INTRODUCTION
Cirrhosis is the result of the progression of many forms of necroinflammatory liver diseases leading to fibrosis, vascular remodeling, portal hypertension development along with its complications, and ultimately liver failure.
The precise prevalence of cirrhosis worldwide is unknown; however, it was estimated at 0.15% or 400000 in North America which accounted for more than 25000 deaths in the late nineties[1]. Importantly, the average survival probabilities for cirrhotic patients at 1- and 5-years are approximately 85% and 65% for patients with no hospital admissions requirements, and 55% and 30% following hospital admissions, respectively. Moreover, hospital admissions in cirrhotic patients substantially impaired prognosis independent of stage of cirrhosis[2]. Accordingly, the social and economic burden of cirrhosis is immense considering the disability for labor, decreased quality, and need for frequent hospitalizations of cirrhotic patients.
At present there is no effective treatment to revert the cirrhosis; therefore, management is generally focused on treating the primary liver disease, screening and controlling the complications of portal hypertension, and consideration of liver transplantation in patients with decompensated cirrhosis. Although, liver transplantation may be considered curative for cirrhosis, this therapeutic option is not available for the majority of cirrhotic patients.
Malnutrition is one of the most common complications in cirrhosis, and despite the important role that the nutrition status has in the prognosis of cirrhosis, it is frequently overlooked as the nutrition assessment could be complex in cirrhotic patients with fluid retention and/or are overweight[3,4].
At present, several methods are available to evaluate the nutrition status of the cirrhotic patient; however, most of these techniques have limitations primarily due to a lack of objectivity, reproducibility, and prognosis discrimination. In this regard, muscle mass quantification with cross-sectional imaging studies [computed tomography (CT) scan, or magnetic resonance imaging (MRI)] constitutes an objective and reproducible technique, and an attractive index of nutritional status in cirrhosis[5].
Moreover, muscularity assessment with cross-sectional imaging studies is not bias by the fluid overload status that frequently is present in decompensated cirrhosis, and sarcopenia seems to reflect a chronic detriment in the general physical condition, rather than acute severity of the liver disease[6].
In general, sarcopenia is defined as a muscle mass two standard deviations below the healthy young adult mean[7], and although it is associated with aging, it can also be present as a result of chronic diseases and malignancy[8], and it ultimately leads to decreased functional capacity and higher risk of morbidity and mortality in different groups of patients[9,10].
In this review, we analyze the current accepted and new potential methods to evaluate the prognosis in cirrhotic patients. Along with the current evidence regarding frequency and clinical impact of sarcopenia in cirrhosis, in order to promote recognition of this complication and lead to strategies in effort to improve survival and reduced morbidity associated with cirrhosis.
PROGNOSTIC EVALUATION OF CIRRHOSIS WITH CONVENTIONAL SCORES
Prognostic assessments of patients with cirrhosis remain a difficult task as the natural history of these patients is particularly variable due to several factors. These factors include etiology of the cirrhosis, the possibility of treatment and resolution of the underlying damaging process of the liver, the level of liver dysfunction, the presence and degree of portal hypertension, and the occurrence of hepatocellular carcinoma (HCC)[11]. For example, in patients with cirrhosis, the onset of complications such as variceal hemorrhage, ascites, encephalopathy, jaundice, and development of HCC changes the stage from compensated cirrhosis to decompensated cirrhosis, and in the absence of liver transplantation this may lead to early mortality. Once patients have developed the first episode of decompensation, complications tend to accumulate and the life expectancy is markedly reduced. In a single-center study, the risk of transitioning from compensated to a decompensated state was 58% over 10 years[12], and in another experience from the United Kingdom general practice research database, the rate of transition from compensated to decompensated state was 12% per year[13].
D’Amico et al[14] from the University of Palermo have further subclassified compensated cirrhosis into stage 1 and 2, and decompensated cirrhosis in stage 3 and 4 as a result of a systematic review of 118 studies. Patients in stage 1 have neither varices nor ascites, and the 1-year risk of mortality is only 1%, and if patients develop varices (stage 2) the 1-year risk of mortality increase to 3.4%. With decompensation and onset of ascites (stage 3) the 1-year risk of mortality increase to 20%, and following a variceal bleeding (stage 4) the 1-year risk of mortality is higher than 50%[14]. Furthermore, currently there is a proposal to include two more stages to this classification; stage 5, patients with infections (such as bacteremia or spontaneous bacterial peritonitis) as 1-year risk of mortality increase from 49% to 66%[15], and stage 6, patients with kidney failure as mortality at 1-year could be up to 70%[16].
Child-Pugh[17] and the Model for End-Stage Liver Disease (MELD)[18] scores constitute the most frequent tools to predict mortality in patients with cirrhosis. Child-Pugh score (initially termed Child-Turcotte) was originally designed to predict mortality during surgery in patients with cirrhosis[19]. This has been shown to be useful in determining prognosis, treatment response, and necessity for liver transplant[20,21]. MELD was originally developed as a prognostic model of early-mortality in patients with cirrhosis who received a transjugular intrahepatic portosystemic shunt (TIPS)[22]. The original MELD has subsequently been simplified[23] and currently it is widely used to predict short-term mortality in different patient populations with cirrhosis[24-27]. Moreover, in most liver transplant centers MELD score has replaced the Child-Pugh score for priority of organ allocation[28], and since 2002 the MELD score has been used for the prioritization of potential liver transplant recipients in North America, mainly because MELD was developed in a statically fashion and includes only objective laboratory parameters. Since implementation of the MELD score, there have been reports of reductions in the number of patients listed for liver transplantation, waiting time for liver transplant, and deaths on the waiting list[29,30].
Although, MELD score has the advantage over Child-Pugh score of being based on objective variables [serum bilirubin, international normalized ratio of prothrombin time (INR), and serum creatinine] rather than on subjective evaluation of the severity of clinical findings (ascites and encephalopathy), the MELD score also has limitations[31,32], the most important of which are the variability of biochemical parameters, the lack of evaluation of the nutritional, and functional status in patients with cirrhosis.
OTHER TESTS TO EVALUATE PROGNOSIS OF CIRRHOTIC PATIENTS
Despite the irrefutable benefits of MELD, limitations of this score have been recognized and attempts are ongoing to improve it[33,34]. One of the major limitations of MELD is not having a component for the evaluation of the nutritional and functional status. As current scores to evaluate the prognosis in patients with cirrhosis need appropriate modifications, previous studies have evaluated the importance of other biochemical parameters.
For example, serum sodium has been shown to be an independent risk factor for mortality in patients with cirrhosis[22,33]; and previous studies reported that the addition of the serum sodium to generate the MELDNa score was more accurate than MELD for predicting short-term mortality on the waiting list[33]. The authors in this study estimated that use of MELDNa might have prevented 7% of deaths that occurred within 90-d of listing for transplantation; though, serum sodium is highly variable in cirrhotic patients using diuretics. Moreover, in one study of patients with cirrhosis we found that hyponatremia was only present in less than 15% of our patients being evaluated for liver transplant[35].
Another study reported a negative prognostic impact of hypoalbuminemia among liver transplant candidates after adjusting for the MELD and serum sodium concentration, and compared with MELD, a novel risk score named the 5-variable MELD (5vMELD, MELDNa scores incorporating albumin), improve the prediction of short-term mortality among cirrhotic patients awaiting liver transplantation[34].
The hepatic venous pressure gradient (HVPG), is an indirect estimation of portal pressure, and has been associated to the development of complications and prognosis in patients with cirrhosis. The HVPG has been associated with the risk of development of varices and variceal bleeding; also to the development hepatocellular carcinoma, requirement of liver transplantation, and survival[36]. Other tests such as the 6-min walk distance[37] seem to identify cirrhotic patients with low functional capacity and increased risk of death which may be due to sarcopenia, and further studies evaluating this test and correlations with sarcopenia are warranted[38].
PREVALENCE AND ORIGIN OF MALNUTRITION IN CIRRHOSIS
Malnutrition is one of the most frequent complications in cirrhotic patients, independently of the etiology[39]. The frequency of malnutrition in cirrhosis is highly variable and has been estimated to affect between 50%-90% of patients. This wide range is explained in part as there are significant differences in the operational definitions of malnutrition in cirrhosis. For example, it is difficult to define the presence of calorie malnutrition and as adipose tissue is the largest pool of calories, fat malnutrition is generally defined as reduction in body fat mass. However, as most proteins are located in the skeletal muscle, a proper definition of clinical protein malnutrition should use depletion of skeletal muscle[40].
Overweight and obesity are now endemic in Western countries. Patients with cirrhosis may develop simultaneous loss of skeletal muscle and gain of adipose tissue, culminating in the condition of sarcopenic obesity. Moreover, muscle depletion is characterized by both a reduction in muscle size and increased proportion of inter- and intra-muscular fat[41].
The etiology of malnutrition in cirrhosis is complex, seems to be multifactorial and tends to be more common as the liver disease progresses, as the factors that lead to malnutrition in the first place become more prominent. The most important factors associated with malnutrition in cirrhosis include metabolic abnormalities, insufficient oral intake mainly due to early satiety in moderate-severe ascites, malabsorption, and impaired capacity of the liver to metabolize and save nutrients.
TESTS USED FOR NUTRITION EVALUATION IN CIRRHOSIS
The nutrition evaluation in cirrhosis should consider various aspects of the patient, including the severity of underlying illness, medical history, physical examination including anthropometric measurements, and biochemical data[3]. The subjective global assessment (SGA) is one of the most frequent instruments used for nutrition assessment in patients with cirrhosis. Since the early eighties, SGA has been used to assess patients for malnutrition at the bedside, without the need for precise body composition analysis[42]. The features of the SGA include a physical exam component that evaluates the loss of subcutaneous fat, peripheral or sacral edema, and muscle wasting. The quantity of muscle and subcutaneous tissue is graded subjectively by the examiner, who then categorizes it as normal, mildly, moderately, or severely decreased. Multiple components on patient history are also evaluated. The first component is the amount of weight loss in the previous 6 months. Less than 5% of body weight loss is consider “small,” 5%-10% is considered “potentially significant”, and > 10% “definitely significant”. Supplementary historical features of the SGA include patient’s dietary intake and the presence of gastrointestinal symptoms experienced daily for at least 2 wk. These symptoms can include anorexia, nausea, vomiting and/or diarrhea. Once the history and physical examination sections are completed, the patients are classified as well nourished (SGA grade A), moderately malnourished or suspected of being malnourished (SGA grade B), or severely malnourished (SGA grade C). However, the SGA is a partially subjective method, constituted by quantitative and qualitative variables subject to varied interpretations, and previous studies reported that SGA has demonstrated low sensitivity in cirrhotic patients, as underestimates the nutritional state in the majority of patients[43].
Some laboratory tests are used as part of the nutritional assessment in cirrhosis, including the prothrombin time or INR, albumin, prealbumin, creatinine height index, and indirect evaluation of the of immune function, like the delayed-type hypersensitivity reactions[39,44]. Nevertheless, as cirrhosis confounds these common parameters of nutritional status, their utility in these patients is limited. For example, patients with cirrhosis may have significant impairment in their hepatic synthetic function that results in low serum albumin, prealbumin, transferrin levels, and prolonged INR, which may lead to an overestimation of the prevalence of malnutrition[45,46]. Also, the creatinine height index is an inaccurate measure of malnutrition in cirrhosis as creatinine levels could be low due to muscle wasting, or alternatively could be high as in renal impairment, which is common in these patients[47]. Lastly, T cell anergy is a tolerance mechanism in which the lymphocytes are inactivated following an antigen encounter, and this phenomenon is frequently present in cirrhotic patients. This makes delayed-type hypersensitivity an inaccurate measurement of malnutrition[45,48].
Therefore, even that several methods to evaluate nutritional and functional capacity status in patients with cirrhosis have been tested, they have reported diverse results[37,49-52], and consequently an optimal index for nutritional status in terms of availability, reproducibility, practicality, and prognostic performance is needed.
BODY COMPOSITION EVALUATION IN CIRRHOSIS
Patients with cirrhosis frequently have significant changes in their body composition, characterized by increased in the extracellular fluids and decrease in muscle and adipose tissue. However, clinical identification of body composition changes in cirrhosis with ascites and edema could be challenging, as fluid gains hide muscle and adipose tissue losses[53,54]. It seems that cirrhotic males have more muscle wasting, whereas females tend to have more depletion of fat tissue[55]. Moreover, previous studies have showed that changes in body composition may progress with the course of the liver disease and correlate with the Child-Pugh score[53].
Numerous indirect methods have been used to quantify body composition in cirrhotics. These methods include total-body electrical conductivity, bioelectrical impedance, dual energy X-ray absorptiometry, air displacement plethysmography, and magnetic resonance spectroscopy[56-58]. These methods are based on the principle that body fat and no fat mass have specific components, such as water, proteins, and minerals[59]. By establishing the total body weight and fat mass, the remaining weight should be lean mass (no fat mass). As approximately 50% of lean body mass is constituted by skeletal muscle mass, quantification of lean body or fat-free mass is considered to be an indirect measure of the body skeletal muscle mass.
Most of these methods lack either availability and/or reproducibility, and their accuracy may be limited in the presence of fluid retention. For example, the dual-energy X-ray absorptiometry assesses body composition through a low-dose X-ray, and the bioelectrical impedance analysis measures the body composition by the flow of a small (less than 1 mA) alternating current that estimates total body water, fat-free mass, and body fat; still, these techniques are not accurate in cirrhotic patients with fluid retention[57,60].
Other methods, such as the body cell mass is preferentially assessed by bioelectrical impedance analysis, which can be performed with a portable equipment in the office setting and is a validated marker used to assess body composition in cirrhotics and seems to be accurate even in fluid retention. Although, this is expensive, not accessible for clinical use, and is usually is only used as a validation tool when analyzing other anthropometric assessments. The air plethysmography measures changes in volume within an organ or whole body (usually resulting from fluctuations in the amount of blood or air it contains), and subsequent calculation of body composition; however, this varies among men and women, and there is limited availability[61,62].
Other techniques, includes the skin-fold thickness measurement that quantify fat mass in the upper-arm (midarm muscle area) using a caliper; however, there have been conflicting reports in the accuracy for predicting malnutrition in cirrhosis due to interobserver variability, and this method did not correlate with Child-Pugh score[60,63]. With regards to the hand grip strength testing used a hand dynamometer to assess grip strength, as decreased grip strength is associated with malnutrition; yet, many factors may influence the results, and this test also did not correlate with the Child-Pugh score[52].
Lastly, a recent study showed that low respiratory quotient (RQs) occurs in cirrhosis, and this finding did not predict mortality. Also, a direct and significant relation between RQ and muscle area was reported, which suggests that altered skeletal muscle protein turnover contributes to this metabolic response[64].
MUSCULARITY ASSESSMENT IN CIRRHOSIS
Cross-sectional imaging studies, including CT scan or MRI are the gold standard tools to quantify skeletal muscle mass[65] and hence constitutes a good resource for objective and detailed nutritional/metabolic assessment of cirrhotic patients, and identification of sarcopenia. Recently, our group performed an analysis of the frequency and impact of sarcopenia in cirrhotic patients being evaluated for liver transplant[35]. We used CT scan at the 3rd lumbar (L3) vertebrae analyzed with the SliceOmatic V4.3 software (Tomovision, Montreal, PQ), which enables specific tissue demarcation using previously reported Hounsfield unit (HU) thresholds. Skeletal muscle was identified and quantified by HU thresholds of -29 to +150[66], and cross-sectional area of muscle and adipose tissue was normalized for stature (cm2/m2) as reported in previous studies[67]. The L3 skeletal muscle index (L3 SMI) was expressed as cross sectional muscle area/height2, and cutoffs for sarcopenia were based on a CT-based sarcopenic study for patients with malignancies (L3 SMI: ≤ 38.5 cm2/m2 for women and ≤ 52.4 cm2/m2 for men[68]; moreover, recently we set up new cutoff values for cirrhotic patients, and values were similar in cirrhosis compared to patients with malignancies (L3 SMI: < 42 cm2/m2 for women and < 50 cm2/m2 for men)[69].
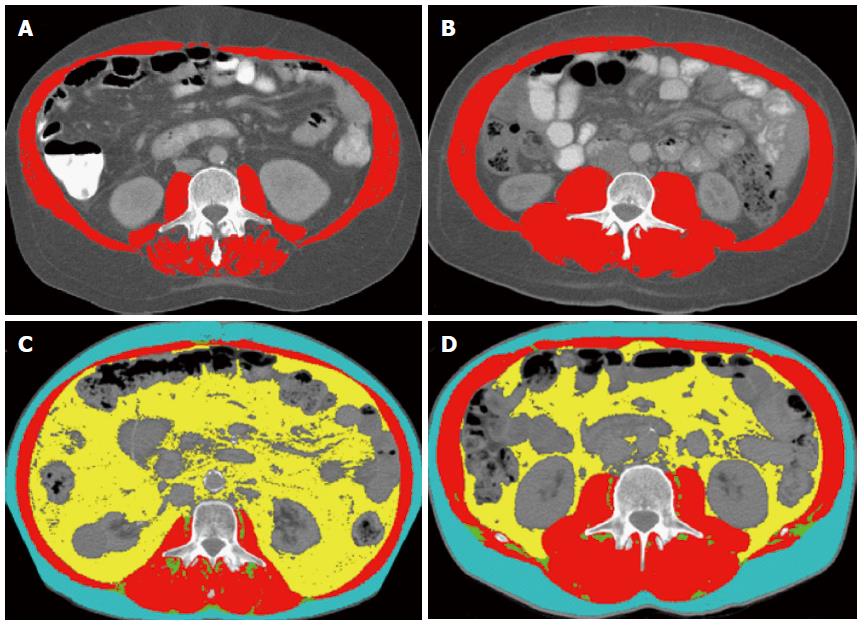
Sarcopenia is present in 40% of patients with cirrhosis being evaluated for liver transplant. To exemplify that there is no adequate correlation of classical anthropometric measurement and sarcopenia, in Figure 1A and B, we present images of the L3 SMI analysis of two cirrhotic patients with identical body mass index, but one with and another without sarcopenia. In Figure 2, we present images of the L3 SMI analysis of two patients with cirrhosis and HCC, and identical body mass index, but one with and another without sarcopenia.
Figure 1 Computed tomography images used for the 3rd lumbar skeletal muscle Index assessment of two patients with cirrhosis (A, B), and two patients with cirrhosis and hepatocellular carcinoma (C, D).
Comparison of two cirrhotic patients with identical body mass index (BMI 32 kg/m2), and two patients with cirrhosis and hepatocellular carcinoma and identical BMI (28 kg/m2). A: The patient at the left is sarcopenic with the 3rd lumbar (L3) skeletal muscle index (L3 SMI) of 50 cm2/m2; B: Patient at the right is not sarcopenic with a L3 SMI of 71 cm2/m2; C: Patient at the left is sarcopenic with L3 SMI of 47 cm2/m2; D: Patient at the right is not sarcopenic with a L3 SMI of 59 cm2/m2. Red color indicates skeletal muscles, green color indicates intermuscular adipose tissue, yellow color indicates visceral adipose tissue, and teal indicates subcutaneous adipose tissue.
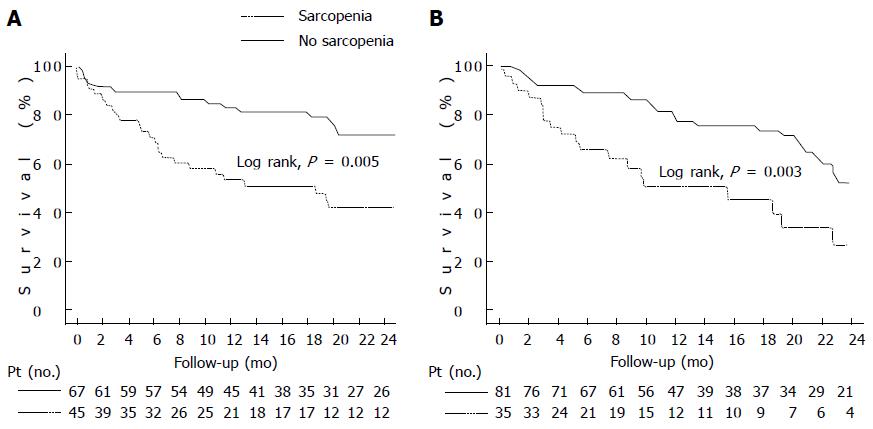
Figure 2 Kaplan-Meier curve indicating the survival of patients with cirrhosis (A) and patients with cirrhosis and hepatocellular carcinoma (B).
A: Kaplan-Meier curve indicating the survival of cirrhotic patients with and without sarcopenia. The 6-m probability of survival was 71% and 90%, respectively (P = 0.005, Log-Rank test); B: Kaplan-Meier curve indicating the survival of patients with cirrhosis and hepatocellular carcinoma with and without sarcopenia. The 6-mo probability of survival was 67% and 90%, respectively (P = 0.003, Log-Rank test).
Importantly, we reported that median survival for sarcopenic patients was significantly worse compared to non-sarcopenic patients (19 ± 6 mo vs 34 ± 11 mo, Log-Rank, P = 0.005) (Figure 2A). Six-month probability of survival was 71% in sarcopenic and 90% in non-sarcopenic patients, respectively; and the frequency of sepsis-related death and was significantly higher in sarcopenic than non-sarcopenic patients. In our study we found that even though the BMI was lower in cirrhotic patients with sarcopenia, only one patient in our cohort would be considered underweight by commonly accepted criteria (BMI ≤ 18.5 kg/m2)[35]. Therefore, sarcopenia is not exclusively present in underweight patients, and constitutes a hidden condition that can be present in cirrhotic patients with any BMI. Furthermore, measurement of sarcopenia is independent of the fluid retention, which plagues accurate measurement of body weight and BMI in cirrhotics.
Subsequently, we found similar results in another study of cirrhotics patients and concurrent hepatocellular carcinoma (HCC) where median survival for sarcopenic patients was 16 ± 6 mo vs 28 ± 3 mo in non-sarcopenic (Log-Rank, P = 0.003, Figure 2B). Also, in the multivariate Cox regression analysis, sarcopenia (HR = 2.04, P = 0.02) was independently associated with mortality[41,70]. We also found that even though sarcopenia is a strong and independent predictor of mortality in patients with HCC and cirrhosis, it did not correlate with tumor-node-metastasis stage, and degree of liver dysfunction evaluated with conventional scores (Child-Pugh and MELD).
Therefore, body composition and muscularity assessment should be considered as part of the nutritional assessment, treatment decision and outcome prognosis in patients with cirrhosis with and without HCC; and further studies including sarcopenia with conventional scores may allow significantly better prediction of mortality among cirrhotic patients.
COMPLICATIONS OF MALNUTRITION AND SARCOPENIA IN CIRRHOSIS
Malnutrition in cirrhosis affects the quality of life, survival, and development of complications in cirrhosis. In one study of cirrhotic patients, those with malnutrition as established by the SGA experience lower quality of life, defined as impairment in 6 of the 8 quality-of-life scales on the short-form health survey with only 36 questions (SF-36), compared to those without malnutrition[71]. Furthermore, in a prospective study to identify risk of variceal bleeding or death in cirrhosis, poor nutritional status was independently associated with a higher risk of variceal bleeding and mortality[72].
Plasma levels of ammonia tend to be high in liver dysfunction and portal hypertension, and as skeletal muscle have a significant role in ammonia detoxification; cirrhotic patients with muscle depletion may be at higher risk of hepatic encephalopathy. Improvement of nutritional status could decrease the prevalence of neurocognitive alterations in these patients[73]. In a prospective study of cirrhotic patients evaluated for the presence of hepatic encephalopathy according to the West-Haven criteria, two psychometric tests, and fasting plasma ammonium concentrations, patients with malnutrition had more frequently hepatic encephalopathy, and in the multivariate analysis the time needed to perform number connection test A was independently correlated to malnutrition[74]. However, another study did not support the hypothesis that malnutrition was an independent risk factor for the presence of hepatic encephalopathy in nonalcoholic cirrhosis[75].
Finally, the clinical significance of malnutrition in cirrhotic patients can be evaluated by the effect of sarcopenia on survival and complications. As previously discussed, patients with cirrhosis and sarcopenia have elevated mortality[35,41]; and this higher mortality risk seems to be related to a higher frequency of sepsis-related death rather than liver failure mortality[35] as there is correlation between protein malnutrition and sepsis in hospitalized cirrhotic patients[76]. This may explain why conventional scores that reflect mainly liver function, such as MELD and Child-Pugh do not detect mortality risks associated with low muscle mass.
THERAPEUTIC OPTIONS FOR MALNOURISHMENT AND SARCOPENIA IN CIRRHOTIC
In patients with cirrhosis, increased protein intake has been demonstrated to be safe, well tolerated, and beneficial; however, the long-term effects on muscle mass are not completely elucidated. Other strategies that have been evaluated include late-evening snacks, repeated snacks, branched chain amino acid, and protein supplementation in general with beneficial results[77-79].
Interestingly, intake of leucine-enriched essential amino acid nutrient may be useful in the treatment of sarcopenia in cirrhosis. Leucine is one essential amino acids substrate for protein synthesis, but also has a key role in the skeletal muscle anabolism, protein synthesis and autophagy regulation. The activation of anabolic signalling occurs via the mammalian target of rapamycin (mTOR) through an undefined mechanism and as amino acid[80,81]. This data suggests a potential role for leucine-rich supplements in the management of muscle wasting in cirrhosis.
Exercise, including aerobic and resistance physical activity are important for the muscle metabolism. Patients with cirrhosis frequently have complications of portal hypertension, such as ascites or hepatic encephalopathy, or symptoms associate to chronic illness including significant fatigue and reduced maximum exercise capacity which significantly reduce the physical activity. Moreover, even moderate exercise augments the portal pressure and may increase the risk of variceal bleeding in patients with esophageal varices; therefore, cirrhotic patients with portal hypertension should be advised of potential risks during exercise[82]. The patients who are able and willing to enter in a exercise program may benefit from pharmacological prophylaxis, as the increase in portal pressure could be prevented by propranolol pretreatment[83].
An interesting therapeutic approach in cirrhotic sarcopenia could be the use of transjugular intrahepatic portosystemic shunt (TIPS). A recent study showed that TIPS may reverse sarcopenia, and failure to improve muscle mass after TIPS is associated with higher mortality[84]; however, the utility of TIPS as an intervention to reverse sarcopenia should be evaluated in future prospective studies. Similar to these findings, our group has recently showed that sarcopenia does not increase mortality after liver transplantation, and may revert after transplant in some cases[85].
New treatments to reverse sarcopenia in cirrhotic patients, including myostatin antagonists are waiting to be evaluated in randomized controlled trials. In a preliminary investigation, myostatin levels in patients undergoing liver transplant evaluation were significantly higher in cirrhotics compared with healthy controls[86]. In addition, animal model studies have shown that myostatin expression can be reversed without significant impairment of liver dysfunction[87].
Finally, one specific problem to be consider in cirrhosis is that division of adiposity and muscle responses to specific interventions may result in the increase of fat tissue, without the proportional reversion of sarcopenia and potential risk to develop sarcopenic obesity.
SARCOPENIA AND LIVER TRANSPLANTATION
Sarcopenia is associated with increased mortality in cirrhosis[35]; however, how it impacts after liver transplantation requires further analysis. Previously, one study showed that central sarcopenia strongly correlates with mortality after liver transplantation[88]. In this study the cross-sectional area of the psoas muscle was measured on CT scans of 163 liver transplant recipients, and after controlling for donor and recipient characteristics using Cox regression models, authors reported a strong association between psoas area and post-transplantation mortality (HR = 3.7/1000 mm2 decrease in psoas area; P < 0.0001). Then, they stratified into quartiles based on psoas area and the 1-year survival ranged from 50% for the quartile with the smallest psoas area to 87% for the quartile with the largest.
We recently presented a study showing that sarcopenia does not increase mortality after liver transplant, and in some cases may resolve after transplantation[85]. In this study we found that median survival after liver transplantation for sarcopenic patients was 115 ± 25 mo, compared to 146 ± 34 mo in non-sarcopenic patients (P = 0.2); however, sarcopenic patients had longer hospitalization following liver transplant compared to non-sarcopenic patients. Interestingly, in a subanalysis of non-protocol CT after liver transplant we found that in patients who had sarcopenia before the transplant sarcopenia resolved in at least 20% after transplantation.
Some differences in protocols may explain these dissimilar results between the two studies[85,88]; for example in first retrospective study, 85% of the patients had a non-protocol CT done in the post-transplantation period, and probably sicker patients with higher risk of mortality were those who need CT after transplant. Moreover, the muscularity assessment technique they used was different to ours, as they evaluated the cross-sectional area of the psoas muscle obtained selecting imaging slice at the superior aspect of fourth lumbar vertebra and outlined borders of the left and right psoas muscle, and the area of the resulting enclosed regions was then computed to generate the cross-sectional area of the psoas muscles. Lastly, we used instead, the L3 SMI, which has been shown to have an excellent correlation with the total muscle mass[89]. Further prospective studies will be necessary to establish the impact of sarcopenia after liver transplantation.
In summary, sarcopenia affects the quality of life, survival and the development of the complications in cirrhosis. Therefore, body composition and muscularity assessment should be considered as part of the nutritional assessment, treatment decision and outcome prognosis in cirrhotic patients. Regardless of previous finding concerning the significance of sarcopenia as prognostic factor in patients with cirrhosis[50,90,91], the general inclusion of sarcopenia into cirrhosis prognostic scores has been limited by lack of dependable and objective method to quantify muscle wasting.
Numerous indirect methods have been used to quantify body composition in cirrhotics; however, most of these methods lack either availability and/or reproducibility, and their accuracy may be limited in the presence of fluid retention. Cross-sectional imaging studies, including CT scan or MRI are the gold standard tools to quantify skeletal muscle mass[65], and hence constitute a good resource for objective and detailed nutritional/metabolic assessment of patients and identification of sarcopenia.
Sarcopenia is not exclusively present in underweight patients, and constitutes a hidden condition that can be present in cirrhotic patients with any BMI. Furthermore, measurement of sarcopenia is independent of the fluid retention which plagues accurate measurement of body weight and BMI in cirrhotics. A recent study from our group showed that modification of MELD to include sarcopenia is associated with a modest improvement in the prediction of mortality in patients with cirrhosis[92]. However, prior to the widespread use of MELD-sarcopenia, additional validation in larger cohorts of patients with cirrhosis is necessary.