Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7079
Revised: January 22, 2014
Accepted: May 1, 2014
Published online: June 21, 2014
Processing time: 253 Days and 14.5 Hours
Until recently the traditional treatment for hepatitis C infection included pegylated interferon and ribavirin combination therapy. The sustained virological response (SVR) seen with this combination is poor and requires lengthy treatment to achieve. Additionally, significant side effects and numerous contraindications prevented many patients from being successfully treated with this therapy. In 2011, two new protease inhibitors, telaprevir and boceprevir, were approved for use with pegylated interferon and ribavirin in the United States by the United States Food and Drug Administration. These agents have significantly improved SVR rates; however significant problems with toxicity remain including severe skin rash and neutropenia. There are a wide range of compounds in late stage development for the future treatment of hepatitis C that exploit many different mechanisms of viral inhibition. Some of these compounds include additional protease inhibitors, like telaprevir and boceprevir, as well as inhibitors of other nonstructural proteins in the viral genome such as NS5A and NS5B, and compounds that target host proteins within the virus. Some of these agents are being developed for oral administration once daily and various combinations are being assessed for use without the need for pegylated interferon and ribavirin. This paper reviews agents in late phase development that may be commercially available within 1-2 years.
Core tip: A plethora of new agents for the management of hepatitis C promising higher response rates and better tolerated side effect profiles is upon us. Many of these new drugs in development utilize novel pharmacologic mechanisms and may replace older more toxic therapies such as interferon and ribavirin. In addition, once daily dosing and shorter treatment durations should help improve adherence and optimize therapeutic outcomes for hepatitis C infection.
- Citation: Thompson JR. Emerging therapeutic options for the management of hepatitis C infection. World J Gastroenterol 2014; 20(23): 7079-7088
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7079.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7079
The hepatitis C virus (HCV) is a major cause of gastrointestinal morbidity and mortality worldwide. According to the World Health Organization (WHO), approximately 170 million people have chronic HCV infection with 3-4 million new infections occurring each year. In the United States, up to four million people have chronic HCV and 18-20000 new infections are diagnosed annually per the Centers for Disease Control (CDC).
Until recently treatment for HCV infection relied upon pegylated-interferon (PEG-IFN) and ribavirin combination therapy. The sustained virological response (SVR) seen with this combination is poor and requires lengthy treatment to achieve[1]. Additionally, significant side effects and numerous contraindications, prevent most patients from being successfully treated with these agents.
Recently two new protease inhibitors, telaprevir and boceprevir, have been approved by the Food and Drug Administration in the United States and the use of direct-acting antiviral (DAA) triple therapy has substantially improved SVRs[2,3]. In addition, a plethora of new chemical entities directed against various components of the nonstructural proteins in the hepatitis C virion are in late phase development and offer the promise of greater efficacy and fewer side effects, with once or twice daily oral administration and perhaps no longer requiring interferon and ribavirin. This paper will review telaprevir and boceprevir as well as agents that are in late phase development and may come available within the next 1-2 years.
The traditional treatment approach to hepatitis C relies heavily on combination therapy with pegylated interferon and ribavirin[4,5]. For genotype I disease, patients less than 75 kg receive peginterferon alpha-2a 180 μg by injection per week plus ribavirin 1000 mg daily in divided doses for 48 wk or peginterferon alpha-2b 1.5 μg/kg per week by injection plus ribavirin 800-1000 mg (based on weight) daily in divided doses for 48 wk. Patients greater than 75 kg receive peginterferon alpha-2a 180 μg by injection per week plus ribavirin 1200 mg daily in divided doses for 48 wk or peginterferon alpha-2b 1.5 μg/kg per week by injection plus ribavirin 1000-1400 mg (based on weight) daily in divided doses for 48 wk. Patients with genotypes 2 or 3 disease may be treated with peginterferon alpha-2a 180 μg by injection per week plus ribavirin 800 mg daily in divided doses for 24 wk or peginterferon alpha-2b 1.5 μg/kg per week by injection plus ribavirin 800-1400 mg (based on weight) daily in divided doses for 24 wk. Dose reductions may be necessary for patients with significant renal disease or those experience serious adverse reactions. For patients with contraindications or intolerance to ribavirin, monotherapy with peginterferon alpha-2a 180 μg by injection per week for 48 wk or peginterferon alpha-2b 1 μg/kg per week by injection for one year may be used.
Clinical success is greater with combination therapy, but SVR rates remain less than optimal at 54%-56%[5]. Numerous contraindications to ribavirin preclude some patients from receiving combination therapy. These contraindications include autoimmune hepatitis, decompensated liver disease, pregnancy, hemoglobinopathy, renal insufficiency, hemodialysis, thyroid disease, diabetes, rheumatoid arthritis, asthma or chronic obstructive pulmonary disease, and ischemic cardiovascular or cerebrovascular disease. Also, side effects associated with both pegylated interferon and ribavirin are substantial and include fatigue, fever, headache, nausea, arthralgia, musculoskeletal pain, insomnia, depression, neutropenia, thrombocytopenia, and anemia. For these reasons, only about 10% of patients with hepatitis C are successfully treated with traditional therapy.
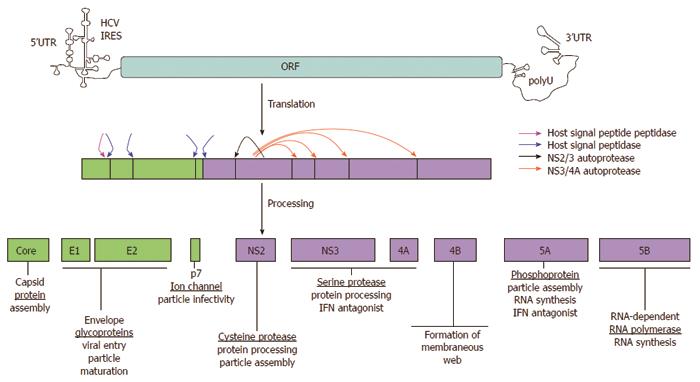
The hepatitis C virus contains six nonstructural HCV proteins that are processed by both viral and host proteases[6]. These nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) are primarily enzymes that are essential in the HCV life cycle (Figure 1). A number of compounds have been developed which target these proteases involved in HCV polyprotein procession. They are divided into two chemical classes, macrocyclic inhibitors and linear tetra-peptide α-ketoamid derivatives. The NS3/4A protease inhibitors are potent anti-viral agents as monotherapy against HCV replication, but may cause selection of resistance species. However, this resistance appears to be attenuated when the drugs are used in combination with standard peginterferon/ribavirin therapy. The first two drugs to reach the commercial marketplace in this class are telaprevir and boceprevir.
Telaprevir is an orally bioavailable NS3 protease inhibitor of the α-ketoamid class that binds the enzyme in a covalent but reversible manner with an enzyme-inhibitor complex half-life of 58 min. A phase I, placebo-controlled dose ranging study compared telaprevir 450 mg or 750 mg every 8 h with 1250 mg every 12 h in treatment-naïve genotype I patients and found the 750 mg dose to be most effective with a median reduction in HCV RNA of 4.4 log10 after 14 d[7]. A subsequent similar phase I study compared this dose following a 1250 mg loading dose either alone or in combination with peginterferon alpha-2a with peginterferon alpha-2a monotherapy for 14 d. The reduction in HCV RNA was 1.09 log10 in the peginterferon alpha-2a/placebo group, 3.99 log10 in the telaprevir/placebo group, and 5.49 log10 in the peginterferon alpha-2a/telaprevir group at the end of therapy. The development of resistant mutants was significantly lower in the peginterferon combination treatment group and no breakthrough of virus was seen throughout the study period[8]. An additional 28 d trial in treatment-naïve genotype I patients showed undetectable HCV RNA serum levels following telaprevir 750 mg every 8 h combined with peginterferon alpha-2a and weight-based dosing of ribavirin[9].
These early results were further validated in two phase II trials, PROVE 1 (American) and PROVE 2 (European) in treatment naïve, genotype I patients. In the PROVE 1 trial, combination therapy with telaprevir 1250 mg loading dose followed by 750 mg every 8 h or placebo, peginterferon alfa-2a 180 μg weekly, and ribavirin 1000-1200 mg/d based on weight for 12 wk was followed by interferon alfa-2a and ribavirin at the same dosages for an additional 0, 12, or 36 wk. Treatment was stopped after 12 or 24 wk only when a rapid virologic response (RVR) was achieved. This produced SVR rates of 35%, 61% and 67% at 12, 24, and 48 wk, respectively, vs 41% SVR with 48 wk standard therapy[10]. The PROVE 2 trial compared telaprevir + peginterferon alone for 12 wk, telaprevir + peginterferon and ribavirin for 12 wk, and telaprevir + peginterferon and ribavirin for 12 wk followed by an additional 12 wk of peginterferon and ribavirin alone, against standard peginterferon and ribavirin therapy. SVRs of 36%, 60%, and 69% respectively, vs 46% with standard therapy were documented[11]. thus, ribavirin appears important to achieve high SVRs and 12 wk of therapy appears insufficient to prevent relapse. In these trials skin rash, anemia, and gastrointestinal disorders were the most common side effects, causing up to 18% of patients to discontinue therapy.
A third trial, PROVE 3, evaluated telaprevir-based combination therapy in patients who had prior non-response or relapse with standard peginterferon and ribavirin therapy[12]. SVRs in patients retreated with telaprevir, interferon and ribavirin for 12 or 24 wk followed by peginterferon and ribavirin alone for up to 24 wk were 51% and 53%, respectively, compared with standard therapy at 14%. However, retreatment with telaprevir and interferon alone for 24 wk followed by peginterferon and ribavirin alone for an additional 24 wk gave only a 24% SVR rate, again demonstrating the need for ribavirin in initial therapy with telaprevir.
Telaprevir has since been evaluated in three large, randomized, controlled trials in both treatment naïve and standard treatment failure patients. The ADVANCE trial enrolled a total of 1095 patients with treatment naïve genotype 1 chronic hepatitis C and compared three treatment arms: telaprevir 750 mg three times daily for 8 wk followed by peginterferon and ribavirin or placebo for an additional 4 wk and then peginterferon and ribavirin in both groups for 12 subsequent weeks (or 36 subsequent weeks in patients who did not have a RVR at 24 wk); telaprevir 750 mg three times daily for 12 wk followed by peginterferon and ribavirin for 12 subsequent weeks (or 36 subsequent weeks in patients who did not have a RVR at 24 wk); and peginterferon and ribavirin or placebo for 12 wk followed by peginterferon and ribavirin for the subsequent 36 wk. SVRs were seen in 72% of the telaprevir 8-wk arm, 79% in the telaprevir 12-wk arm, but only 46% in the peginterferon and ribavirin or placebo 48-wk group (P < 0.001)[13]. A 24-wk treatment period appeared sufficient for patients who achieved an early RVR.
The REALIZE trial evaluated telaprevir in 662 patients of which 354 were prior relapsers and 308 were prior non-responders to standard treatment with peginterferon and ribavirin. This study compared telaprevir 750 mg three times daily with peginterferon and ribavirin for 12 wk followed by peginterferon and ribavirin for an additional 36 wk; peginterferon and ribavirin for 4 wk followed by telaprevir 750 mg three times daily with peginterferon and ribavirin for an additional 12 wk, and then peginterferon and ribavirin alone for a subsequent 32 wk; or standard therapy with peginterferon and ribavirin for a full 48 wk course. In patients who had previously relapsed, SVR rates were 84%-88% in the telaprevir groups compared with only 22% in the placebo or peginterferon and ribavirin standard therapy groups[14]. In patients who had previously partially responded to standard therapy, SVR rates were 56%-61% in telaprevir treated patients compared with 15% in placebo or peginterferon and ribavirin treated patients. Previous nonresponders to standard therapy achieved SVRs of 31%-33% with telaprevir compared with only 5% with placebo or peginterferon and ribavirin standard therapy. The 4 wk lead-in exposure to interferon and ribavirin did not produce substantially different results than starting simultaneously with telaprevir. Patients with on-treatment virologic failure were fewer in telaprevir groups and relapse rates were lower than the control subjects for prior relapsers and prior non-responders.
A third phase III clinical trial, ILLUMINATE, evaluated the efficacy of telaprevir therapy for 12 wk with either 24 or 48 wk of peginterferon and ribavirin based upon an achievement of extended RVR (eRVR) at 24 wk. Treatment naïve patients with genotype I chronic hepatitis C were given telaprevir 750 mg three times daily for 12 wk with peginterferon and ribavirin for at least 24 wk. Patients who achieved an extended rapid virologic response as evidenced by undetectable HCV RNA levels at weeks 4 and 12 were randomized to either stop treatment at week 24 or continue peginterferon and ribavirin therapy for a full 48 wk. Patients who did not achieve an eRVR continued peginterferon and ribavirin therapy for the full 48 wk as well. SVRs were at least 90% in both groups and total treatment for 24 wk was non-inferior to 48 wk for those achieving an extended rapid virologic response. The SVR rate was > 70% for all groups, compared with historical standards of 46%-52%, and this study population included patients with historically lower SVRs with standard therapy. In addition, the relapse rate was low for both eRVR+ and eRVR- patients at an overall rate of 9.2%[15].
The safety profile of telaprevir was evaluated in a pooled analysis of adverse events reported in all five phase II and III placebo-controlled trials[16]. During these trials, 2012 patients received at least one dose of telaprevir and 1346 patients were randomized to receive telaprevir 750 mg three times daily for 12 wk with peginterferon and ribavirin for 24-48 wk and 764 patients were randomized to receive placebo with peginterferon and ribavirin. A total of 73% of telaprevir-treated patients and 49.1% of placebo-treated patients completed the full duration of therapy. The most frequently occurring adverse events in the telaprevir group (> 20%) included pruritus, nausea, rash, anemia, and diarrhea. Hemorrhoids, anorectal discomfort, anal pruritus, dysgeusia, and generalized pruritus occurred less frequently. Anemia caused discontinuation of participation in 2.7% of telaprevir treated patients and 0.5% of placebo treated patients. Hemoglobin concentrations decreased rapidly over the first four weeks of treatment in both groups, but continued to decrease to a greater extent thereafter in telaprevir treated patients. The initial onset of rash occurred at any time following treatment with telaprevir, but most commonly occurred within the first four weeks. Progression of severity was reported for < 10% of cases, and many cases of rash resolved over the first 24 wk of therapy. A Dermatology Expert Panel reviewed the rashes and determined that the visual appearance of rash in telaprevir-treated patients was virtually indistinguishable from that seen in peginterferon and ribavirin-treated patients. One case of rash was suggestive of Stevens-Johnson syndrome, but it was not thought to be drug related as it occurred 11 wk after the last dose of telaprevir.
Boceprevir is another orally bioavailable NS3 protease inhibitor of the α-ketoamid class that binds the enzyme in a covalent but reversible manner. In an early dose ranging trial of 100-400 mg daily for 14 d as monotherapy in genotype I patients with prior treatment failure on standard therapy, the maximum dose achieved a 2.06 log10 reduction in HCV RNA load and was well tolerated. Viral breakthrough occurred in some patients, however[17]. A later phase I trial compared boceprevir 200 or 400 mg every 8 h for 7 d alone or in combination with peginterferon alpha 2b for 14 d with peginterferon alpha 2b monotherapy for 14 d in genotype I patients who were nonresponders to standard therapy. This approach achieved maximal reductions in HCV RNA of 2.45 and 2.88 log10 for boceprevir 200 and 400 mg, respectively, with peginterferon alpha 2b, 1.08 and 1.61 log10 for boceprevir 200 and 400 mg monotherapy, and 1.08 and 1.26 log10 for peginterferon alpha 2b alone in the respective boceprevir dose groups[18]. Boceprevir was well tolerated in this trial as well, both alone and in combination with peginterferon alpha-2b, but viral breakthrough was observed again, primarily in patients receiving monotherapy.
The addition of ribavirin to boceprevir and peginterferon alpha-2b was evaluated in a phase II trial (SPRINT-1) in treatment naïve, genotype I patients. In this trial patients received 28 or 48 wk of boceprevir 800 mg three times daily, peginterferon alpha-2b, and ribavirin or a 4 wk lead-in treatment of peginterferon alpha-2b with ribavirin followed by 24 or 44 wk of boceprevir 800 mg three times daily, peginterferon alpha-2b, and ribavirin compared with standard therapy with peginterferon alpha-2b and ribavirin for a full 48 wk. The four week lead-in treatment with peginterferon alpha-2b and ribavirin boosted SVR rates from 54% to 56% at 28 wk and from 67% to 75% at 48 wk, compared with 38% with standard peginterferon alpha-2b and ribavirin therapy for 48 wk[19]. However, RVR rates with boceprevir triple therapy were only 38% compared with 70% seen in telaprevir triple therapy trials. The most common side effects seen with boceprevir in this trial were anemia, nausea, vomiting, and dysgeusia. A subsequent phase II trial evaluated boceprevir triple therapy in HCV genotype I nonresponders, but SVR rates were poor, ranging from 2% for control to 14% with boceprevir[20].
Boceprevir has subsequently been evaluated in two large, well-controlled, multicenter clinical trials in both untreated and previously treated patients with genotype I chronic HCV infection. The SPRINT-2 trial enrolled a total of 1099 patients with untreated genotype I HCV infection who were randomized to receive triple therapy with boceprevir 800 mg three times daily with peginterferon alpha-2b and ribavirin for 24 or 44 wk following a lead-in treatment of 4 wk with standard peginterferon alpha-2b and ribavirin therapy and compared with a full 48 wk course of standard peginterferon alpha-2b and ribavirin therapy. One arm of the trial was allowed to discontinue therapy at 24 wk if HCV RNA levels were undetectable, while those with detectable levels received additional interferon alpha-2b and ribavirin with placebo from weeks 28 to 48. Black patients and nonblack patients were enrolled and analyzed separately. In the larger, nonblack cohort, an SVR rate of 40% was achieved with standard pegylated interferon alpha-2b and ribavirin for 48 wk, compared with 67% in patients treated with boceprevir for 24 wk and 68% in patients treated with boceprevir for 44 wk (P < 0.001)[21]. In the black cohort, an SVR rate of 23% was achieved with standard pegylated interferon alpha-2b and ribavirin for 48 wk, compared with 42% in patients treated with boceprevir for 24 wk (P < 0.04) and 53% in patients treated with boceprevir for 44 wk (P < 0.004). In the variable duration arm, 44% of patients had nondetectable HCV RNA levels and were able to discontinue therapy at 24 wk. Adverse events occurred in 98% of patients and were similar in number across groups. Fatigue, headache, and nausea were the most common clinical adverse events. Dysgeusia was twice as frequent in boceprevir treated patients and anemia occurred in 49% of boceprevir treated patients compared with 29% of those receiving peginterferon alpha-2b only. This led to dose reduction in 13% of the control patients and 21% of boceprevir treated patients and discontinuation of therapy in 1% and 2%, respectively.
The RESPOND-2 trial provided a similar evaluation of boceprevir in patients with chronic HCV genotype I infection, who had been previously treated with standard therapy and experienced either a nonresponse or relapse. This trial enrolled 403 patients and randomized them in a 1:2:2 fashion to standard therapy with peginterferon alpha-2b and ribavirin for 48 wk, boceprevir 800 mg three times daily with peginterferon alpha-2b and ribavirin for 32 wk, or boceprevir 800 mg three times daily with peginterferon alpha-2b and ribavirin for 44 wk. Both boceprevir triple therapy arms were preceded by 4 wk of treatment with standard peginterferon alpha-2b and ribavirin therapy. One arm of the trial was allowed to discontinue therapy at 32 wk if HCV RNA levels were undetectable, while those with detectable levels received additional interferon alpha-2b and ribavirin with placebo from weeks 36 to 48. The overall rate of SVR for boceprevir treated patients was significantly higher in this trial (59% at 32 wk and 66% at 44 wk) compared with standard therapy (21%, P < 0.001) for the full 48 wk[22]. Patients who had undetectable HCV RNA levels at week 8 had SVR rates of 86% after 32 wk of boceprevir triple therapy and 88% after 44 wk. Patients whose HCV RNA level decreased by less than 1 log10 at 4 wk had SVR rates of 33% and 34% after boceprevir triple therapy compared with 0% after standard therapy. Side effects seen in this trial were similar to those seen in SPRINT-2, with anemia being more common in boceprevir treated patients (43%-46%) compared with standard therapy (20%). Erythropoeitin was required to manage the anemia in 41%-46% of boceprevir treated patients compared with 21% with standard therapy.
Protease inhibitors are classified as 1st generation or 2nd generation based upon resistance profiles. The generations are further subdivided into waves based on improved potency and dosing[23]. Telaprevir and boceprevir represent the first wave of the first generation of protease inhibitors. A number of agents are currently being investigated, which constitute the second wave. These agents have resistance profiles similar to telaprevir and boceprevir. Second generation protease inhibitors have pan-genotypic activity and a higher barrier for resistance than first generation PIs. Table 1 lists protease inhibitors currently under development.
| First generation (wave 2) |
| Simeprevir |
| Faldaprevir |
| Danoprevir |
| Vaniprevir |
| ABT-450/ABT-450r |
| Asunaprevir |
| Second generation |
| MK-5172 |
| ACH-2684 |
Simeprevir is a macrocyclic NS3/4A protease inhibitor that is active against all genotypes of hepatitis C except genotype 3. It undergoes hepatic metabolism through cytochrome p4503A with an elimination half-life of 40 h, which makes it suitable for once daily dosing[24]. In a large phase III clinical trial, simeprevir 150 mg once daily with pegylated interferon and ribavirin for 12 wk followed by pegylated interferon and ribavirin alone for an additional 12-36 wk produced SVRs at 12 wk post-treatment (SVR12) of 81% in genotype 1, treatment naïve patients[25]. Response rates were even higher in patients with the IL28B polymorphism and lower stage liver fibrosis. Frequently reported adverse effects included rash and indirect hyperbilirubinemia.
Faldaprevir is another potent NS3 protease inhibitor that can be dosed on a once daily basis. However, its activity is limited to genotype 1 disease. In a large phase III trial of genotype 1, treatment-naïve patients, faldaprevir 120 or 240 mg once daily with pegylated interferon and ribavirin for 12 wk achieved 80% SVR12 with even higher responses seen in patients with the IL28B polymorphism[26]. Indirect hyperbilirubinemia was reported in this trial as well.
Danoprevir is also a potent macrocyclic protease inhibitor with activity against HCV genotypes 1, 4, and 6. Unlike simeprevir and faldaprevir, however, danoprevir requires twice daily dosing. In a recent phase IIb study, danoprevir 300 mg every 8 h, 600 mg every 12 h, 900 mg every 12 h or placebo was given with pegylated interferon and ribavirin for 12 wk in genotype 1 patients and followed with pegylated interferon/ribavirin alone for an additional 48 wk[27]. Treatment was stopped at 24 wk if an extended rapid virologic response (eRVR) with HCV RNA below 15 IU/mL during weeks 4-20 was achieved. This occurred in 65% of the 300 mg group and 70% of the 600 mg group. Unfortunately, the 900 mg group was discontinued early due to reversible, grade 4 increases in alanine aminotransferase in three patients. SVR at 24 wk post-treatment was 68%, 85%, and 76% in the 300 mg, 600 mg, and 900 mg groups, respectively, vs 42% in the placebo group. Serious adverse effects occurred in 19% of patients in the placebo group vs 7%-8% of patients in the active treatment groups.
Vaniprevir is a potent NS3/4A protease inhibitor in genotypes 1 and 2 that has shown efficacy in phase II trials when given twice daily to non-cirrhotic patients who failed previous pegylated interferon/ribavirin therapy. Vaniprevir 300 mg and 600 mg twice daily for 24-48 wk with pegylated interferon/ribavirin therapy produced SVR rates at 24 wk post-treatment ranging from 66.7%-78% vs only 19% with placebo and pegylated interferon/ribavirin[28]. Higher rates of gastrointestinal adverse events were seen with vaniprevir, but no difference in rash or anemia occurred between the two groups.
ABT-450 is an NS3/4A protease inhibitor that is metabolized by cytochrome p4503A and coadministered with ritonavir 100 mg (ABT-450r) to allow once daily dosing. It has been studied in combination with ABT-333, an NS5B nonnucleoside polymerase inhibitor, as part of an interferon-free regimen[29]. Clinical trial results of this combination will be discussed later.
Asunaprevir is also a potent NS3 inhibitor that is dosed twice daily, but is limited in efficacy to genotype 1 disease. It has been studied in combination with daclatasvir, a potent NS5A replication complex inhibitor, as a part of an interferon-free regimen[30]. Clinical trial results of this combination will be discussed later.
MK-5172 is a second generation protease inhibitor with pan-genotypic activity. In a phase II trial, MK-5172 100, 200, 400 and 800 mg once daily combined with pegylated interferon and ribavirin for 12 wk gave SVRs of 86%, 92%, 91%, and 87%, respectively, compared with 54% in the control group receiving triple therapy with pegylated interferon/ribavirin and boceprevir[31]. Elevations in bilirubin and serum transaminases were seen mostly in the higher dose groups. Rates of serious adverse events were similar between all groups but half as many patients discontinued therapy due to adverse events in the MK-5172 treatment arms (7% vs 14%).
ACH-2684 is also a pan-genotypic, highly potent second generation protease inhibitor. This compound can be dosed orally once daily and does not inhibit cytochrome p450 microsomal enzymes or activate transcription[32]. ACH-2684 has completed phase I trials and is currently being evaluated in phase II.
Unlike protease inhibitors that generally interfere with protein processing within the HCV genome, a number of compounds in development target nonstructural proteins involved in viral replication. (Table 2) Daclatasvir works by inhibiting the function of a viral replication complex by binding to the NS5A protein. It is highly potent orally, pan-genotypic in coverage and can be dosed once daily, but offers a lower barrier to resistance so will likely be used as combination therapy. Initial studies in combination with pegylated interferon/ribavirin showed 10 and 60 mg doses once daily produced SVRs at 24 wk post-treatment of 83% compared to 25% with standard therapy alone[33]. More recently, daclatasvir combined with sofosbuvir, an NS5B polymerase inhibitor discussed below, was shown to produce a 100% SVR at 24 wk in genotype 1 patients who had failed triple therapy with pegylated interferon/ribavirin and telaprevir or boceprevir without ribavirin[34]. The combination was generally well tolerated without serious adverse events or discontinuations related to adverse events. Daclatasvir has also been studied in combination with asunaprevir and another NS5B polymerase inhibitor (BMS-791325) in treatment-naïve, genotype 1 non-cirrhotic patients for 12 and 24 wk with SVRs of 88% and 94%, respectively[30].
| NS5A hepatitis C virus replication inhibitors |
| Daclatasvir |
| Ledipasvir |
| ACH-3102 |
| ABT-267 |
| NS5B RNA dependent RNA polymerase inhibitors |
| Sofosbuvir |
| Mericitabine |
| ABT-333 |
| Host targeted agents |
| Alisporivir |
| Miravirsen |
| Interferon-λ |
Ledipasvir is also an NS5A replication inhibitor that has been studied in combination with sofosbuvir, an NS5B polymerase inhibitor, in phase II trials. Ledipasvir 90 mg orally once daily in combination with both sofosbuvir and ribavirin in genotype 1 treatment-naïve and prior nonresponse patients increased SVRs to 100% at 12 wk post-treatment vs 84% in naïve patients given sofosbuvir and ribavirin alone and 10% in sofosbuvir/ribavirin nonresponders[35]. These agents are currently in phase III trials in a fixed-dose, once daily oral combination formulation.
ACH-3102 is a structurally distinct, pan-genotypic, second generation NS5A replication inhibitor with a high barrier to resistance. An ongoing phase II clinical trial is evaluating an oral, interferon-free combination regimen of ACH-3102 and sovaprevir with and without ribavirin for 12 and 8 wk durations of treatment in genotype 1 patients with HCV[36]. ACH-3102 has been granted fast-track status from the United States Food and Drug Administration.
ABT-267 is an additional NS5A replication inhibitor that has been studied at a dose of 25 mg once daily in combination with ABT-450/r and ABT-333, an NS5B polymerase inhibitor, and ribavirin in non-cirrhotic, treatment-naïve patients with genotype 1 disease and prior pegylated interferon/ribavirin nonresponders. Phase II trial data showed a greater than 90% SVR in both groups at both 12 and 24 wk post-treatment[37]. This combination regimen is currently being evaluated in a large phase III trial.
Nucleoside/tide inhibitors (NIs) block HCV RNA transcription and elongation by acting as chain terminators. Because of the highly conserved nature of the polymerase catalytic site, NIs as a class are pan-genotypic in coverage and they have the highest barrier to resistance. Non-NIs (NNIs) bind allosteric polymerase sites away from the catalytic site and, while potent, have a much lower barrier to resistance.
Sofosbuvir is an NS5B nucleoside inhibitor that is pan-genotypic, highly potent, and suitable for once daily oral dosing. This compound has now completed study in four large, phase III trials and has been awarded priority review status by the United States Food and Drug Administration. Additionally, phase II studies of sofosbuvir combination therapies as interferon-free regimens are currently underway.
Sofosbuvir 400 mg once daily was initially studied in combination with pegylated interferon/ribavirin therapy in 327 HCV patients with genotypes 1, 4, 5, or 6 for 12 wk (98% of patients were genotype 1 or 4)[38]. A SVR of 90% was achieved in these patients. A subsequent follow up study compared 499 patients with HCV genotypes 2 or 3 receiving sofosbuvir 400 mg once daily plus ribavirin or pegylated interferon plus ribavirin for 12 wk. The sofosbuvir/ribavirin regimen was shown to be non-inferior to the pegylated interferon/ribavirin regimen achieving as SVR of 67%[38]. Adverse events were less common with sofosbuvir than pegylated interferon and consisted primarily of headache, fatigue, nausea, and neutropenia.
Sofosbuvir has since been evaluated in HCV patients with genotype 2 or 3 who were not eligible to receive pegylated interferon or who had previous failed therapy with this agent. Sofosbuvir 400 mg once daily in combination with ribavirin or matching placebos was studied in 278 patients who had previously discontinued pegylated interferon therapy secondary to side effects, who had a current medical condition precluding treatment with pegylated interferon, or who decided against treatment with pegylated interferon for other reasons. Sofosbuvir produced a SVR at 12 wk of 78% vs 0% with placebo in these patients[39]. In a study of 201 similar patients who had failed previous therapy with pegylated interferon, sofosbuvir 400 mg once daily plus ribavirin was compared in 12 and 16 wk treatment groups. Sofosbuvir achieved SVR rates of 50% and 73% in these treatment arms respectively, compared with historical controls of 25%[39]. The drug was well tolerated in both of these trials with primary side effects of fatigue and insomnia occurring in 3%-5% of patients and few patients discontinuing use because of adverse effects.
Mericitabine is also an NS5B nucleoside inhibitor with pan-genotypic activity, but requiring twice daily dosing. Mericitabine 1000 mg twice daily or placebo was given with pegylated interferon/ribavirin to 166 patients with HCV genotypes 1 or 4 for 24 wk. Patients who achieved an HCV RNA level < 15 IU/mL (eRVR) from weeks 4 to 22 stopped all treatment at that time, but all other patients continued to receive pegylated interferon/ribavirin for a full 48 wk of therapy. Mericitabine-treated patients achieved a SVR at 24 wk post-treatment of 56.8%, compared with 36.5% of placebo-treated patients[40]. Relapse rates were 27.7% and 32% in patients treated with mericitabine and placebo, respectively. The safety profile was similar in both groups, but fewer patients in the mericitabine group discontinued therapy for reasons of safety.
ABT-333 is a NS5B non-nucleoside polymerase inhibitor that has been evaluated in combination with the protease inhibitor, ABT450/r, and ribavirin in HCV genotype 1 treatment-naïve patients, as well as null or partial prior responders to pegylated interferon and ribavirin. ABT-333 at a dose of 400 mg twice daily plus ABT-450/r 150 or 250 mg and ribavirin produced SVRs of 93%-95% at 12 wk post-treatment in previously untreated patients[41]. Only 47% of prior null or partial responders to pegylated interferon and ribavirin achieved an SVR12, however. As mentioned previously, this drug is currently being studied in a phase III trial in combination with ABT-450/r and ABT-267, with or without ribavirin in genotype 1b patients with HCV.
Alisporivir is a nonimmunosuppressive form of cyclosporine that blocks HCV replication by neutralizing the peptidyl-prolyl isomerase activity of the host protein, cyclophilin, which is required by NS5B for maximum RNA binding[42]. Alisporivir 600 mg twice daily for one week followed by 600 mg daily thereafter or placebo combined with pegylated interferon and ribavirin was evaluated in 288 patients with treatment-naïve genotype 1 HCV for 24 and 48 wk of therapy[43]. SVR rates at 24 wk post-treatment were 76% in patients receiving triple therapy with alisporivir and pegylated interferon/ribavirin for 48 wk vs 55% in the control arm. The drug was well tolerated overall with serious adverse events occurring in 7% of patients treated with alisporivir for 24 wk and 10% of patients treated with alisporivir for 48 wk. Subsequent to this trial, 3 patients developed pancreatitis, including one who died, and the FDA asked the manufacturer to place further trials on clinical hold until it can be determined if alisporivir potentiates the risk of hepatitis that is known with interferon therapy.
Miravirsen is an antisense oligonucleotide that works by inhibiting miR-122, a micro-RNA found in the liver essential to the stability and propagation of HCV RNA. Miravirsen can be dose subcutaneously once daily, is pan-genotypic in activity, and has a high barrier to resistance. In a phase IIa dose-ranging trial, miravirsen resulted in dose-dependent reduction in HCV RNA levels that were still not present 14 wk post-treatment[44]. No dose-limiting adverse events or escape mutations were noted in this small trial.
Pegylated interferon lambda is a type 3 interferon that signals through a different receptor than type 1 interferon, but can inhibit HCV viral replication in vitro. In early trials it was well tolerated without the usual flu-like syndrome and hematopoietic effects typically seen with interferon alpha. A phase II trial comparing pegylated interferon lambda with pegylated interferon alpha each in combination with ribavirin found comparable SVR rates but less interferon side effects with pegylated interferon lambda[45]. This could prove to be a useful option for patients who do not respond to interferon-free regimens currently in development.
Clearly the availability of both telaprevir and boceprevir, as well as other anti-HCV drugs in development, will vastly improve our antiviral armamentarium for patients with hepatitis C. Telaprevir and boceprevir must be used in conjunction with standard pegylated interferon and ribavirin therapy. However, the duration of treatment may be reduced substantially for many patients, based upon the clinical trial results from patients receiving a rapid virologic response. Substantial improvement in the SVR should be seen with these new agents for both treatment-naïve as well as prior relapsed or non-responsive patients. The further development of non-protease inhibitor based therapy offers the potential for interferon and ribavirin free therapy with once or twice daily oral dosing and a better tolerated side-effect profile. Additional studies are needed to determine which agent or agents should be used initially and the optimal combination regimen for patients needing additional therapy.
At this point in time, it appears that second generation protease inhibitors will supplant current protease inhibitor therapy on the basis of broader genotypic coverage and a higher barrier to resistance. Both NS5A replication inhibitors and NS5B RNA dependent RNA polymerase inhibitors offer greater potency than currently available drugs, but a lower barrier to resistance with the NS5As will likely require combination therapy. The availability of these new agents should preclude the continued need for interferon therapy and possibly for ribavirin therapy as well.
P- Reviewer: Scuteri A S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2239] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 2. | INCIVEK. Cambridge, MA: Vertex Pharmaceuticals Incorporated, 2011. Available from: http://en.wikipedia.org/wiki/Vertex_Pharmaceuticals. |
| 3. | VICTRELIS. Whitehouse Station, NJ: Schering Corporation, 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020503s046lbl.pdf. |
| 4. | PEGASYS®. Nutley, NJ: Hoffman-La Roche Inc. 2011. Available from: http://www.manta.com/c/mmbpq0d/hoffmann-la-roche-inc. |
| 5. | PegIntron®. Whitehouse Station, NJ: Merck & Co., Inc., 2011. Available from: http://www.merck.com/index.html. |
| 6. | Tencate V, Sainz B Jr, Cotler SJ, Uprichard SL. Potential treatment options and future research to increase hepatitis C virus treatment response rate. Hepat Med. 2010;2010:125-145. [PubMed] |
| 7. | Reesink HW, Zeuzem S, Weegink CJ, Forestier N, van Vliet A, van de Wetering de Rooij J, McNair L, Purdy S, Kauffman R, Alam J. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology. 2006;131:997-1002. [PubMed] |
| 8. | Forestier N, Reesink HW, Weegink CJ, McNair L, Kieffer TL, Chu HM, Purdy S, Jansen PL, Zeuzem S. Antiviral activity of telaprevir (VX-950) and peginterferon alfa-2a in patients with hepatitis C. Hepatology. 2007;46:640-648. [PubMed] |
| 9. | Lawitz E, Rodriguez-Torres M, Muir AJ, Kieffer TL, McNair L, Khunvichai A, McHutchison JG. Antiviral effects and safety of telaprevir, peginterferon alfa-2a, and ribavirin for 28 days in hepatitis C patients. J Hepatol. 2008;49:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 11. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 793] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 12. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 13. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1860] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 14. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1212] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 15. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 601] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 16. | U.S. Food and Drug Administration. Briefing Information for the April 28, 2011 Meeting of the Antiviral Drugs Advisory Committee. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/ucm252559.htm. |
| 17. | Susser S, Welsch C, Wang Y, Zettler M, Domingues FS, Karey U, Hughes E, Ralston R, Tong X, Herrmann E. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology. 2009;50:1709-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 18. | Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK, Hussain M, Shah A, Cutler D, Zhang J. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology. 2007;132:1270-1278. [PubMed] |
| 19. | Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 20. | Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, Poynard T, Morgan TR, Molony C, Pedicone LD. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608-618.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1978] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 22. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1307] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 23. | Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J Viral Hepat. 2012;19:449-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Tanwar S, Trembling PM, Dusheiko GM. TMC435 for the treatment of chronic hepatitis C. Expert Opin Investig Drugs. 2012;21:1193-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Manns PM, Poordad F. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment-resistant patients: results from QUEST-2 a phase III trial. Presented at 48th Annual Meeting of the European Association for the Study of the Liver: 2013 Apr 24-28; . |
| 26. | Ferenci P, Asselah T, Foster GR. Faldaprevir plus pegylated interferon alpha-2A and ribavirin in chronic HCV genotype-1 treatment-naïve patients: final results from STARTVerso1, a randomized double blind placebo-controlled phase III trial. Presented at 48th Annual Meeting of the European Association for the Study of the Liver: 2013 Apr 24-28; . |
| 27. | Marcellin P, Cooper C, Balart L, Larrey D, Box T, Yoshida E, Lawitz E, Buggisch P, Ferenci P, Weltman M. Randomized controlled trial of danoprevir plus peginterferon alfa-2a and ribavirin in treatment-naïve patients with hepatitis C virus genotype 1 infection. Gastroenterology. 2013;145:790-800.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Park JS, Chang DY, Kim JH, Jung JH, Park J, Kim SH, Lee YD, Kim SS, Suh-Kim H. Retrovirus-mediated transduction of a cytosine deaminase gene preserves the stemness of mesenchymal stem cells. Exp Mol Med. 2013;45:e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Lawitz E, Poordad F, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, Larsen L, Menon R, Koev G, Tripathi R. A phase 2a trial of 12-week interferon-free therapy with two direct-acting antivirals (ABT-450/r, ABT-072) and ribavirin in IL28B C/C patients with chronic hepatitis C genotype 1. J Hepatol. 2013;59:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Everson GT, Sims KD, Rodriguez-Torres M. Interim analysis of an interferon (IFN)- and ribavirin (RBV)-free regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 in treatment-naive, hepatitis C virus genotype 1-infected patients. Presented at 48th Annual Meeting of the European Association for the Study of the Liver: 2013 Apr 24-28; . |
| 31. | Manns M, Vierling JM, Bacon BR. High sustained viral response at 12- and 24-week follow-up of MK-5172 with pegylated interferon alfa-2b and ribavirin (PR) in HCV genotype 1 treatment-naive non-cirrhotic patient. Presented at 48th Annual Meeting of the European Association for the Study of the Liver (EASL 2013): 2013 Apr 24-28; . |
| 32. | Lawitz E, Hill J, Vince B. ACH-2684 demonstrates potent viral suppression in genotype 1 hepatitis C patients with and without cirrhosis: safety, pharmacokinetic, and viral kinetic analysis. Presented at 48th Annual Meeting of the European Association for the Study of the Liver: 2013 April 24-28; . |
| 33. | Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, Hézode C, Lim JK, Bronowicki JP, Abrams GA. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M. Sustained virologic response with daclatasvir plus sofosbuvir ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC). Presented at 48th Annual Meeting of the European Association for the Study of the Liver: 2013 Apr 24-28; . |
| 35. | Gane EJ, Stedman CA, Hyland RH. ELECTRON: 100% SVR rate for once-daily sofosbuvir plus ledipasvir plus ribavirin given for 12 weeks in treatment- naive and previously treated patients with HCV GT 1. Presented at 20th Annual Conference on Retroviruses and Opportunistic Infections: 2013 Mar 3-6; . |
| 36. | HCV NS5A Inhibitor – ACH-3102. Available from: http://www.achillion.com/ACH3102. Accessed Sep 28, 2013. |
| 37. | Kowdley KV, Lawitz E, Poordad F. Safety and efficacy of interferon-free regimens of ABT-450/r, ABT-267 and ABT-333 /- ribavirin in patients with chronic genotype 1 infection: results from the Aviator study. Presented at 48th Annual Meeting of the European Association for the Study of the Liver (EASL 2013): 2013 Apr 24-28; . |
| 38. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1324] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 39. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 837] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 40. | Pockros PJ, Jensen D, Tsai N, Taylor R, Ramji A, Cooper C, Dickson R, Tice A, Kulkarni R, Vierling JM. JUMP-C: a randomized trial of mericitabine plus pegylated interferon alpha-2a/ribavirin for 24 weeks in treatment-naïve HCV genotype 1/4 patients. Hepatology. 2013;58:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, Heckaman M, Larsen L, Menon R, Koev G. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 42. | Schultheiss C, Schauer T, Nahrstaedt H, Seidl RO. Evaluation of an EMG bioimpedance measurement system for recording and analysing the pharyngeal phase of swallowing. Eur Arch Otorhinolaryngol. 2013;270:2149-2156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Flisiak R. Cyclophilin inhibitor alisporivir active against HCV in phase 2b, HBV in lab. Presented at 46th Annual Meeting of the European Association for the Study of the Liver (EASL 2011): 2011 Mar 30-Apr 3; . |
| 44. | Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1665] [Cited by in RCA: 1694] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 45. | Muir A, Hillson J, Gray T. Peginterferon lambda-1a (Lambda) compared to peginterferon alpha-2a (Alfa) in treatment-naïve patients with HCV genotypes (GT) 1 or 4: SVR24 results from EMERGE Phase 2b. Presented at 63rd Annual Meeting of the American Association for the Study of Liver Diseases: 2012 Nov 9-12; . |