Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8102
Revised: March 31, 2014
Accepted: April 28, 2014
Published online: July 7, 2014
Processing time: 226 Days and 10.9 Hours
Thyroid hormones are totally involved in the regulation of body weight, lipid metabolism, and insulin resistance. Therefore it is anticipated that thyroid hormones may have a role in the pathogenesis of non alcoholic fatty liver disease (NAFLD) and non alcoholic steatohepatitis (NASH). In this study, we reviewed the current literature on the association between thyroid dysfunction and NAFLD/NASH. A search for English language medical literature reporting an association between thyroid dysfunction and NAFLD/NASH in humans was conducted across PubMed, ISI Web of Science, and Scopus in August, 2013. Out of 140 studies initially identified through the search, 11 relevant articles were included in the final review. Thyroid dysfunctions in the form of overt or subclinical hypothyroidism are prevalent among patients with NAFLD/NASH. Hypothyroidism appears to be an independent risk factor for NAFLD/NASH in some studies; however, other newly published studies failed to find such an association. The results of the studies on the role of thyroid abnormalities in NAFLD/NASH are inconsistent, and further research is recommended to determine the relationship between hypothyroidism and NAFLD/NASH and the underlying mechanisms.
Core tip: Thyroid hormones help regulate of body weight, lipid metabolism, and insulin resistance. Therefore, thyroid hormones may have a role in the pathogenesis of non alcoholic fatty liver disease (NAFLD) and non alcoholic steatohepatitis (NASH). Following a systematic review of human studies in the English language medical literature, eleven articles were found to address this relationship. Hypothyroidism appears to be an independent risk factor for NAFLD/NASH in some studies; however, other studies failed to find such an association. As the results of these studies are not consistent, further research is recommended to determine the relationship hypothyroidism and NAFLD/NASH and the underlying mechanisms.
- Citation: Eshraghian A, Jahromi AH. Non-alcoholic fatty liver disease and thyroid dysfunction: A systematic review. World J Gastroenterol 2014; 20(25): 8102-8109
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8102.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8102
Non alcoholic fatty liver disease (NAFLD) represents a broad clinical spectrum ranging from simple fatty liver to non alcoholic steatohepatitis (NASH), which may progress to liver fibrosis, cirrhosis and hepatocellular carcinoma[1]. NAFLD is a rapidly growing diagnosis, and it is the most common cause of abnormal liver function tests worldwide[2]. The growing pattern of NAFLD prevalence is generally attributed to a global increase in the prevalence of obesity and other metabolic risk factors[3]. Advanced age and metabolic disorders, such as diabetes type 2, impaired glucose tolerance, and central obesity, are among the risk factors for NAFLD[4-6]. Cryptogenic cirrhosis is a term used for those patients with liver cirrhosis who lack any identifiable viral, alcoholic, autoimmune or drug-related cause of the condition. Many clinicians now believe that a considerable number of these patients have cirrhosis due to NASH[7]. Considering the increasing incidence of NAFLD/NASH, especially in developed and developing countries, it is anticipated that cirrhosis due to these conditions may surpass other causes of cirrhosis in a near future. Therefore, understanding the pathophysiology, risk factors and new treatment options of NAFLD/NASH should be among the priorities in the field of hepatology.
Endocrine hormones are generally involved in cell metabolism, regulation of energy expenditure and fat distribution in the human body and thereby play an important role in the development of metabolic abnormalities. The thyroid gland is significantly involved in energy homeostasis, lipid and carbohydrate metabolism, regulation of body weight and adipogenesis[8,9]. In a clinical setting, subclinical hypothyroidism has been associated with metabolic syndrome, cardiovascular mortality and disturbance of lipid metabolism[10,11]. In recent years, growing body of evidence has led to speculation on the association between NAFLD/NASH and thyroid dysfunction. Herein, we review current English language medical literature on the association between NAFLD/NASH and thyroid dysfunction, and on the proposed underlying mechanisms of this relationship.
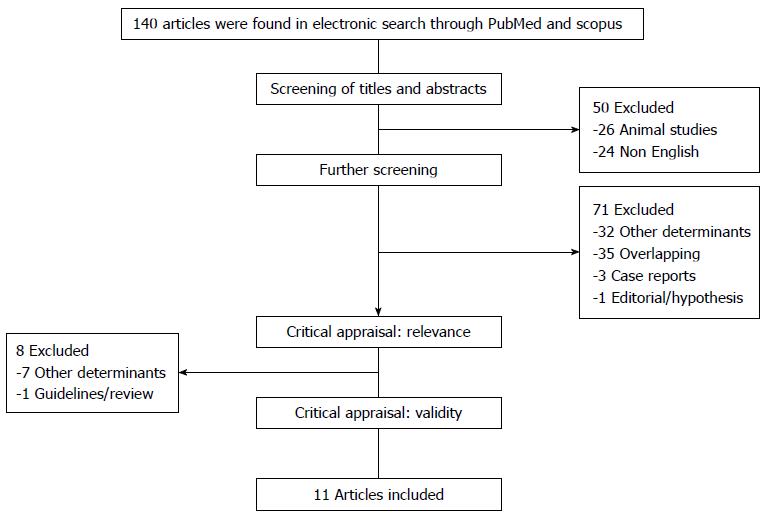
The study was conducted using the PRISMA (Preferred reporting items for systematic review and meta-analyses) guidelines, flow diagram and checklist[12]. A computerized English language literature search of ISI Web of Science, PubMed and Scopus was performed in August 2013. No time limitation was applied, and studies on animal models were excluded. After a preliminary search in the Medical Subject Headings database, search terms were selected. The terms “non alcoholic fatty liver disease” and “thyroid”, “NAFLD” and “thyroid”, “non alcoholic steatohepatitis” and “thyroid”, and “NASH” and “thyroid” were applied as keywords in the titles and/or abstracts.
All studies were reviewed and carefully appraised for inclusion in this review. All descriptive or analytical cross-sectional studies, case-control studies, and clinical trials with proper methods for assessment of NAFLD and NASH and that evaluated thyroid function were included. Additionally, only studies that used either ultrasonography or liver biopsy for the assessment of NAFLD were included. Editorials, case reports, letters to the editor, hypotheses, studies on animals or cell lines, abstracts from conferences or unpublished reports were excluded. Studies on patients with liver disease other than NAFLD/NASH or on NAFLD/NASH in the context of other liver diseases i.e., chronic viral hepatitis, liver cirrhosis, acute liver failure, hepatocellular carcinoma or drug-induced hepatitis were excluded (Figure 1). Studies on the pediatric age group (age < 18 years) were also excluded.
Data were extracted from the full text of relevant articles. Relevant data from articles reporting thyroid hormone abnormalities and NAFLD/NASH were outlined in a table.
The final literature search returned 140 articles, of which 84 were reviewed and appraised for relevance and validity. After excluding studies with other determinants, studies that were not representative of our aims, and the case reports, editorials, overlapping studies, 11 studies[13-23] were included, and the results were organized into Table 1. No new articles were found by reviewing the cited references of the included studies. A flow chart showing detailed search results and the identification of eligible studies is outlined in Figure 1.
| Study | Design | Number | Method for diagnosis of NAFLD/NASH | Definition of thyroid dysfunction | Main Findings |
| Chung et al[13] | Cross-sectional | 4648 (2324 Hypothyroidism vs 2324 euthyroidism) | Ultrasonography | Subclinical hypothyroidism: TSH > 4.1 mIU/L and normal fT | Prevalence of NAFLD increased with severity of hypothyroidism (subclinical: 29.9%, overt: 36.3%) |
| Overt hypothyroidism: TSH > 4.1 mIU/L and fT4 < 0.7 ng/Dl | Prevalence of NAFLD plus elevated ALT was higher in patients with hypothyroidism (P < 0.001) | ||||
| Hypothyroidism is an independent risk factor for increased prevalence of NAFLD (OR = 1.38, 95%CI: 1.17-1.67) | |||||
| Liangpunsakul et al[14] | Case-control | 174 NASH patients compared with 442 controls | Liver biopsy Liver biopsy | Previous history of hypothyroidism on T4 replacement therapy | Prevalence of hypothyroidism was 15 % compared to 7.2% in controls (P < 0.001) |
| In multivariate analysis, hypothyroidism was more prevalent than controls (OR = 2.3, 95%CI: 1.2-4.2, P = 0.008) | |||||
| Silveira et al[15] | Cross-sectional | 97 patients with NAFLD Compared with 67 PBC, and 79PSC | Liver biopsy | TSH > 5 mIU/L or < 0.3 mIU/L | The prevalence of hypothyroidism in patients with NAFLD was 20% |
| Total thyroxine> 12.5 μg/dL or < 5 μg/dL | Five patients had hyperthyroidism in NAFLD group | ||||
| History of hyper/hypo thyroidism | The prevalence of thyroid dysfunction was not different in three group | ||||
| Pagadala et al[16] | Cross-sectional | 233 patients with NAFLD Compared to 430 controls | Liver biopsy | Clinical diagnosis of hypothyroidism and on thyroid replacement therapy | The prevalence of hypothyroidism was higher in NAFLD patients compared to controls (21.1% vs 9.5%, P < 0.001) |
| Hypothyroidism was more common in NASH compared to patients without NASH (P = 0.03) | |||||
| Xu et al[17] | Cross-sectional | 227 patients with NAFLD Compared with 651 controls | Ultrasonography | TSH > 4.5 mIU/L or < 0.5 mIU/L | Patients with lower FT4 or higher TSH are more likely to develop NAFLD (P < 0.001) |
| fT4 > 14.4 pmol/L or < 7.85 pmol/L | in logistic regression analysis Ft4 was a risk factor for NAFLD (OR = 0.847, 95%CI: 0.743-0.966) | ||||
| Mazo et al[18] | Retrospective | 33 patients with steatosis Compared with 70 NASH patients | Liver biopsy | History of hypothyroidism on thyroid replacement therapy | Prevalence of hypothyroidism was 15.5% in NAFLD (15.2% in steatosis and 15.7% in NASH) |
| In multivariate analysis insulin, HOMA index and AST were correlated with hypothyroidism | |||||
| No direct association between NASH and hypothyroidism | |||||
| Moustafa et al[19] | Cross-sectional | 90 patients with NASH, Chronic HCV, HCV cirrhosis compared to 20 healthy controls | Ultrasonography | Only determined thyroid hormone without normal range | The serum TSH level in NASH patients was higher than healthy controls (2.1 ± 0.75 μIU/mL vs 1.75 ± 0.9 μIU/mL |
| Carulli et al[20] | Cross-sectional | 69 NAFLD, 25 steatosis, 44 NASH | Liver biopsy | Normal range: TSH: 0.35-4.5 μIU/mL | TSH level was significantly higher in NASH compared to steatosis group |
| FT4: 6.1-16.6 pg/mL; FT3: 1.7-4.2 pg/mL | TSH level was an independent positive risk factor for NASH in logistic regression analysis (OR = 2.34, 95%CI: 1.15-4.776) | ||||
| Zhang et al[21] | Cross-sectional | 1322 participants including 266 patients with NAFLD | Ultrasonography | Normal TSH range: 0.71-6.25 mIU/mL | In female patient with NAFLD serum TSH level was significantly higher than controls (P < 0.05) |
| In logistic regression analysis TSH level was not an independent risk factor for NAFLD | |||||
| Ittermann et al[22] | Cross-sectional | 3661 healthy appearing participants | Ultrasonography | Thyroid hormone and TSH below or Above normal range | Low FT4 concentrations are associated with hepatic steatosis |
| No consistent association between TSH and hepatic steatosis | |||||
| No association between hyper- or hypothyroidism and hepatic steatosis | |||||
| Eshraghian et al[23] | Cross-sectional | 832 healthy appearing participants | Ultrasonography | Normal TSH range: 0.2- 5.2 mIU/mL | No association between hyper- or hypothyroidism and NAFLD |
| -FT4: 11.5-23 pmol/L | No association between thyroid autoimmunity and NAFLD | ||||
| The diagnosis of NAFLD was higher among low TSH group | |||||
| The thyroid hormone abnormalities may be due to sick euthyroid syndrome |
The main characteristics and findings of the studies were summarized in Table 1. The 11 articles included in this review were published between 2003 and 2013. Three studies were done in the United States[14-16], two in China[17,21] and one each in Egypt[19], South Korea[13], Italy[20], Brazil[18], Germany[22] and Iran[23]. The eleven studies included 12924 participants when combined. Ten studies[13,15-23] were cross- sectional, and one study used case-control design[14]. In six studies[13,17,19,21-23]. NAFLD was diagnosed based on ultrasonographic criteria and the absence of evidence of viral, autoimmune or alcohol- or drug-induced liver disease. Five other studies[14,15,16,18,20] used liver biopsy and the above criteria for diagnosis of NAFLD/NASH. Thyroid dysfunction was defined as a history of present thyroid disease and thyroid replacement therapy in three studies[14,16,18] while eight studies checked the thyroid hormone profile of all patients. The methods used to check the thyroid hormone profile was not uniform across studies. The thyroid hormone profile was checked using immunoradiometric assay (n = 2), solid phase enzyme linked immunosorbent assay (n = 1) and chemilumiscence enzyme immunoassay (n = 4). One study did not mention the laboratory method used to check thyroid hormone levels.
Participants in the included studies were adults (> 18 years) and of both sexes. One study was conducted in elderly euthyroid subjects attending their annual routine health examination[17]. Eight studies had apparently healthy individuals as control groups[13,14,16,17,19,21-23]. Participants in five studies came from the general population that were seen for routine a health check-up[13,17,21-23]. Participants in other studies were mainly collected from hepatology clinics.
One study investigated thyroid dysfunction in NAFLD patients and compared this group with patients with primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC), but did not compare them to healthy controls[15]. Another study compared hypothyroidism in patients with simple steatosis and patients with NASH to find a relationship between thyroid dysfunction and severity of fatty infiltration of the liver[18].
Study populations were divided based on presence or absence of NAFLD/NASH or hypothyroidism. Among the 11 studies, three studies reported no association between hypothyroidism and NASH[18,22,23]. In 2 studies hypothyroidism was an independent risk factor for developing NAFLD/NASH[13,14]. Three studies indicated that lower free T4 (FT4) was an independent risk factor for NAFLD[13,17,22]. One study showed that increased serum levels of thyroid stimulating hormone (TSH) is an independent risk factor for NASH[20].
This review summarized the evidence for an association between thyroid dysfunction and NAFLD/NASH. To the best of the author’s knowledge, this is the first systematic review of clinical studies on this topic. As outlined in table there is growing data about higher prevalence of thyroid dysfunction in the form of overt or subclinical hypothyroidism among patients with NAFLD/NASH. The prevalence of hypothyroidism was reported to range from 15.2 % to 36.3 % among patients with NAFLD/NASH. Several studies that used healthy controls showed a significantly higher prevalence of hypothyroidism in patients with NAFLD/NASH compared to the controls. Several studies demonstrated that hypothyroidism is an independent risk factor for NAFLD. This indicates that hypothyroidism may directly result in NAFLD irrespective of other metabolic risk factors. Considering the results of these studies, hypothyroidism may be added to risk factors of NAFLD/NASH.
The other question with multiple diagnostic and therapeutic implications is whether hypothyroidism can predict the severity of fatty liver i.e., presence of steatohepatitis. Three studies tried to answer this question with conflicting results. Chung et al[13], in their population based study, evaluated a relatively large numbers of healthy individuals and showed that prevalence of NAFLD plus elevated alanine aminotransferase (ALT) was higher in patient with hypothyroidism. An increased serum ALT level is a surrogate biomarker for NAFLD in the absence of other causes of liver disease and an indicator for the development of diabetes, cardiovascular disease and long term adverse complications from metabolic syndrome. Therefore, this study confirms the association between the severity of NAFLD and hypothyroidism.
Pagadala et al[16] reported that hypothyroidism was more common in patients with NASH compared to patients with NAFLD. This finding remained statistically significant after adjusting for other variables including age, diabetes, dyslipidemia and hypertension but not gender. This study, which used liver biopsy and the NAFLD activity score to distinguish NASH from NAFLD, provides additional evidence for the association between the severity of liver fatty infiltration and hypothyroidism. Contrary to these 2 studies, results of the study by Mazo and co-workers did not show a statistically significant association between hypothyroidism, simple steatosis and NASH[18]. Furthermore, two other recently published studies of healthy participants, also confirmed the Mazo et al findings[22,23]. We have recently shown that thyroid hormone abnormalities in patients with NAFLD may simply be due to alterations in thyroid hormones occurring in non-thyroidal illnesses[23]. Consequently we must still be conservative about reporting any contribution of hypothyroidism to NAFLD/NASH. Carulli et al[20] reported that an increased serum TSH level is an independent risk factor for NASH compared to patients with NAFLD.
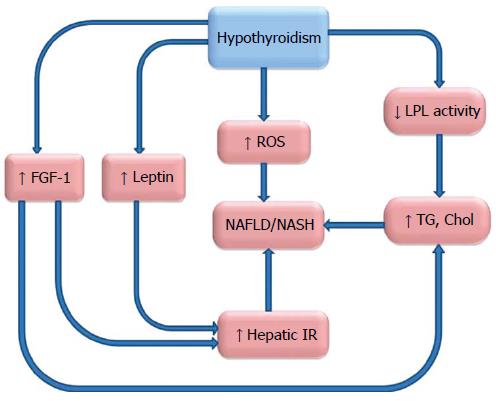
NAFLD/NASH is supposed to be a hepatic feature of metabolic syndrome and insulin resistance[24,25]. Hypothyroidism has been reported to be associated with obesity and metabolic syndrome[26-28]. Insulin resistance in the context of hypothyroidism and its alleviation with treatment of the hypothyroidism has been reported[29]. Hypothyroidism was more prevalent in patients with type 2 diabetes and in some reports was associated with diabetic microangiopathy[30,31]. Associations of hypothyroidism with these metabolic abnormalities, which are frequently accompanied by NAFLD/NASH, strengthen the notion of association between hypothyroidism and NAFLD/NASH. While the underlying pathophysiology for this association is still not clear, several mechanisms have been proposed. The role of adipocytokines in NAFLD[32,33] has been established previously, and some studies aimed to find a relationship between adipocytokines and hypothyroidism to clarify the mechanism of thyroid dysfunction and NAFLD. These studies failed to find an association between serum levels of adiponectin and hypothyroidism[34,35]. However, results of these studies were controversial for visfatin, an adipocytokine involved in energy homeostasis[36,37]. An increased level of leptin has been identified in patients with hypothyroidism, and it may be responsible for the development of NAFLD/NASH in this context[38,39]. Leptin is an adipocytokine involved in the regulation of appetite, with an increased level seen in cases of obesity, can induce collagen synthesis in the liver and promotes hepatic insulin resistance[40-42].
Patients with NAFLD/NASH have abnormal lipid profiles notable for elevated cholesterol, low density lipoproteine and triglyceride levels[43]. Thyroid hormones induce their effects on lipid metabolism via thyroid hormone receptor β, which is expressed in liver[44]. Thyroid hormone receptor activation results in a reduction in body weight and fat as well as a decrease in cholesterol and triglyceride levels, which takes place only in hepatocytes[45,46].
Cable et al[47] showed that liver steatosis will reduce after treatment of animal models with liver-targeted thyroid hormone receptor agonist. Furthermore, hypothyroidism and elevated TSH result in diminished hepatic lipoproteine lipase activity and cause elevated serum triglyceride levels[48,49].
The role of Fibroblast growth factor-21 (FGF-21) has been recently proposed in NAFLD/NASH[50]. FGF-21 is a member of endocrine FGF family that has several hormone like activities[51]. FGF-21 induces glucose uptake in mouse and human adipocytes, which can improve glucose homeostasis after administration to obese mice[52,53]. Improved insulin sensitivity, glucose clearance and reduced plasma triglyceride level have been observed in transgenic mice with over expression of FGF-21 in the liver[54]. Increased serum levels of FGF-21 in NAFLD have been described in several human studies[55-57], indicating a relative FGF-21 resistance in these patients[58].
Adams et al[59] have recently shown that administration of tri-iodothyroine (T3) to mice can induce specific hepatic expression of FGF-21 in a dose dependent manner[59]. An increased plasma level of FGF-21 in patients with hypothyroidism has been also observed in a recent study by Lee and co-workers[60]. These findings together are indicative of FGF-21 pathway in NAFLD, although the precise mechanism remains to be elucidate.
The other theory is based on hepatic damage through mitochondrial dysfunction, oxidative stress and reactive oxygen species (ROS) production. Oxidative stress as a phenomenon refers to the aerobic nature of cellular metabolism in which a redox imbalance between antioxidants and pro-oxidants occurs in favor of pro oxidants[61]. Mitochondria are the principle organelle for oxidative reactions like β- oxidation, and they are a main source of ROS[62]. Free fatty acids (FFA) undergo β- oxidation in the mitochondria in physiologic conditions. With excessive accumulation of FFA in the hepatocytes, there is excessive oxidation of FFA in mitochondria, microsomes and peroxisomes leading to overproduction of ROS[63]. ROS activates lipid peroxidation that is accompanied by activation of Kupffer cells and hepatic satellite cells[64]. Kupffer cells induce secretion of inflammatory cytokines like tumor necrosis factor alpha and transforming growth factor β that both activate hepatic satellite cells and promote fibrosis[65,66]. Previous studies described increased markers of oxidative stress such as serum malondialdehyde in hypothyroidism[67]. Elevated serum markers of oxidative stress have been observed in Hashimoto’s thyroiditis patients with hypothyroidism[68,69].
A summary of possible underlying mechanisms is outlined in Figure 2. Despite the growing evidence of an association between hypothyroidism and NAFLD, drawing a firm conclusion is going to be difficult.
Results of current studies are conflicting about the association between thyroid abnormalities and NAFLD/NASH. Results of some of the reviewed studies propose hypothyroidism as a risk factor for NAFLD/NASH. This may lead us to test thyroid hormone profiles as a part of initial clinical assessment in patients with NAFLD/NASH. As hypothyroidism is a modifiable risk factor and can easily be treated with thyroid replacement therapy, the interesting issue for future studies will be to evaluate whether treatment of hypothyroidism in patients with NAFLD/NASH will improve disease progression and outcome. So far some studies have failed to show an association between hypothyroidism and NAFLD. None of the present studies are interventional trials and placebo controlled clinical trials should be conducted to further elucidate the issue.
P- Reviewers: Hashimoto N, Jutavijittum P, Narciso-Schiavon JL, Zhong JH S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Law K, Brunt EM. Nonalcoholic fatty liver disease. Clin Liver Dis. 2010;14:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [PubMed] |
| 3. | Day CP. Non-alcoholic fatty liver disease: a massive problem. Clin Med. 2011;11:176-178. [PubMed] |
| 4. | Amarapurkar D, Kamani P, Patel N, Gupte P, Kumar P, Agal S, Baijal R, Lala S, Chaudhary D, Deshpande A. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161-163. [PubMed] |
| 5. | Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, Hardies J, Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Yamada T, Fukatsu M, Suzuki S, Wada T, Yoshida T, Joh T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J Gastroenterol Hepatol. 2010;25:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Caldwell SH, Lee VD, Kleiner DE, Al-Osaimi AM, Argo CK, Northup PG, Berg CL. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:346-352. [PubMed] |
| 8. | Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, Psyrogiannis AI, Kalfarentzos FE, Kyriazopoulou VE. Thyroid function in humans with morbid obesity. Thyroid. 2006;16:73-78. [PubMed] |
| 9. | Raftopoulos Y, Gagné DJ, Papasavas P, Hayetian F, Maurer J, Bononi P, Caushaj PF. Improvement of hypothyroidism after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2004;14:509-513. [PubMed] |
| 10. | Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 828] [Cited by in RCA: 774] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 11. | Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S109-S112. [PubMed] |
| 12. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11038] [Article Influence: 689.9] [Reference Citation Analysis (0)] |
| 13. | Chung GE, Kim D, Kim W, Yim JY, Park MJ, Kim YJ, Yoon JH, Lee HS. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non-alcoholic steatohepatitis? J Clin Gastroenterol. 2003;37:340-343. [PubMed] |
| 15. | Silveira MG, Mendes FD, Diehl NN, Enders FT, Lindor KD. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int. 2009;29:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Pagadala MR, Zein CO, Dasarathy S, Yerian LM, Lopez R, McCullough AJ. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Xu C, Xu L, Yu C, Miao M, Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clin Endocrinol (Oxf). 2011;75:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Mazo DF, Lima VM, Stefano JT, Rabelo F, Faintuch J, Oliveira CP. Gluco-lipidic indices in treated hypothyroidism associated with nonalcoholic fatty liver disease. Arq Gastroenterol. 2011;48:186-189. [PubMed] |
| 19. | Moustafa AH, Ali EM, Mohamed TM, Abdou HI. Oxidative stress and thyroid hormones in patients with liver diseases. Eur J Intern Med. 2009;20:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Carulli L, Ballestri S, Lonardo A, Lami F, Violi E, Losi L, Bonilauri L, Verrone AM, Odoardi MR, Scaglioni F. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern Emerg Med. 2013;8:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Zhang J, Sun H, Chen L, Zheng J, Hu X, Wang S, Chen T. Relationship between serum TSH level with obesity and NAFLD in euthyroid subjects. J Huazhong Univ Sci Technolog Med Sci. 2012;32:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Ittermann T, Haring R, Wallaschofski H, Baumeister SE, Nauck M, Dörr M, Lerch MM, Meyer zu Schwabedissen HE, Rosskopf D, Völzke H. Inverse association between serum free thyroxine levels and hepatic steatosis: results from the Study of Health in Pomerania. Thyroid. 2012;22:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Eshraghian A, Dabbaghmanesh MH, Eshraghian H, Fattahi MR, Omrani GR. Nonalcoholic fatty liver disease in a cluster of Iranian population: thyroid status and metabolic risk factors. Arch Iran Med. 2013;16:584-589. [PubMed] |
| 24. | Yilmaz Y. NAFLD in the absence of metabolic syndrome: different epidemiology, pathogenetic mechanisms, risk factors for disease progression? Semin Liver Dis. 2012;32:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Collantes RS, Ong JP, Younossi ZM. The metabolic syndrome and nonalcoholic fatty liver disease. Panminerva Med. 2006;48:41-48. [PubMed] |
| 26. | Pearce EN. Thyroid hormone and obesity. Curr Opin Endocrinol Diabetes Obes. 2012;19:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Pacifico L, Anania C, Ferraro F, Andreoli GM, Chiesa C. Thyroid function in childhood obesity and metabolic comorbidity. Clin Chim Acta. 2012;413:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I, Satterfield S, Newman AB, Bauer DC. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf). 2012;76:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Kowalska I, Borawski J, Nikołajuk A, Budlewski T, Otziomek E, Górska M, Strączkowski M. Insulin sensitivity, plasma adiponectin and sICAM-1 concentrations in patients with subclinical hypothyroidism: response to levothyroxine therapy. Endocrine. 2011;40:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Smithson MJ. Screening for thyroid dysfunction in a community population of diabetic patients. Diabet Med. 1998;15:148-150. [PubMed] |
| 31. | Díez JJ, Iglesias P. Subclinical hyperthyroidism in patients with type 2 diabetes. Endocrine. 2012;42:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412-421. [PubMed] |
| 33. | Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Rabbione L, Premoli A. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42:1175-1183. [PubMed] |
| 34. | Altinova AE, Törüner FB, Aktürk M, Bukan N, Cakir N, Ayvaz G, Arslan M. Adiponectin levels and cardiovascular risk factors in hypothyroidism and hyperthyroidism. Clin Endocrinol (Oxf). 2006;65:530-535. [PubMed] |
| 35. | Aragão CN, Souza LL, Cabanelas A, Oliveira KJ, Pazos-Moura CC. Effect of experimental hypo- and hyperthyroidism on serum adiponectin. Metabolism. 2007;56:6-11. [PubMed] |
| 36. | Ozkaya M, Sahin M, Cakal E, Yuzbasioglu F, Sezer K, Kilinc M, Imrek SS. Visfatin plasma concentrations in patients with hyperthyroidism and hypothyroidism before and after control of thyroid function. J Endocrinol Invest. 2009;32:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 37. | Caixàs A, Tirado R, Vendrell J, Gallart L, Megía A, Simón I, Llauradó G, González-Clemente JM, Giménez-Palop O. Plasma visfatin concentrations increase in both hyper and hypothyroid subjects after normalization of thyroid function and are not related to insulin resistance, anthropometric or inflammatory parameters. Clin Endocrinol (Oxf). 2009;71:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Leonhardt U, Ritzel U, Schäfer G, Becker W, Ramadori G. Serum leptin levels in hypo- and hyperthyroidism. J Endocrinol. 1998;157:75-79. [PubMed] |
| 39. | Kautzky-Willer A, Ludwig C, Nowotny P, Roden A, Huemer C, Widhalm K, Vierhapper H, Waldhäusl W, Roden M. Elevation of plasma leptin concentrations in obese hyperinsulinaemic hypothyroidism before and after treatment. Eur J Clin Invest. 1999;29:395-403. [PubMed] |
| 40. | Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity (Silver Spring). 2010;18:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762-771. [PubMed] |
| 42. | Benomar Y, Wetzler S, Larue-Achagiotis C, Djiane J, Tomé D, Taouis M. In vivo leptin infusion impairs insulin and leptin signalling in liver and hypothalamus. Mol Cell Endocrinol. 2005;242:59-66. [PubMed] |
| 43. | Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009;48:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 533] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 44. | Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biol Rev Camb Philos Soc. 2000;75:519-631. [PubMed] |
| 45. | Grover GJ, Mellström K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennström B. Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA. 2003;100:10067-10072. [PubMed] |
| 46. | Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA. 2007;104:15490-15495. [PubMed] |
| 47. | Cable EE, Finn PD, Stebbins JW, Hou J, Ito BR, van Poelje PD, Linemeyer DL, Erion MD. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Brenta G, Berg G, Arias P, Zago V, Schnitman M, Muzzio ML, Sinay I, Schreier L. Lipoprotein alterations, hepatic lipase activity, and insulin sensitivity in subclinical hypothyroidism: response to L-T(4) treatment. Thyroid. 2007;17:453-460. [PubMed] |
| 50. | Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab. 2012;97:2143-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Kharitonenkov A, Shanafelt AB. FGF21: a novel prospect for the treatment of metabolic diseases. Curr Opin Investig Drugs. 2009;10:359-364. [PubMed] |
| 52. | Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774-781. [PubMed] |
| 53. | Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 950] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 54. | Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627-1635. [PubMed] |
| 55. | Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 56. | Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C, Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Yan H, Xia M, Chang X, Xu Q, Bian H, Zeng M, Rao S, Yao X, Tu Y, Jia W. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 2011;6:e24895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Samson SL, Sathyanarayana P, Jogi M, Gonzalez EV, Gutierrez A, Krishnamurthy R, Muthupillai R, Chan L, Bajaj M. Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia. 2011;54:3093-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Adams AC, Astapova I, Fisher FM, Badman MK, Kurgansky KE, Flier JS, Hollenberg AN, Maratos-Flier E. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARalpha-dependent manner. J Biol Chem. 2010;285:14078-14082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Lee Y, Park YJ, Ahn HY, Lim JA, Park KU, Choi SH, Park do J, Oh BC, Jang HC, Yi KH. Plasma FGF21 levels are increased in patients with hypothyroidism independently of lipid profile. Endocr J. 2013;60:977-983. [PubMed] |
| 61. | D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813-824. [PubMed] |
| 62. | Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6484] [Cited by in RCA: 6093] [Article Influence: 380.8] [Reference Citation Analysis (0)] |
| 63. | Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 719] [Article Influence: 55.3] [Reference Citation Analysis (1)] |
| 64. | Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194:1044-1050. [PubMed] |
| 65. | Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol. 2008;103:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851-857. [PubMed] |
| 67. | Torun AN, Kulaksizoglu S, Kulaksizoglu M, Pamuk BO, Isbilen E, Tutuncu NB. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin Endocrinol (Oxf). 2009;70:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Öztürk Ü, Vural P, Özderya A, Karadağ B, Doğru-Abbasoğlu S, Uysal M. Oxidative stress parameters in serum and low density lipoproteins of Hashimoto’s thyroiditis patients with subclinical and overt hypothyroidism. Int Immunopharmacol. 2012;14:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 69. | Baskol G, Atmaca H, Tanriverdi F, Baskol M, Kocer D, Bayram F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp Clin Endocrinol Diabetes. 2007;115:522-526. [PubMed] |