Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6953
Revised: February 13, 2014
Accepted: March 12, 2014
Published online: June 14, 2014
Processing time: 223 Days and 9.4 Hours
AIM: To investigate whether early liver regeneration after resection in patients with hepatic tumors might be influenced by post-operative infective complications.
METHODS: A retrospective analysis of 27 liver resections for tumors performed in a single referral center from November 2004 to January 2010. Regeneration was evaluated by multidetector computed tomography at a mean follow-up of 43.85 d. The Clavien-Dindo classification was used to evaluate postoperative events in the first 6 mo after transplantation, and Centers for Disease Control and Prevention definitions were used for healthcare associated infections data. Generalized linear regression models with Gaussian family distribution and log link function were used to reveal the principal promoters of early liver regeneration.
RESULTS: Ten of the 27 patients (37%) underwent chemotherapy prior to surgery, with a statistically significant prevalence of patients with metastasis (P = 0.007). Eight patients (30%) underwent embolization, 3 with primary tumors, and 5 with secondary tumors. Twenty patients (74%) experienced complications, with 12 (60%) experiencing Clavien-Dindo Grade 3a to 5 complications. Regeneration ≥ 100% occurred in 10 (37%) patients. The predictors were smaller future remnant liver volume (-0.002; P < 0.001), and a greater spleen volume/future remnant liver volume ratio (0.499; P = 0.01). Patients with a resection of ≥ 5 Couinaud segments experienced greater early regeneration (P = 0.04). Nine patients experienced surgical site infections, and in 7 cases Clavien-Dindo Grade 3a to 4 complications were detected (P = 0.016). There were no significant differences between patients with primary or secondary tumors, and either onset or infections or severity of surgical complications.
CONCLUSION: Regardless of the onset of infective complications, future remnant liver and spleen volumes may be reliable predictors of early liver regeneration after hepatic resection on an otherwise healthy liver.
Core tip: Our study of 27 patients who underwent hepatectomies focuses on early liver regeneration after resection in patients with hepatic tumors but no underlying liver disease and on determining if post-operative infective complications might influence it. We identify three predictive factors for optimal early regeneration.
- Citation: Pagano D, Spada M, Parikh V, Tuzzolino F, Cintorino D, Maruzzelli L, Vizzini G, Luca A, Mularoni A, Grossi P, Gridelli B, Gruttadauria S. Liver regeneration after liver resection: Clinical aspects and correlation with infective complications. World J Gastroenterol 2014; 20(22): 6953-6960
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6953.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6953
The success of liver resection for the treatment of hepatic malignancies depends on the remnant liver’s ability to regenerate after major tissue loss. Human liver is able to regenerate thanks to a hyperplastic reaction in the remnant liver[1]. However, if after resection, the remnant liver is less than 20% of the original liver volume, liver function might not sustain metabolic, synthetic, and detoxifying needs, leading to post-resection liver failure[2].
Since Lortat-Jacob reported the first anatomic right hepatectomy, in 1952[3], and particularly in the last two decades, hepatic surgery has achieved important technical breakthroughs, such as intermittent portal triad clamping, total vascular exclusion, preoperative portal vein embolization with two-stage hepatectomy, and sophisticated methods of parenchymal transection[1,4,5].
These changes have resulted in a significant reduction in post-resection liver failure, and the related onset of complications, resulting in improved post-resection survival[6-8]. In addition, pre- and postoperative multidetector computed tomography (MDCT) scans have become essential tools for both estimating and assessing the volume of the remnant liver[9-11]. Yet, despite these advances, few studies have investigated the impact of post-operative infective complications on early liver regeneration after resection for hepatic tumors. Healthcare-associated infections (HAIs) are a major cause of increased length of hospital stay and mortality[12]. Hospitals with surgeons who treat patients with multiple no modifiable risk factors would expect higher HAIs rates; moreover, risk adjustment that accounts for differences in patient case mix is critical to allow for more meaningful comparisons between surgeons and/or hospitals, when using infection data as a performance evaluation. Clinical variables may reflect surgical technique more than patient case mix for calculating any risk adjustment strategy, and surgical technique analyses can inappropriately allow for adjusting rates down among surgeons with poor technique. In addition, the presence of more complex procedure-specific HAIs might delay adjuvant treatment following radical liver resections that would include chemotherapy or radiation oncologic treatments. These issues are highlighted in the National Healthcare Safety Network, a Web-based system used by the Centers for Disease Control and Prevention (CDC)[13]. The aims of this single center, retrospective study were to analyze the impact of surgical infections on early liver regeneration after liver resection in patients with hepatic malignancies, primary or secondary, but no underlying liver disease, and determine which, if any, preoperative variables have significant predictive value for liver regeneration soon after surgery.
Twenty-seven patients with no underlying chronic liver disease who underwent liver resection of at least 2 Couinaud classification[14] segments between November 2004 and January 2010 were retrospectively identified from a prospectively collected data base (Table 1). The study was approved by our Institutional Research Review Board.
| Patients (n) | Right hepatectomy | Extended right hepatectomy | Left hepatectomy | Left lobectomy |
| Overall Tumor patients (27) | 5 (18.5) | 13 (48.1) | 8 (29.6) | 1 (3.7) |
| Primary liver tumor (14) | 1 (20.0) | 7 (46.6) | 5 (33.3) | 1 (6.7) |
| Secondary liver tumor (13) | 4 (25.0) | 6 (50.0) | 3 (25.0) | 0 |
Patients were imaged pre- and postoperatively with 64-slice MDCT (VCT, GE Medical Systems, Milwaukee, United States). The protocol consisted of the acquisition of basal images of the entire superior abdomen, followed by acquisition of triple-phase, contrast-enhanced images during the hepatic arterial phase, portal venous phase, and delayed phase. All MDCT acquisitions were started at the top of the liver and proceeded in a cranio-caudal direction during a single breath-hold on inspiration. Contrast-enhanced images were obtained with a dose of 1.8 mL/kg body weight of non-ionic iodinated contrast material (iopramide 370 mg I/mL, Ultravist, BayerShering Pharma, Berlin, Germany) administered with a power injector (Stellant, Medrad, Pittsburgh, United States) at a flow rate of 5 mL/s through an 18-gauge intravenous catheter placed in an antecubital vein.
Calculation of the future remnant liver volume (FRLV) was made according to the number of Couinaud segments to be resected. Major vessels, including the inferior vena cava and the extrahepatic portal vein, as well as major fissures, such as the fissure for the ligamentum teres, were excluded. Regeneration of the remnant liver volume (RLV), expressed as percentage, was calculated with the following formula: Percent liver regeneration = (RLVday, follow-up - FRLV) × 100/FRLV.
Preoperative blood biochemical tests were done in all 27 patients. Values drawn from electronic medical records included alanine aminotransferase, aspartate aminotransferase, albumin, total bilirubin, gamma-glutamyl transpeptidase, glucose, platelet count, prothrombin time in (%), and international normalized ratio (INR) (Table 2). The Clavien-Dindo classification was used to evaluate postoperative events in the first 6 mo after transplantation[15].
| Preoperative factor | Patients with malignancies |
| Age (yr) | 61.04 ± 11.40 |
| Weight (kg) | 71.71 ± 12.33 |
| Height (cm) | 165.74 ± 8.82 |
| BMI | 26.02 ± 3.43 |
| LV (cc) | 2036.47 ± 804.95 |
| FRLV (cc) | 701.45 ± 343.56 |
| Bilirubin (mg/dL) | 0.88 ± 1.86 |
| ALT (u/L) | 57.26 ± 33.14 |
| Albumin (g/dL) | 3.44 ± 0.67 |
| AST (u/L) | 45.78 ± 38.28 |
| Creatinine(mg/dL) | 0.94 ± 0.24 |
| GGT (u/L) | 179. 33 ± 164.98 |
| Platelet (103/μL) | 278.96 ± 107.22 |
| Prothrombin time (%) | 95.93 ± 21.56 |
| INR | 1.05 ± 0.14 |
| Steatosis (Hounsfield units) | 1.10 ± 0.18 |
| Portal vein diameter (mm) | 13.83 ± 2.01 |
| SV (cc) | 315.38 ± 129.06 |
| Glucose (mg/dL) | 110.19 ± 38.08 |
| Numbers of resected liver segments | 4.67 ± 1.07 |
When patients had more than one complication, only the seminal one was counted, or the most serious one if complications occurred contemporaneously. The onset of the complication was defined as the time when the resulting organ dysfunction began, or the corrective treatment was started. For infections data, Centers for Disease Control and Prevention definitions were used[13].
The Couinaud classification was used to define and describe resection. Fourteen patients had a primary neoplasm on otherwise healthy liver (hepatocellular carcinoma in 5 cases, intrahepatic cholangiocarcinoma in 6, hilar cholangiocarcinoma in 1, gallbladder cancer in 1, and huge hepatobiliary cystadenoma in 1). The other 13 had liver metastases (from colorectal cancer in 10 cases, ileal neuroendocrine tumor in 1 case, gastrointestinal stromal tumor in 1 case, and ovarian leiomyosarcoma in 1 case).
Surgical site infections (SSIs) were defined according National nosocomial infections surveillance system[16,17]. Criteria for SSIs included infection occurring at the incision site within 30 d after surgery. In addition the onset time after surgery and the causative bacteria were identified. Due to the small number of infection episodes no comparison between groups of causative bacteria was performed.
Quantitative variables are expressed as mean ± SD, and qualitative variables as absolute and relative frequencies. Comparisons between groups of quantitative and qualitative variables were done with the t test, and Fisher’s exact test. Generalized linear regression models with Gaussian family distribution and log link function were used to reveal the principal promoters of early liver regeneration. The residuals analyses were graphically assessed and the predictor coefficients and corresponding 95%CI were meter adjusted for follow-up time, which was assumed as a linear predictor in the regression models. Table 3 is a stepwise multiple generalized linear regression with a significance level of 0.2 for removing variables from the model. Because we considered the entire cohort of patients who underwent resection for primary or secondary tumors on otherwise healthy livers between November 2004 and January 2010, no formal sample-size calculation was made beforehand. Statistical tests were considered significant when the corresponding P value was less than 5%. Data handling and analyses were done with R 2.14[18] and SPSS version 17.0 software.
| Post-surgical factors | Minor liver regeneration | Major regeneration | P value |
| Total bilirubin (mg/dL) | 2.37 ± 4.75 | 2.99 ± 4.68 | 0.745 |
| Direct bilirubin (mg/dL) | 1.44 ± 2.78 | 2.13 ± 3.64 | 0.586 |
| Albumin (g/dL) | 2.73 ± 0.67 | 2.76 ± 0.51 | 0.912 |
| Creatinine (mg/dL) | 0.89 ± 0.41 | 0.92 ± 0.27 | 0.817 |
| Prothrombin time (%) | 81.58 ± 25.84 | 80.61 ± 17.16 | 0.917 |
| PT/INR | 1.41 ± 0.71 | 1.16 ± 0.21 | 0.306 |
Based on preliminary analysis, and solely for exploratory purposes, a regeneration ≥ 100% was used as a cutoff to divide all 27 patients into two groups: major regeneration (10 patients, 37%) and minor regeneration (17 patients, 63%). Moreover, because the Vauthey formula was used for establishing minimal FRLV, we considered 100% regeneration of FRLV in the early postoperative period a more than acceptable result.
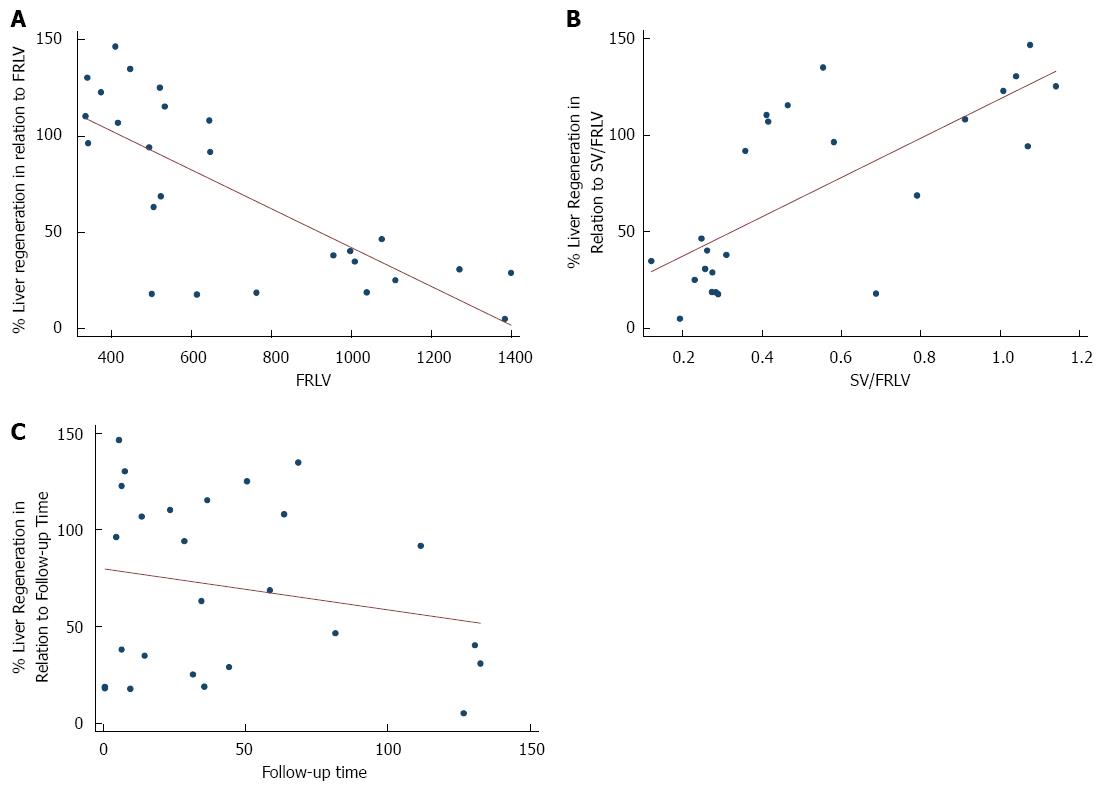
Generalized linear regression analysis, adjusted for follow-up time, found that among all the preoperative variables the only statistically significant predictors of early liver regeneration were smaller FRLV (-0.0023; P < 0.001), smaller BMI (-0.1155, P < 0.001), and greater spleen volume (SV)/FRLV ratio (0.4999; P = 0.016) (Table 4).
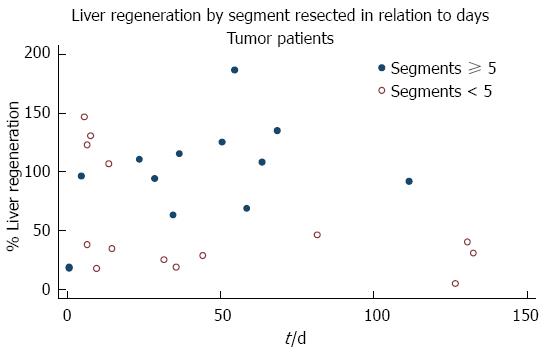
Preoperative weight, serum bilirubin level, prothrombin time in (%), and diameter of portal vein were similar in all patients. The mean follow-up time was 43.85 d and, on stepwise multiple regression, there was an association between follow-up time and greater percentage of liver regeneration (P = 0.002) (Figure 1, Table 5).
| Preoperative factors | Coefficient | SE | P value | 95%CI |
| FRLV | -0.00232 | 0.00054 | < 0.001 | (-0.00337 - -0.00127) |
| SV/FRLV | 0.49988 | 0.20731 | 0.016 | (0.09357-0.90619) |
| Follow-up time | 0.00704 | 0.00232 | 0.002 | (0.00249-0.01159) |
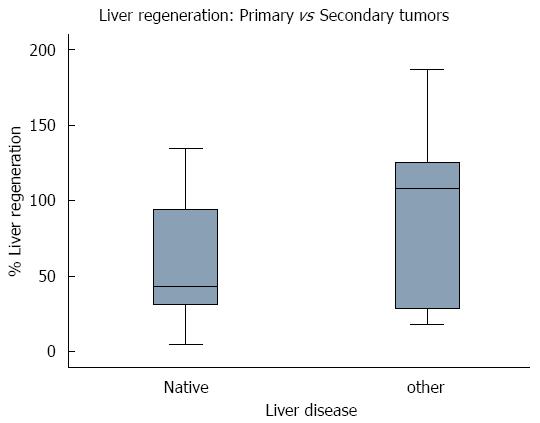
Thirteen patients (48%) underwent a resection of ≥ 5 Couinaud segments, with a mean 94.75% ± 45.72% of liver parenchymal regeneration, while the 14 patients (52%) with a resection of < 5 Couinaud segments had a mean percentage of regeneration of 56.64% ± 47.72%. Comparison of the percentage of regeneration in relation to the number of segments resected showed that patients with a resection of ≥ 5 Couinaud segments experienced greater early regeneration (P = 0.0446). The 14 patients resected for primary liver malignancy had a mean of 60.32% ± 41.23% of liver regeneration but, when compared with the other 13, resected for secondary tumors (90.79 ± 54.86), no significant difference was detected (P = 0.1136) (Table 6).
| Preoperative factors | Coefficient | P value | 95%CI |
| FRLV | -0.0027 | < 0.001 | (-0.0036- -0.0018) |
| BMI | -0.1155 | < 0.001 | (-0.1763- -0.0548) |
| SV/FRLV | 1.0925 | < 0.001 | (0.7356-1.4494) |
| Bilirubin | -0.0885 | 0.547 | (-0.3762-0.1993) |
| Albumin | 0.0003 | 0.999 | (-0.3945-0.3951) |
| GGT | 0 | 0.993 | (-0.0016-0.0016) |
| Creatinine | 0.7841 | 0.194 | (-0.4001-1.9683) |
| INR | 0.7054 | 0.435 | (-1.0666-2.4775) |
| Age | -0.0032 | 0.744 | (-0.0224-0.0160) |
| Steatosis (Hounsfield units) | 0.1326 | 0.857 | (-1.3137-1.5788) |
| HAIs | 0.2739 | 0.402 | (0.3661-0.9141) |
Ten of the 27 patients (37%) underwent chemotherapy prior to surgery, with a statistically significant prevalence of patients with metastasis (P = 0.007). Eight patients (30%) underwent embolization, 3 with primary tumors, and 5 with secondary tumors.
Twenty patients (74%) experienced complications, with 12 (60%) experiencing Clavien-Dindo Grade 3a to 5 complications. Interestingly, no significant association was found between early regeneration and either onset or severity of surgical complications, classified according to the Clavien-Dindo model, with a P value > 0.999 for both. No remarkable discrepancies were showed in terms of preoperative blood biochemical tests between patients with early minor and major liver regeneration (Table 3).
Clinically relevant infections developed in 9 patients (33%): they all experienced SSIs and 2 of them had secondary bloodstream infections. Median SSI onset time was 10 d (range 4-30 d). Five patients had plurimicrobial infection. The most commonly causative bacteria were Enterococcus spp. and Enterobacteriaceae. SSIs were not associated with early liver regeneration.
On the other hand, SSIs were significantly associated with the onset of surgical complications: 9 patients experienced SSIs, and in 7 cases Clavien-Dindo Grade 3a to 4 complications were detected (P = 0.016).
According to the NNIS system, the United States Centers for Disease Control and Prevention (CDC) reported that SSIs are the third most common nosocomial infection, accounting for 14%-16% of all nosocomial infections in the United States. In surgical patients, SSIs are the most common nosocomial infection, and are still a common cause of major morbidity after hepatectomy for hepatic tumors[19].
In recent years, attention has increasingly been focused on the accurate identification and monitoring of SSIs. The NNIS system established by the CDC in the United States provides a comprehensive monitoring system that reports on trends in SSIs. Risk factors for SSIs after hepatectomy for hepatic tumor under CDC guidelines have not yet been fully investigated[20].
To the best of our knowledge, there is a scarcity of data in the English literature on the impact of infection on early liver regeneration after resection for hepatic tumors. The human liver is able to regenerate thanks to a hyperplastic reaction in the residual liver[1], with portal flow volume considered the most important factor for liver regeneration[21,22]. Postoperative liver failure is currently defined as impaired ability of the liver to maintain its synthetic, excretory, and detoxifying functions, and is characterized by increased INR and bilirubin levels on or after postoperative day 5. Severity is graded according to its impact on clinical management[23].
Some studies have suggested that preoperative variables such as age, gender, BMI, native liver disease, chemotherapy, platelet count and steatosis may have a significant influence on human liver regeneration[2,24-27]. With this in mind we wanted to investigate whether post-operative infective complications would have any impact of early liver regeneration in patients resected for hepatic tumors on otherwise healthy livers. Moreover, the key aim was to identify which, if any, preoperative factors may influence early liver regeneration after hepatic resection.
Olthoff cites a number of studies that show the release of pro-inflammatory cytokines such as tumor necrosis factor alpha and interleukin-6 after injury due to resection as initiators of the regenerative process[27], and like many, we postulated that larger resections may lead to a greater concentration of cytokines, and promote growth[28,29]. Indeed, the patients in our study who underwent a resection of ≥ 5 Couinaud segments had a greater percentage of early liver regeneration (P = 0.0446) than those with a resection of < 5 segments.
Interestingly, we found no significant differences in early regeneration between patients with primary or secondary tumors (P = 0.1136), suggesting that the type of malignancy may not have an effect on early liver regeneration in resected patients (Figure 2).
Wang et al[30] have hypothesized that inflammatory response was strong and persistent when biliary leakage occurred, and it may promote the restoration of liver function after partial hepatectomy, but we did not recognize any protective role of HAIs or major complication grades. On stepwise generalized linear regression analysis, the only independent variables (adjusted for follow-up time) significantly associated with major early regeneration were a smaller FRLV, and a greater SV/FRLV ratio, which could be interpreted as a result of portal hyperperfusion, itself the result of parenchymal decurtation, and not portal hypertension, given that our patients were not suffering from chronic liver disease (Figure 3).
A previous study of ours, a retrospective investigation of early regeneration in 70 living donors who underwent right hepatectomy, also found a statistically significant association between smaller FRLV, greater SV/FRLV ratio, and early regeneration[31]. Of note here is the fact that nearly half of our tumor patients underwent extended right hepatectomy, with removal of the middle hepatic vein, while in all 70 of our living donors the middle hepatic vein was preserved. Though there is a considerable difference in the number of patients (27 vs 70), there was no association between either onset or severity of Clavien-Dindo classification of complications or infective complications[32], and early regeneration, and in both study cohorts early regeneration was associated with larger resection.
We undertook a stepwise generalized linear regression analysis of the 4 variables that had significant value on bivariate generalized liner regressions analysis, adding follow-up time as a 5th variable. We were fully aware of the fact that this was a far statistical overreach for just 27 patients. Nonetheless, we undertook this analysis as an exploration of potential factors associated with early regeneration, and not looking to suggest causality.
This study has a number of inherent limitations, the most important of which is its retrospective design, and the fact that we studied a small group of patients, though we feel this is partially mitigated by the fact that cancer, primary or secondary, on an otherwise healthy liver is a relatively rare condition. The small group of patients studied also posed limits to the number of variables that could be simultaneously investigated with multiple linear regression analysis. In addition, this is a single center study, and was performed in a small, highly specialized transplant hospital, perhaps rendering the associations that we found to be significant ungeneralizable to larger, more heterogeneous settings. Future prospective, multicenter, studies will be needed to confirm our results. Our findings would seem to underscore the importance of pre-operative imaging tools not only for estimating the volume of the residual liver, but also for predicting the impact of portal blood flow on the functional quality of the regenerating liver. This information, together with knowledge of the anatomic vascular and biliary variations potentially detected by preoperative MDCT scan, can allow surgeons and clinicians to plan safer resective surgery for patients with hepatic neoplasms on otherwise healthy livers. We can conclude by suggesting that in this relatively rare group of patients, with primary or secondary tumors and no underlying chronic liver disease, a smaller FRLV, and a greater SV/FRLV ratio may indicate those patients who will experience greater early regeneration after major hepatectomy. We have not detected any crucial role of HAIs or surgical complications on early liver regeneration after resection for hepatic tumors.
The authors would like to thank Warren Blumberg for his help in editing this paper.
Liver regeneration follows a similar pathway in living donors and in patients resected for cancer. After liver resection if the remnant liver volume is less than 20% of the standard liver volume, liver function might fails to sustain metabolic needs leading to the “small-for-size” syndrome.
The authors have recently put emphasis not only on the evaluation of the ratio between donor and recipient liver volume but also on the predictors of optimal early liver regeneration in the donors, and the degree of portal hypertension and the stage of the liver disease in the recipients of a 12 years single Italian center experience.
The authors have identified three predictive factors for optimal early regeneration: the future remnant liver volume, body habitus, and spleen volume proved to be markers of liver regeneration. Preoperative multi-detector computed tomography volumetry is an essential tool to assess future remnant liver volume. The alterations of hepatic blood flow have a negative impact on the progression of liver regeneration that always follows major hepatic resection, therefore impairing liver function with varying degrees of severity. Furthermore, biliary system ischemic damage could cause biliary leakages and probably favors the occurrence of biliary complications and sepsis that plague the post-operative clinical course.
To further improve the outcome of these complex procedures, refinements in the surgical technique and better comprehension of the interrelations between liver regeneration, portal hypertension and healthcare-associated infections will be needed.
Liver regeneration: The success of liver resection for the treatment of liver malignancies relies on the remnant liver’s ability to regenerate after major tissue loss. However, after liver resection, if the remnant liver is less than 20% of the original liver volume, liver function might not sustain metabolic, synthetic, and detoxifying needs, leading to the post-resection liver failure. Healthcare-associated infections: are infections that patients acquire during the course of receiving healthcare treatment for other conditions. Every day, about 1 in every 20 hospitalized patients has an infection caused by receiving medical care. These infections related to medical care can be devastating and even deadly.
The article is important guidance to clinicians who know about the clinical aspects correlation with infective complications of liver regeneration after liver resection.
P- Reviewers: Qin JM, Yagi H S- Editor: Zhai HH L- Editor: O’Neill M E- Editor: Ma S
| 1. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 2. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 4. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 5. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-80; discussion 680-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 9. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Scott II RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. 2009. Accessed December 26, 2012. Available from: http://www.cdc.gov/ncidod/dhqp.. |
| 13. | Centers for Disease Control and Prevention. National Healthcare Safety Network: surgical site infection (SSI) event. Accessed December 26, 2012. Available from: http://www.cdc.gov/nhsn. |
| 14. | Couinaud C. Surgical Anatomy of the Liver Revisited. Paris: Couinaud 1989; . |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24842] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 16. | Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 375] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Jarvis WR. Benchmarking for prevention: the Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance (NNIS) system experience. Infection. 2003;31 Suppl 2:44-48. [PubMed] |
| 18. | R Development Core Team (2007). R: a language and environment for statistical computing. Available from: http://www.R-project.org. |
| 19. | Sadamori H, Yagi T, Shinoura S, Umeda Y, Yoshida R, Satoh D, Nobuoka D, Utsumi M, Yoshida K, Fujiwara T. Risk factors for organ/space surgical site infection after hepatectomy for hepatocellular carcinoma in 359 recent cases. J Hepatobiliary Pancreat Sci. 2013;20:186-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Sadamori H, Yagi T, Shinoura S, Umeda Y, Yoshida R, Satoh D, Nobuoka D, Utsumi M, Yoshida K, Fujiwara T. Risk factors for organ/space surgical site infection after hepatectomy for hepatocellular carcinoma in 359 recent cases. J Hepatobiliary Pancreat Sci. 2013;20:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Child CG, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann Surg. 1953;138:600-608. [PubMed] |
| 22. | Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1727] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 24. | Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 595] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Clavien PA, Graf R. Liver regeneration and platelets. Br J Surg. 2009;96:965-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Olthoff KM. Hepatic regeneration in living donor liver transplantation. Liver Transpl. 2003;9:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Nishizaki T, Ikegami T, Hiroshige S, Hashimoto K, Uchiyama H, Yoshizumi T, Kishikawa K, Shimada M, Sugimachi K. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Fausto N, Riehle KJ. Mechanisms of liver regeneration and their clinical implications. J Hepatobiliary Pancreat Surg. 2005;12:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Wang X, Liu H, Yuan T, Tang C, Liu M, Chen P. Biliary leakage might promote liver regeneration following hepatectomy. Med Hypotheses. 2009;73:925-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Gruttadauria S, Parikh V, Pagano D, Tuzzolino F, Cintorino D, Miraglia R, Spada M, Vizzini G, Luca A, Gridelli B. Early regeneration of the remnant liver volume after right hepatectomy for living donation: a multiple regression analysis. Liver Transpl. 2012;18:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Gridelli B, Panarello G, Gruttadauria S, Marcos A, Grossi P. Infections after living-donor liver transplantation. Surg Infect (Larchmt). 2006;7 Suppl 2:S105-S108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |