Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7461
Revised: May 7, 2014
Accepted: May 19, 2014
Published online: June 21, 2014
Processing time: 75 Days and 2.2 Hours
AIM: To investigate the clinical significance of expression of tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in ulcerative colitis (UC).
METHODS: Thirty UC specimens taken by colonoscopy from patients with active UC treated at the Department of Pathology, Central Hospital Affiliated to Shenyang Medical College from February 2010 to January 2012 were included in an experimental group, and 30 normal colon tissue samples taken by colonoscopy from non-UC patients were included in a control group. Expression of TF and TFPI in UC and normal colon tissue samples was detected by immunohistochemistry.
RESULTS: The positive rate of TF in UC was significantly higher than that in normal colon tissue (63% vs 33%, χ2 = 5.41, P < 0.05). The positive rate of TFPI in UC was also significantly higher than that in normal colon tissue (43% vs 17%, χ2 = 5.08, P < 0.05).
CONCLUSION: Positive rates of TF and TFPI expression in UC are significantly higher than those in normal colon tissue. TF and TFPI may play an important role in the pathogenesis of UC.
Core tip: Recent research showed that tissue factor (TF) and tissue factor pathway inhibitor (TFPI) are associated with inflammatory reactions, and they are expressed in many kinds of tissue and cells. There are few reports about the relationship between TF and TFPI and ulcerative colitis (UC). In this study, we performed immunohistochemical staining for TF and TFPI expression in tissue of UC and normal colon, to determine their association with UC and their significances in pathogenesis. The study results suggest that TF and TFPI may play an important role in the pathogenesis of UC.
- Citation: He HL, Zhang JB, Li Q. Clinical significance of expression of tissue factor and tissue factor pathway inhibitor in ulcerative colitis. World J Gastroenterol 2014; 20(23): 7461-7465
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7461
Ulcerative colitis (UC) is a chronic, nonspecific intestinal inflammatory disease of unknown causes, with the main clinical symptoms being abdominal pain, diarrhea, and blood and mucus in the stool. The etiology and pathogenesis of UC is very complex and not yet completely understood. A better understanding of the pathogenesis of UC will bring new clues to UC treatment. In recent years, many studies have shown that there is a correlation between UC and hypercoagulable states. Microthrombus formation may be one of the important mechanisms responsible for the pathogenesis of UC, and ongoing hypercoagulable states may promote the development and progression of inflammation and be related to UC progression[1]. Studies have found that the pathological process and clinical complications of diseases with thrombosis are closely associated with tissue factor (TF) and tissue factor pathway inhibitor (TFPI)[2,3]. Several studies have demonstrated the presence of TF and TFPI in many human diseases with thrombosis[4,5]. Recent studies revealed a correlation between TF and TFPI and inflammation[6]. TF and TFPI are expressed in a variety of tissues and cells[5]. Currently, there have been few reports on the correlation between TF and TFPI and UC. In the present study, immunohistochemistry was used to detect the expression of TF and TFPI in UC to reveal their relationship with UC and to elucidate their role in the pathogenesis of UC.
Thirty UC specimens taken by colonoscopy from patients with active UC treated at Department of Pathology, Central Hospital Affiliated to Shenyang Medical College from February 2010 to January 2012 were included in an experimental group, and 30 normal colon tissue samples taken by colonoscopy from non-UC patients were included in a control group. The pathological diagnosis of UC was made by a pathologist. The experimental group contained 12 males and 18 females, and the control group contained 16 males and 14 females. The subjects ranged in age from 35 to 52 years, with a mean age of 49 years. In the experimental group, there were 3 cases of entire colon lesions, 8 cases of left-sided colon lesions, 15 cases of sigmoid lesions, and 4 cases of rectal lesions. UC was diagnosed based on the diagnostic criteria in “Analysis on Chinese Consensus on Standard Diagnosis and Treatment of Inflammatory Bowel Disease” developed by the Inflammatory Bowel Disease Collaborative Group of the Chinese Society of Gastroenterology[7]. The study protocol was approved by the Ethics Committee of Central Hospital Affiliated to Shenyang Medical College, and all subjects signed informed consent.
Rabbit anti-human TF monoclonal antibody and mouse anti-human TFPI monoclonal antibody were purchased from Boermei (Shenyang, China). SP kit and AEC kit were purchased from Zhongshan Golden Bridge Technology (Beijing, China). An optical microscope (Olympus BX40) and microtome (Shandon AS325) were also used.
Intestinal mucosal tissues were obtained during colonoscopy from the lesion having the most severe inflammation in patients with active UC. For patients in the control group, tissue samples were taken from a site showing no abnormalities (25 cm from the anus) in the sigmoid colon. Four tissue samples were taken from each patient and stored in a refrigerator.
After dewaxing and hydration, paraffin sections were soaked in phosphate-buffered saline for 5 min. Antigen retrieval was then achieved using the microwave method, and endogenous peroxidase activity was inhibited by incubation with an endogenous peroxidase blocking agent (reagent A in the SP Kit) at room temperature for 10 min. After washing with PBS 3 times for 3 min each time, nonspecific binding was blocked by incubation with normal goat serum (reagent B in the SP Kit) at room temperature for 10 min. Sections were then incubated with primary antibody TF or TFPI overnight at 4 °C. After washing, sections were incubated with a biotinylated secondary antibody (reagent C in the Universal SP Kit) at room temperature for 10 min, followed by incubation with horseradish peroxidase-labeled streptavidin (reagent D in the Universal SP Kit) at room temperature for 10 min. After washing again, the signal was developed by incubation with AEC reagent for 5-10 min. Nuclei were counterstained with hematoxylin, and mounted on slides.
(1) evaluation criteria for positive TF expression: TF was localized in the membrane and cytoplasm of cells in the mucosal tissue around the ulcer, and positive immunohistochemical signals were brown. Ten typical high power fields (HPF × 400) were selected from each slide for evaluation. The extent of the immunohistochemical signal was divided into 4 levels: 0%-5% (-), 5%-10% (+), 10%-40% (++), > 40% (+++); (2) evaluation criteria for positive TFPI expression: TFPI was localized in the cytoplasm of cells in the mucosal tissue around the ulcer, and positive immunohistochemical signals were yellowish brown. Ten typical HPF (× 400) were selected from each slice for evaluation. The extent of the immunohistochemical signal was divided into 4 levels as above; and (3) positive rate: Ten HPF were observed for each case. The percentage of positive cells were divided into 4 levels as above.
Statistical analyses were performed using SPSS 11.0 for Windows software (SPSS Inc., Chicago, IL, United States). Percentages were compared using the χ2 test. P values < 0.05 were considered statistically significant.
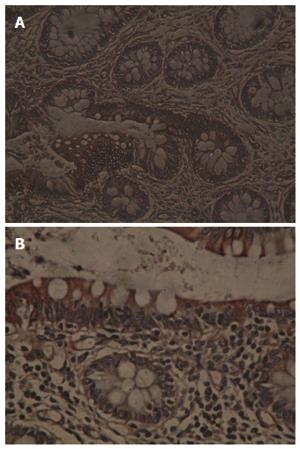
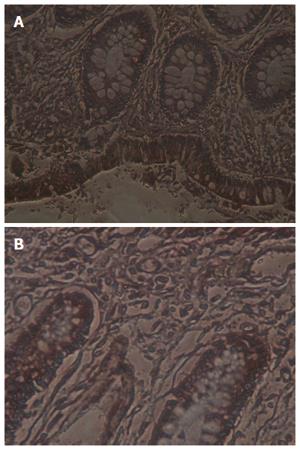
The positive rate of TF expression in UC was 63% (19/30), significantly higher than that in normal colon tissues (33%, 10/30) (P < 0.05, Table 1, Figure 1A and B).
| Group | Number of total cases | Number of positive cases | Number of negative cases | Positive rate |
| Experimental | 30 | 19 | 11 | 63% |
| Control | 30 | 10 | 20 | 33% |
The positive rate of TFPI expression in UC was 43% (13/30), significantly higher than that in normal colon tissues (17%, 5/30) (P < 0.05, Table 2, Figure 2A and B).
| Group | Number of total cases | Number of positive cases | Number of negative cases | Positive rate |
| Experimental | 30 | 13 | 17 | 43% |
| Control | 30 | 5 | 25 | 17% |
The etiology and pathogenesis of UC are very complex and are still not very clear. It is currently believed that inflammatory processes elicited by mucosal immune system abnormalities caused by multiple factors, including environmental, immunological and genetic factors, play an important role in the pathogenesis of UC[8,9]. In recent years, the incidence of UC has increased, and there are more reported cases of critically ill patients. Clinical studies indicate that there exist hypercoagulable states and a potential risk of thrombosis in UC patients. UC complicated with thromboembolic diseases are not uncommon, and autopsies revealed that up to 39% of UC patients have thromboembolic diseases. Thromboembolic diseases have become the third leading cause of death in patients with UC[10,11]. Intestinal multifocal infarction caused by microvascular inflammation has become an important mechanism in the pathogenesis of UC. Persistent hypercoagulable states may be related to the clinical progression of UC[1], and have a role in promoting the development and progression of inflammation. In this study, immunohistochemistry was used to detect the expression of molecular biomarkers TF and TFPI in UC tissues to provide further information in the elucidation of the pathogenesis of UC.
Studies have found that the pathological process and clinical complications of diseases with thrombosis are closed associated with TF and TFPI. Studies have demonstrated the presence of TF and TFPI in many human diseases with thrombosis. Recent studies have also found that there exists an association between TF and TFPI and inflammation.
TF is a protein with 236 amino acids, and contains an extracellular domain, a transmembrane domain and a cytoplasmic domain. It is now clear that the extracellular domain is critical for the binding to FVII and the initiation of the extrinsic coagulation pathway, and has procoagulant activity and proteolytic functions[12]. The TF-initiated coagulation process was formerly known as the extrinsic coagulation pathway. Research advances over the past decade have shown that this is a start-up phase of coagulation, and the intrinsic coagulation pathway belongs to the maintenance phase of coagulation. TF has important significance in physiological hemostasis and pathological thrombosis and participates in the inflammatory response. The transmembrane domain may also play a role in signal transduction, but the function of the intracellular domain has been a hotspot of TF research in recent years. The phosphorylation of 3 serine residues in the terminus of the cytoplasmic domain can mediate intracellular signal transduction and promote the transcription and synthesis of vascular endothelial growth factor (VEGF)[13,14]. Ollivier et al[15] found that, in human pulmonary fibroblasts, TF-dependent VEGF expression requires binding between FVII and TF, and binding of FVII with inactivated active sites and TF inhibits VEGF production. Bulut et al[16] suggested that VEGF is involved in the pathogenesis of UC and is related to disease severity. TF binding to its receptor FVIIa triggers intracellular signal transduction mechanisms and induces the upregulation of matrix metalloproteinases (MMPs), which are involved in tissue repair and have very important significance in inflammatory diseases. A large number of studies have confirmed that MMPs are involved in basal inflammatory tissue repair, angiogenesis and leukocyte chemotaxis in UC patients, and MMPs are highly expressed in UC tissues and are enhanced with the aggravation of inflammation[17,18]. The results of the present study showed that the positive rate of TF expression in UC tissue (63%) was significantly higher than that in normal colon tissue (33%) (P < 0.05), indicating that the expression level of TF differs between UC tissue and normal colon tissue. This finding suggests that TF may be associated with the occurrence of UC, and hypercoagulable states and intestinal microthrombosis may mediate the role of TF in UC. In addition, TF, as an indirect inflammatory mediator to stimulate the release of other inflammatory mediators and induce inflammatory responses, may be involved in this process.
As the only physiological inhibitor of TF-FVIIa complex, TFPI inhibits TF-induced thrombus formation. It depends on the feedback inhibition of FXa to regulate the extrinsic coagulation pathway, preventing the unlimited expansion of clotting[19]. TFPI plays an important role not only in the extrinsic coagulation pathway[20,21], but also in inflammatory responses. In the process of inflammatory responses, when endothelial cells contact with tumor necrosis factor (TNF), endotoxin and thrombin, TFPI output on the cell surface significantly increases, and TF activity is increased[22]. Animal experiments and clinical studies showed that TFPI has anti-inflammatory effects, and it can inhibit leukocyte activation as well as the synthesis and expression of inflammatory mediators including TNF-α, interleukin (IL)-6 and IL-1. These inflammatory mediators have a clear association with the occurrence of UC and are important in the pathogenesis of UC[23,24]. TFPI not only has inhibitory effects on the TF-FVIIa complex, FXIa and cathepsin G[25], as well as serine proteases, plasmin, trypsin, chymotrypsin, plasma kallikrein and FXa[26], but also is an indirect inhibitor of MMPs in the extracellular matrix[27]. Studies have demonstrated that the expression of MMP-3 and MMP-9 is significantly increased in the mucosal inflammatory area in UC patients, and is enhanced with aggravation of inflammation. Clinical trials showed that blood levels of TFPI were significantly higher in UC patients than in normal controls, and in patients with active UC than in those with the disease in the remission period. The results of the present study showed that the positive rate of TFPI in UC tissue (43%) was significantly higher than that in normal colon tissue (17%) (P < 0.05), indicating that the expression level of TFPI differs between UC tissue and normal colon tissue. This finding suggests that TFPI may have a role in the occurrence of UC, possibly by inhibition of TF-induced thrombosis and suppression of inflammatory mediator release to inhibit inflammation. Therefore, the application of recombinant TFPI to inhibit the activation of inflammatory cells and the release of inflammatory mediators can be considered a strategy to treat UC[28], and this may provide a new avenue for the treatment of UC.
In the present study, immunohistochemistry was used to investigate the expression of TF and TFPI in UC tissue, and the results may help understand the molecular mechanisms of pathogenesis of UC. Clinical detection of TF and TFPI may be helpful in the diagnosis of UC. The present study explored the mechanisms of pathogenesis of UC from another perspective, and the results obtained are expected to be able to guide the selection of reasonable treatment regimens and provide new ideas for the clinical treatment of UC. However, due to the limitations of experimental conditions and funding, the present study did not conduct a deeper exploration of UC pathogenesis. Future studies are required to further explore the pathogenesis, diagnosis, treatment and prognosis of UC.
Ulcerative colitis (UC) is associated with a blood hypercoagulable state and microthrombus formation may be one aspect of the pathogenesis of UC. Thrombus formation is closely associated with tissue factor (TF) and tissue factor pathway inhibitor (TFPI).
A persistently hypercoagulable state may be related to the clinical progression of UC, which can promote inflammation take place and progress. Recent research showed that TF and TFPI expression was associated with thrombus formation and also inflammatory reactions, with expression in many kinds of tissue and cells.
There are few reports about the relations between TF and TFPI and UC. In this study, authors performed immunohistochemical staining for TF and TFPI expression in tissue of UC and normal colon, and found significantly higher expression in UC tissue.
The study results suggest that TF and TFPI may play an important role in the pathogenesis of UC. TF and TFPI expression in tissue can help diagnosis of UC TFPI and TF research may provide new ideas for clinical treatment.
This is a good study in which authors analyze the expression of TF and TFPI in tissue of UC. The results are interesting and suggest that TF and TFPI may play an important role in the pathogenesis of UC.
P- Reviewers: Andersen V, Krista R S- Editor: Qi Y L- Editor: Cant MR E- Editor: Wang CH
| 1. | Jiang ZX, Liu CY, Sui T. The levels of molecular markers of prethrombotic state in patients with ulcerative colitis and its significance. Weichangbingxue He Ganbingxue Zazhi. 2007;16:187-189. |
| 2. | Palmier MO, Hall LJ, Reisch CM, Baldwin MK, Wilson AG, Wun TC. Clearance of recombinant tissue factor pathway inhibitor (TFPI) in rabbits. Thromb Haemost. 1992;68:33-36. [PubMed] |
| 3. | Zhu FM. Physiological and clinical significance of tissue factor pathway. Guowai Yixue Shuxue Ji Xueyexue Fence. 1997;1:52-54. |
| 4. | Drew AF, Davenport P, Apostolopoulos J, Tipping PG. Tissue factor pathway inhibitor expression in atherosclerosis. Lab Invest. 1997;77:291-298. [PubMed] |
| 5. | Kaikita K, Takeya M, Ogawa H, Suefuji H, Yasue H, Takahashi K. Co-localization of tissue factor and tissue factor pathway inhibitor in coronary atherosclerosis. J Pathol. 1999;188:180-188. [PubMed] |
| 6. | Chen J, Bierhaus A, Schiekofer S, Andrassy M, Chen B, Stern DM, Nawroth PP. Tissue factor--a receptor involved in the control of cellular properties, including angiogenesis. Thromb Haemost. 2001;86:334-345. [PubMed] |
| 7. | Inflammatory Bowel Disease Co-operation Group. Chinese Society of Gastroenterology. Chinese consensus on standard diagnosis and treatment of inflammatory bowel diseases. Weichangbingxue. 2007;12:488-495. |
| 8. | Jiang XL, Qin CY, Li GQ. Other treatments for ulcerative colitis. Shijie Huaren Xiaohua Zazhi. 2000;8:341-342. |
| 9. | Larsen TB, Nielsen JN, Fredholm L, Lund ED, Brandslund I, Munkholm P, Hey H. Platelets and anticoagulant capacity in patients with inflammatory bowel disease. Pathophysiol Haemost Thromb. 2002;32:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Fägerstam JP, Whiss PA, Ström M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res. 2000;49:466-472. [PubMed] |
| 11. | Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840-845. [PubMed] |
| 12. | Mackman N. Alternatively spliced tissue factor - one cut too many? Thromb Haemost. 2007;97:5-8. [PubMed] |
| 13. | Ruf W, Edgington TS. Structural biology of tissue factor, the initiator of thrombogenesis in vivo. FASEB J. 1994;8:385-390. [PubMed] |
| 14. | Ruf W, Edgington TS. Two sites in the tissue factor extracellular domain mediate the recognition of the ligand factor VIIa. Proc Natl Acad Sci USA. 1991;88:8430-8434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Ollivier V, Bentolila S, Chabbat J, Hakim J, de Prost D. Tissue factor-dependent vascular endothelial growth factor production by human fibroblasts in response to activated factor VII. Blood. 1998;91:2698-2703. [PubMed] |
| 16. | Bulut K, Pennartz C, Felderbauer P, Ansorge N, Banasch M, Schmitz F, Schmidt WE, Hoffmann P. Vascular endothelial growth factor (VEGF164) ameliorates intestinal epithelial injury in vitro in IEC-18 and Caco-2 monolayers via induction of TGF-beta release from epithelial cells. Scand J Gastroenterol. 2006;41:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zhang WJ, Xu GM. Expression of MMP-3 and MMP-9 in ulcerative colitis. Zhonghua Xiaohua Zazhi. 2003;23:187-188. |
| 18. | von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Zhao B, Katsuhiko S. Advances in research of tissue factor pathway inhibitor. Guowai Yixue Linchuang Shengwuhuaxue Yu Jianyanxue Fence. 1998;19:97-98. |
| 20. | Jeske W, Hoppensteadt D, Callas D, Koza MJ, Fareed J. Pharmacological profiling of recombinant tissue factor pathway inhibitor. Semin Thromb Hemost. 1996;22:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Liang J, Yang Q. The functional state of platelet and tissue factor pathway inhibitor in the patients with ulcerative colitis. Linchuang Neike Zazhi. 2004;21:405-406. |
| 22. | Abraham E. Tissue factor inhibition and clinical trial results of tissue factor pathway inhibitor in sepsis. Crit Care Med. 2000;28:S31-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Wang ZF. Advances and trend in thrombosis and hemostasis. Zhonghua Xueyexue Zazhi. 2004;25:190-191. |
| 24. | Ding WQ, Lin GJ, Xu SR. Changes of interleukin level in ulcerative colitis patients. Fudan Xuebao (Yixue Ban). 2001;7:330. |
| 25. | Miao HW, Luo XP. Advances in research of tissue factor pathway inhibitor 2. Yixue Zongshu. 2009;15:1286-1287. |
| 26. | Crawley JT, Goulding DA, Ferreira V, Severs NJ, Lupu F. Expression and localization of tissue factor pathway inhibitor-2 in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2002;22:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Herman MP, Sukhova GK, Kisiel W, Foster D, Kehry MR, Libby P, Schönbeck U. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Enkhbaatar P, Okajima K, Murakami K, Uchiba M, Okabe H, Okabe K, Yamaguchi Y. Recombinant tissue factor pathway inhibitor reduces lipopolysaccharide-induced pulmonary vascular injury by inhibiting leukocyte activation. Am J Respir Crit Care Med. 2000;162:1752-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |