar of three or five mm to grasp the terminal ileum and the diverticulum.
The most common complication after a Meckel’s diverticulectomy is adhesive intestinal obstruction, which occurs in 5%-10% of the patients[3,6]. The main symptom is gastrointestinal bleeding; these complications generally occur in patients with ischemic and congested intestine[3]. Chan et al[12] presented a series of 20 patients with complicated MD and reported that, after laparoscopy, none of the patients were readmitted for rebleeding or adhesive intestinal obstruction. In our series, no complications were registered at follow-up except for one patient, who developed a fever one week after surgery.
In conclusion, laparoscopy must be considered as the technique of choice in the management of MD because it permits the easy diagnosis and identification of the diverticulum, and it is fundamental in surgical planning, even in an emergency situation. Trans-umbilical laparoscopic-assisted diverticulectomy is safe and effective in the management of MDs.
Meckel’s diverticulum (MD) is one of the most common malformations of the small intestine (2%-4%). In most cases it is clinically silent and found incidentally during abdominal exploration for other pathologies. Its presence is related to an obliteration defect of the omphalomesenteric duct, and for this reason it is localized on the antimesenteric border of the ileum, up to 100 cm from the ileocecal valve. When the MD is symptomatic, it may be responsible for severe episodes of intestinal bleeding, intussusception, bowel obstruction, or recurrent abdominal pain with repeated vomiting and/or nausea.
Pediatric laparoscopy has been widely adopted in the treatment of many intra-abdominal diseases and it has given satisfactory results. Advances in laparoscopy have significantly aided the diagnosis and surgical treatment of MD, with excellent cosmetic results, reduction in hospitalization and improved post-operative pain. The authors experience highlights the benefit of trans-umbilical laparoscopic assisted approach (TULA).
The TULA approach could be defined as the synthesis between an excellent modern diagnostic technique, laparoscopy, and the traditional surgical approach. In fact, it allows to exteriorize the intestinal loop through the navel, to palpate for the presence of ectopic mucosa at the base of the diverticulum and to reconstruct the intestinal continuity outside of the abdomen with minimal risk for the patient. This technique has the advantage of combining diagnosis and treatment, making it effective and suitable for the patient.
Trains-umbilical laparoscopic-assisted diverticulectomy is safe and effective in the management of MD and could be considered as a treatment of choice in pediatric patients.
TULA Meckel’s diverticulectomy is a surgical technique that allows to exteriorize the diverticulum through the navel and to perform the diverticulectomy outside of the abdominal cavity.
Scintigraphy with Tc-99m pertechnetate MS is a diagnostic technique useful for the research of ectopic gastric mucosa, because pertechnetate is actively accumulated and secreted by the mucoid cells of the gastric mucosa but has false positive and negative rate.
The incorporeal anastomosis has any significant better results in the management of these patients.
P- Reviewers: Flessas II, Yao YM S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Ruscher KA, Fisher JN, Hughes CD, Neff S, Lerer TJ, Hight DW, Bourque MD, Campbell BT. National trends in the surgical management of Meckel’s diverticulum. J Pediatr Surg. 2011;46:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Menezes M, Tareen F, Saeed A, Khan N, Puri P. Symptomatic Meckel’s diverticulum in children: a 16-year review. Pediatr Surg Int. 2008;24:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Prasad TR, Chui CH, Jacobsen AS. Laparoscopic-assisted resection of Meckel’s diverticulum in children. JSLS. 2006;10:310-316. [PubMed] |
| 4. | Mukai M, Takamatsu H, Noguchi H, Fukushige T, Tahara H, Kaji T. Does the external appearance of a Meckel’s diverticulum assist in choice of the laparoscopic procedure? Pediatr Surg Int. 2002;18:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Cobellis G, Cruccetti A, Mastroianni L, Amici G, Martino A. One-trocar transumbilical laparoscopic-assisted management of Meckel’s diverticulum in children. J Laparoendosc Adv Surg Tech A. 2007;17:238-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Sai Prasad TR, Chui CH, Singaporewalla FR, Ong CP, Low Y, Yap TL, Jacobsen AS. Meckel’s diverticular complications in children: is laparoscopy the order of the day? Pediatr Surg Int. 2007;23:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Rho JH, Kim JS, Kim SY, Kim SK, Choi YM, Kim SM, Tchah H, Jeon IS, Son DW, Ryoo E. Clinical Features of Symptomatic Meckel’s Diverticulum in Children: Comparison of Scintigraphic and Non-scintigraphic Diagnosis. Pediatr Gastroenterol Hepatol Nutr. 2013;16:41-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Rashid OM, Ku JK, Nagahashi M, Yamada A, Takabe K. Inverted Meckel’s diverticulum as a cause of occult lower gastrointestinal hemorrhage. World J Gastroenterol. 2012;18:6155-6159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Shalaby RY, Soliman SM, Fawy M, Samaha A. Laparoscopic management of Meckel’s diverticulum in children. J Pediatr Surg. 2005;40:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Tam YH, Lee KH, Sihoe JD, Chan KW, Cheung ST, Pang KK. Initial experience in children using conventional laparoscopic instruments in single-incision laparoscopic surgery. J Pediatr Surg. 2010;45:2381-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Palanivelu C, Rangarajan M, Senthilkumar R, Madankumar MV, Kavalakat AJ. Laparoscopic management of symptomatic Meckel’s diverticula: a simple tangential stapler excision. JSLS. 2008;12:66-70. [PubMed] |
| 12. | Chan KW, Lee KH, Mou JW, Cheung ST, Tam YH. Laparoscopic management of complicated Meckel’s diverticulum in children: a 10-year review. Surg Endosc. 2008;22:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Martino A, Zamparelli M, Cobellis G, Mastroianni L, Amici G. One-trocar surgery: a less invasive videosurgical approach in childhood. J Pediatr Surg. 2001;36:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Cobellis G, Torino G, Noviello C, Cruccetti A, Mastroianni L, Amici G, Martino A. Versatility of one-trocar surgery in children. J Laparoendosc Adv Surg Tech A. 2011;21:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Loh DL, Munro FD. The role of laparoscopy in the management of lower gastro-intestinal bleeding. Pediatr Surg Int. 2003;19:266-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Attwood SE, McGrath J, Hill AD, Stephens RB. Laparoscopic approach to Meckel’s diverticulectomy. Br J Surg. 1992;79:211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |