Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6869
Revised: February 26, 2014
Accepted: March 6, 2014
Published online: June 14, 2014
Processing time: 199 Days and 15.5 Hours
AIM: To construct and evaluate the functionality of a choanoid-fluidized bed bioreactor (CFBB) based on microencapsulated immortalized human hepatocytes.
METHODS: Encapsulated hepatocytes were placed in the constructed CFBB and circulated through Dulbecco’s Modified Eagle’s Medium (DMEM) for 12 h, and then through exchanged plasma for 6 h, and compared with encapsulated cells cultivated under static conditions in a spinner flask. Levels of alanine aminotransferase (ALT) and albumin were used to evaluate the CFBB during media circulation, whereas levels of ALT, total bilirubin (TBil), and albumin were used to evaluate it during plasma circulation. Mass transfer and hepatocyte injury were evaluated by comparing the results from the two experimental conditions. In addition, the viability and microstructure of encapsulated cells were observed in the different environments.
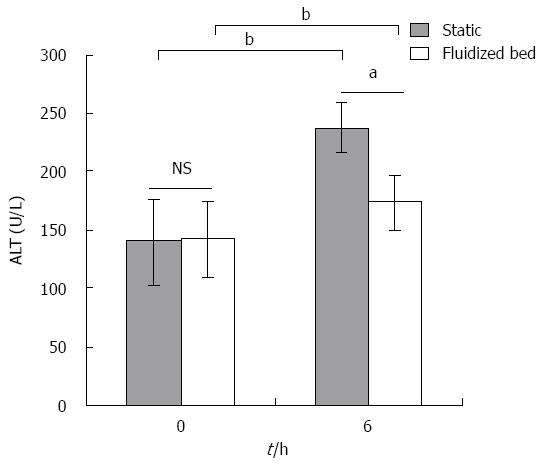
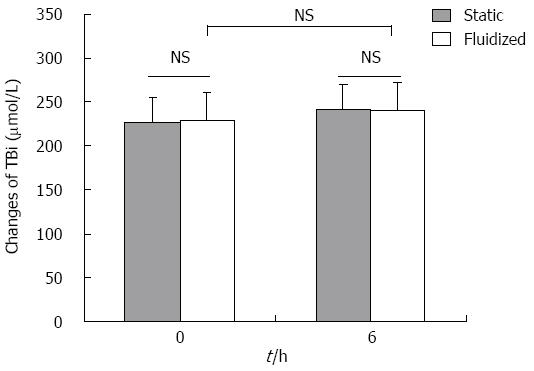
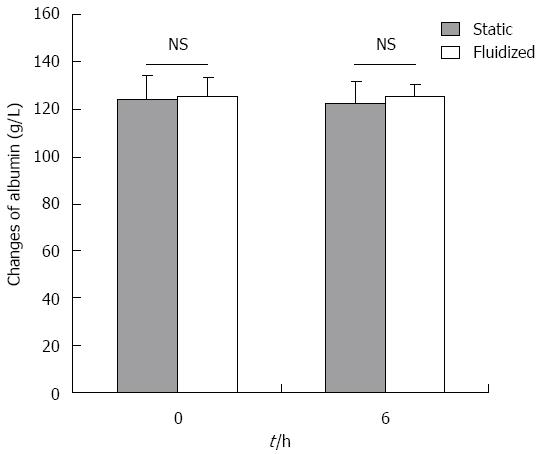
RESULTS: The bioartificial liver model based on a CFBB was verified by in vitro experiments. The viability of encapsulated cells accounting for 84.6% ± 3.7% in CFBB plasma perfusion was higher than the 74.8% ± 3.1% in the static culture group (P < 0.05) after 6 h. ALT release from cells was 29 ± 3.5 U/L vs 40.6 ± 3.2 U/L at 12 h (P < 0.01) in the CFBB medium circulation and static medium culture groups, respectively. Albumin secretion from cells was 234.2 ± 27.8 μg/1 × 107 cells vs 167.8 ± 29.3 μg/1 × 107 cells at 6 h (P < 0.01), 274.4 ± 34.6 μg/1 × 107 cells vs 208.4 ± 49.3 μg/1 × 107 cells (P < 0.05) at 12 h, in the two medium circulation/culture groups, respectively. Furthermore, ALT and TBil levels were 172.3 ± 24.1 U/L vs 236.3 ± 21.5 U/L (P < 0.05), 240.1 ± 23.9 μmol/L vs 241.9 ± 31.4 μmol/L (P > 0.05) at 6 h in the CFBB plasma perfusion and static plasma culture groups, respectively. There was no significant difference in albumin concentration between the two experimental plasma groups at any time point. The microstructure of the encapsulated hepatocytes remained healthier in the CFBB group compared with the static culture group after 6 h of plasma perfusion.
CONCLUSION: The CFBB can function as a bioartificial liver based on a bioreactor. The efficacy of this novel bioreactor is promising for the study of liver failure.
Core tip: We constructed a novel choanoid fluidized bed bioreactor (CFBB) and received a national invention patent. We evaluated the CFBB functionality based on microencapsulated immortalized human hepatocytes. For this purpose, we placed the cultured hepatocytes into the CFBB and circulated through Dulbecco’s Modified Eagle’s Medium media for 12 h and through exchanged plasma for 6 h. The bioartificial liver based on a fluidized-bed bioreactor with microencapsulated immortalized human hepatocytes appeared to be effective for improving severe hepatitis plasma parameters. The efficacy of this novel bioreactor is promising for the study of liver failure.
- Citation: Yu CB, Pan XP, Yu L, Yu XP, Du WB, Cao HC, Li J, Chen P, Li LJ. Evaluation of a novel choanoid fluidized bed bioreactor for future bioartificial livers. World J Gastroenterol 2014; 20(22): 6869-6877
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6869.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6869
Despite recent considerable improvements in supportive therapy, fulminant hepatic failure has a high mortality worldwide[1]. To date, the only effective long-term treatment is orthotropic liver transplantation. However, there is a worldwide shortage of organ donors. A bioartificial liver (BAL) support system represents a new tool for end-stage liver failure after injury or for bridge-to-transplantation use[2,3]. Researchers have developed extracorporeal BALs to investigate the potential performance of hepatocytes immobilized in a bioreactor. Such devices should be able to replace all or most liver functions[4,5].
Presently, various configurations of BALs have been proposed using flat plate and monolayer bioreactors[6,7], hollow fiber[8,9], perfused beds or scaffolds[10-12], beds with encapsulated or suspended cells[13,14], and other complicated configurations with compound bioreactors[15]. The most widely investigated bioreactor at present is based on a hollow fiber membrane. However, this model is difficult to scale-up. Moreover, the semipermeable membrane can act as a diffusion barrier between the hepatocytes and the plasma of patients; therefore, the xenogenic hepatocytes are placed directly in contact with patient plasma without an impermeable immunological barrier. As such, hepatocytes immobilized into alginate/chitosan (AC) microcapsules might be an interesting alternative.
Encapsulated cells hosted in bioreactors may enhance mammalian cell culture within a controlled and efficient environment[16]. At present, AC has been widely applied for immobilization or encapsulation of biological constituents because of its stability, compatibility and simplicity of use[17]. This immobilization allows growth of hepatocytes in a favorable environment: the porous structure of AC maintains a close interaction with the external medium. Exchange between beads and medium can be achieved in flasks or bioreactors. Within a bioreactor, the microcapsules are usually hosted either in a fixed or fluidized bed. In a fixed bed, the beads cannot withstand the extreme stresses caused by high perfusion velocity. The fluid often mobilizes through channels in the space that is devoid of beads, which results in formation of a void volume or dead space in the traditional fixed bed bioreactors. We developed a BAL based on a choanoid-fluidized bed bioreactor (CFBB) configuration that allowed the fluidization of AC beads in which cells can be immobilized. In terms of liver support, substances such as albumin and other macromolecules should be exchanged between the ambient media (the patient’s plasma) and the encapsulated cells. Mass transfer occurs in both directions: from the surrounding fluid into the beads (oxygen, nutrients, or substances to be metabolized by hepatocytes), and from the beads into the surrounding fluid (substances secreted by the cells).
To confirm the functionality of the CFBB, it is important to demonstrate stable function of the encapsulated immortalized human hepatocyte line during perfusion in culture medium or potentially toxic liver failure plasma. So far, only a few groups have attempted to assess its effects on cells grown in 3D cultures in the context of a BAL[18,19]. Other studies that assessed functionality of 3D hepatocyte cultures in putative BALs perfused their systems with culture medium[20]. In this study, we evaluated the newly constructed bioreactor using AC microcapsules that contained the immortalized HepLi4 human hepatocyte line established in our laboratory.
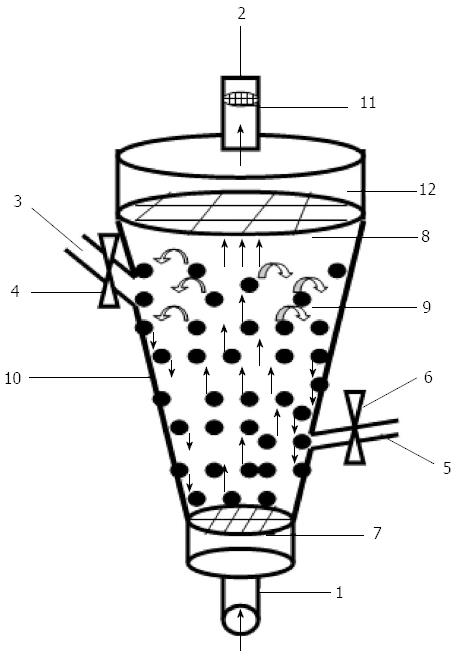
The CFBB consists of a column containing AC beads. The column size was 500 mL, which was designed for a perfusion plasma flow rate ranging from 80 to 120 mL/min and a bead volume of 400 mL. The bioreactor height allowed for a safety volume theoretically free of beads at the top of the column. The materials used for the cylinders were biocompatible. The different components are shown in Figure 1 and are labeled for reference. The microcapsule access port enabled ease of filling of microencapsulated hepatocytes, while the blood sample port allowed fluid samples to be taken during operation. In addition, the cell sieve prevented cells or cell debris from being released into the fluid circulation. The total length of the CFBB was 250 mm. This bioreactor device has received a national invention patent (patent number: ZL200710070279.0).
The immortalization of HepLi4 was established from a brain-dead male by transfection with pcDNA 3.1 (-) recombinant plasmid containing the genes encoding SV40 LT antigen.
The hepatocytes were cultured for 4 d, isolated, and then suspended in 1.8% high-viscosity sodium alginate solution (Sigma, St Louis, MO, United States). The concentration of hepatocytes in the final solution was 2 × 106 viable cells/mL. Droplets of cell solution formed by a drop generator fell into a 0.6% (w/v) chitosan solution; the size of droplets ranged from 500 to 600 mm. The microencapsulation procedure has been described previously [21]. After culturing in special spinners for 2 wk, the microencapsulated hepatocytes achieved their maximum functionality[22]. Viable hepatocytes (5 × 109 cells) were loaded into the CFBB.
The CFBB was treated with hypochlorite solution, ethanol, and 0.02% collagen solution (Nichirei, Tokyo, Japan). Dulbecco’s Modified Eagle’s Medium (DMEM) was passed through the circuit for 2 h prior to the experiment. Microencapsulated hepatocytes (5 × 109), which had a viability consistently > 90%, were loaded into the CFBB. A CFBB containing AC microcapsules encapsulating viable hepatocytes was inserted in the flow circuit containing a medium reservoir and peristaltic pump.
Group 1 (Control group): AC microcapsules hosting cells in static medium/plasma (n = 5 tests). Group 2 (Experimental group): AC microcapsules hosting cells in the fluidized bed bioreactor (n = 5 tests)
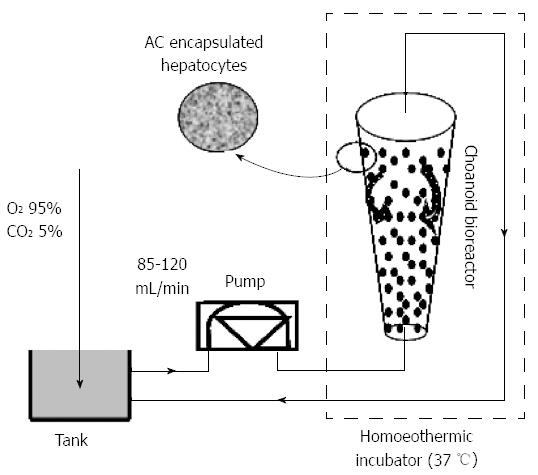
Culture medium was introduced at the bottom of the bioreactor. For the in vitro experiment, a 1500-mL reservoir was installed between the peristaltic pump and BAL module. Oxygen was supplied by bringing the culture medium into contact with a gas mixture of 95% O2 and 5% CO2, using silastic tubing. The optimal flow rate of fluid for proper fluidization of entrapped hepatocytes was 100 mL/min, and the temperature was maintained at 37 °C (Figure 2).
Cell viability: Cell viability was assessed by trypan blue exclusion before and after medium perfusion. The cell count was performed with a hemocytometer as hepatocytes were detached after AC dissolution in citrate buffer (pH 7.4).
Function of the BAL medium perfusion system: Independent experiments were performed five times with the BAL device. Samples of microencapsulated hepatocytes in DMEM were obtained at 2-h intervals during 12 h of circulation. Changes in alanine aminotransferase (ALT) and albumin levels were determined with an automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan).
Exchanged plasma perfusion in vitro: The exchanged plasma, collected from patients with severe hepatitis, was diluted twice with physiological saline, which was set up at the bottom of the bioreactor, as described above, for the in vitro experiments (Figure 2). The optimal flow rate of fluid for proper fluidization of entrapped hepatocytes was 85 mL/min, and the temperature was maintained at 37 °C.
Cell viability: Cell viability was assessed by trypan blue exclusion before and after plasma perfusion, as described above.
Function of the BAL plasma perfusion system: Independent experiments were performed five times with the BAL device. Plasma samples were obtained before and after circulation. Changes in ALT, total bilirubin (TBil) and albumin levels were determined.
Transmission electron microscopy: Microencapsulated hepatocytes in the fluidized bed and static culture groups obtained before and after circulation were observed by transmission electron microscopy (TEM) (JEM-1200EXII; Jeol). Specimens were fixed in 2.5% glutaraldehyde (pH 7.4) overnight at 4 °C, followed by post-fixation in 1% osmium tetroxide for 1 h and by progressive dehydration in an ethanol series (50%-100%, v/v), and then finally embedded in Epon (Hexion Specialty Chemicals, Columbus, OH, United States), followed by pathological serial thin sectioning. Thin sections were observed and photographed using an electron microscope (JEM-1200EXII; Jeol). The specimens were examined by TEM after plating with gold under a vacuum. Areas for examination were randomly chosen.
Measurements are presented as mean ± SD and comparisons were made using Student’s t test. In univariate analyses significance was accepted at P≤ 0.05. SPSS for Windows Version 16.0 (Chicago, IL, United States) was used for data analysis.
The viability of encapsulated cells in the CFBB group was 93.5% ± 3.2% at the beginning of the experiment and 90.6% ± 6.5% after 12 h (P > 0.05). The viability of encapsulated hepatocytes in the static culture flask decreased from 93.9% ± 4.4% to 86.8% ± 3.8% after 12 h (P > 0.05).
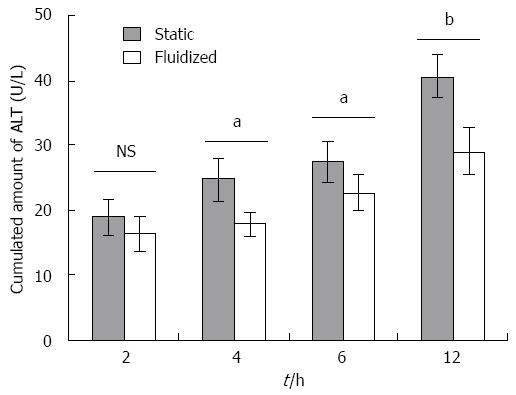
ALT levels: As shown in Figure 3, ALT was released from beads containing hepatocytes maintained under static culture conditions and culture medium in the CTBB. In the fluidized bioreactor group, the ALT release was 16.4 ± 2.7 U/L after 2 h, which increased to 29 ± 3.5 U/L after 12 h. ALT released from the cells in the static culture group was 19 ± 2.7 U/L at 2 h and 40.6 ± 3.2 U/L at 12 h. These data indicate that ALT release from the fluidized bioreactor was significantly lower compared with that from the static medium culture group from 4 to 12 h (4 h, 12 h, P < 0.01; 6 h, P < 0.05).
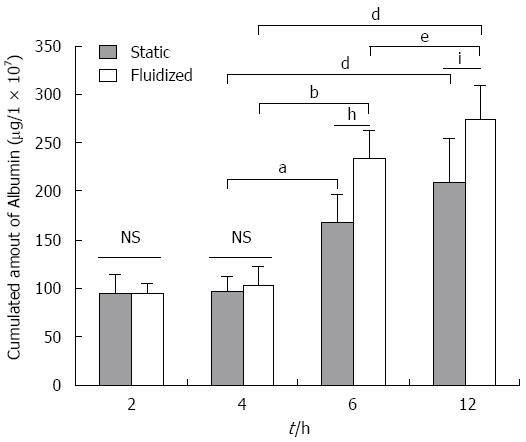
Albumin secretion: As indicated in Figure 4, the amount of albumin secreted after 2 h (94.4 ± 11.1 μg/1 × 107 cells) significantly increased after 6 h in the cells within the CFBB (234.2 ± 27.8 μg/1 × 107 cells) (P < 0.01), and further increased to 274.4 ± 34.6 μg/1 × 107 cells (P < 0.01) after 12 h. The amount of albumin secreted from cells in the static culture group was 94.6 ± 19.8 μg/1 × 107 cells, 167.8 ± 29.3 μg/1 × 107 cells, and 208.4 ± 49.3 μg/1 × 107 cells at 2 h, 6 h, and 12 h, respectively. Albumin secretion from cells in the CFBB group was significantly higher than that from cells in the static culture group over 6-12 h (6 h, P < 0.01; 12 h, P < 0.05).
Cell viability: The viability of encapsulated cells in the fluidized bed plasma perfusion group was 94.5% ± 3.2% at the beginning of the experiment, which decreased to 84.6% ± 3.7% after 6 h (P < 0.05). The viability of encapsulated hepatocytes in the static culture flask decreased from 95.2% ± 4.4% at the beginning of the experiment to 74.8% ± 3.1% after 6 h (P < 0.01). The two groups were significantly different after 6 h (P < 0.05).
ALT levels: As shown in Figure 5, ALT levels before and after plasma perfusion through the fluidized bed increased after 6 h (from 141.7 ± 31.9 U/L to 172.3 ± 24.1 U/L, P < 0.05). There was a significant increase in ALT levels in the plasma of the static culture group after 6 h (139 ± 37.6 to 236.3 ± 21.5 U/L, P < 0.01). The level of ALT after the experiment in the static plasma culture group was significantly higher than in the plasma in the fluidized bed group (P < 0.05).
TBil concentration: As shown in Figure 6, the concentration of TBil before and after plasma perfusion through the fluidized bed decreased (228.7 ± 24.3 μmol/L to 240.1 ± 23.9 μmol/L, P > 0.05), while the concentration in the plasma before and after static culture showed no significant change (226.6 ± 27.8 μmol/L to 241.9 ± 31.4 μmol/L, P > 0.05). The TBil concentration in the plasma perfusion group after the experiment was not significantly different compared with that of the static plasma culture group (P > 0.05).
ALB concentration: As shown in Figure 7, there was no significant difference in albumin concentration between the two experimental groups at any time point.
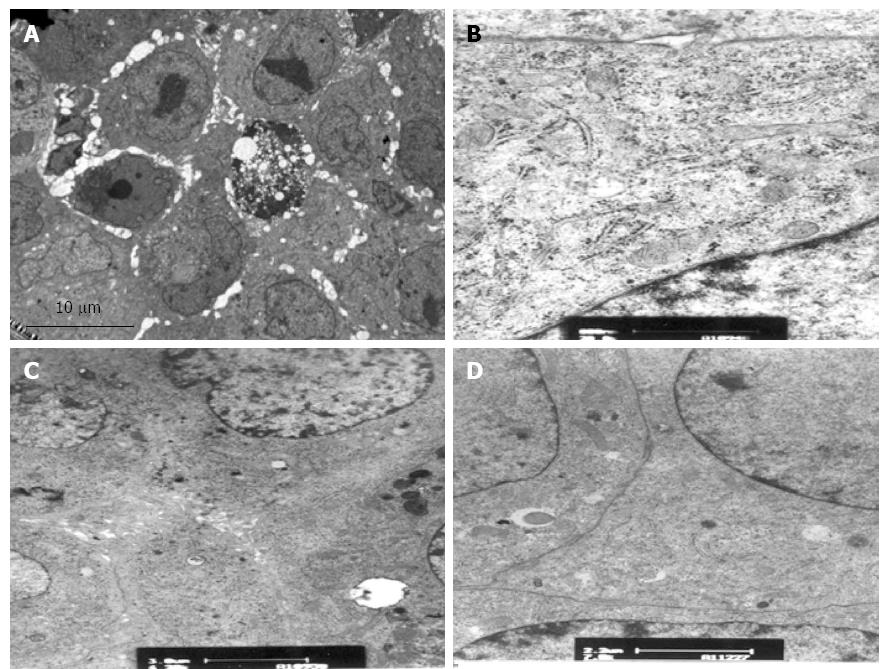
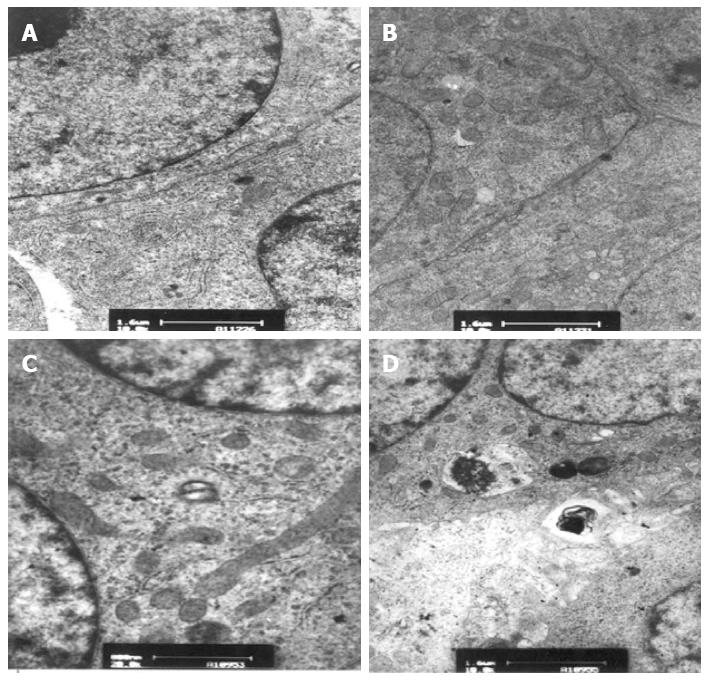
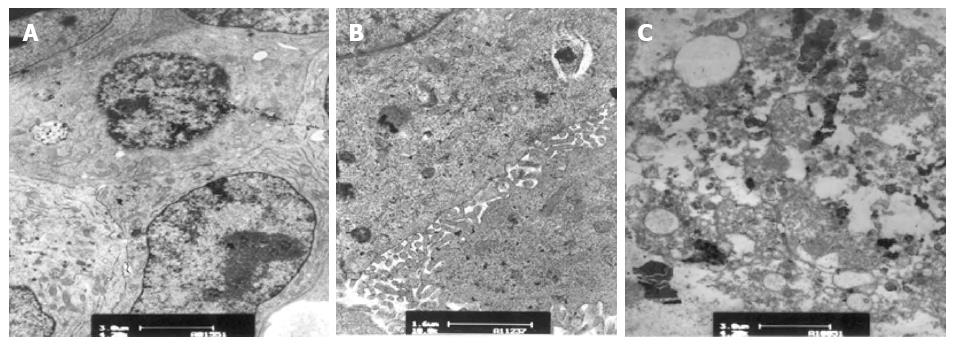
Before the experiments, encapsulated hepatocytes were maintained in a healthy growth state with cohesive colonies. TEM showed normal hepatocyte subcellular architecture with mitochondria, endoplasmic reticulum, nuclei with prominent nucleoli, lipid vacuoles, and pools of glycogen. In addition, desmosomes, hemidesmosomes, bile canaliculi-like formation, and junction complexes were well formed, and there was striking formation of canaliculi lined with microvilli (Figure 8). After the experiments, encapsulated hepatocytes in the CFBB group still possessed numerous mitochondria, endoplasmic reticulum, desmosomes, bile canaliculi-like formation, and junction complexes, with little vacuolization (Figure 9). However, some cells in the static plasma culture group had shrunken nuclei and some cells showed vacuolar degeneration, although there were cells with many organelles, mitochondria, microvilli, and tight junctions (Figure 10).
Day 14 was determined to be the optimal time to use the cells for the CFBB devices, which were constructed by scaling up and evaluating the encapsulated immortalized human hepatocyte cells with spinner cultivation (data not shown). The work described here will provide an important foundation to evaluate the bioreactor for future use as a BAL. We also investigated the structural parameters of the bioreactor, which play a key role in providing beneficial technical support for the bioreactor assessment. Using the CFBB, we investigated the effect of fluidization on the metabolism of the encapsulated hepatocytes by circulating culture medium for 6-12 h, and to verify whether this bioreactor could replace part of the normal liver and serve as an extracorporeal BAL.
For the objective and comprehensive study of the bioreactor, we studied medium circulation and the exchange of plasma isolated from patients with severe hepatitis while circulating through the CFBB. The bioactive component was in the encapsulated immortalized human hepatocytes, which was circulated in the CFBB. For the control group, the beads containing hepatocytes were maintained in a spinner culture flask under static conditions. For the CFBB group, the beads containing hepatocytes were circulated in the fluidized bed. The hepatocytes (5 × 109) were placed in the CFBB, and DMEM with high glucose concentration was circulated through the bioreactor for 12 h to determine the effect of fluidization on hepatocyte metabolism. The exchanged plasma was perfused in vitro for 6 h to determine the effect of encapsulated hepatocytes on the metabolism of plasma from patients with severe hepatitis, and the toxic effect of this plasma on the encapsulated hepatocytes in the CFBB. We determined whether hepatocytes could function properly in the fluidization state compared with those in the static culture conditions, in the presence of plasma isolated from patients with severe hepatitis. Perfusion was conducted for 6-12 h, which corresponds to the length of a session of plasma exchange in combination with continuous renal replacement therapy. It is generally recognized that the extracorporeal circulation should be interrupted after this time period and that several consecutive sessions, using several bioreactors, should be performed before transplantation[23,24]. Therefore, we did not study the long-term metabolism of encapsulated hepatocytes because the application of the bioreactor is not recommended as a long-term culture device.
In vitro media circulation is easier to perform; therefore, we compared the function of the encapsulated hepatocytes under fluidized vs static culture conditions. The index of encapsulated hepatocyte injury, such as the release of ALT, was significantly lower in the fluidized bed group because microcapsules provided a 3D growth environment, and the fluidization state of the encapsulated hepatocytes in the CFBB promoted more rapid bidirectional mass exchange. In contrast, diffusion of metabolites from encapsulated hepatocytes was slower from the microcapsules in the static culture group, which could lead to accumulation of metabolites and perhaps damage to the hepatocytes. Furthermore, the hepatocytes in the static culture lacked effective nutrition because they clustered together. Therefore, the level of ALT increased in the static culture group, while there was no significant increase observed in the CFBB group. This indicated increased mass exchange caused by the fluidization and diffusion through the myriad of micropores in the microcapsules. Similarly, the level of albumin released from hepatocytes in the CFBB group was higher compared with that in the static culture group. These data indicate that fluidization in the CFBB could improve the function and viability of encapsulated hepatocytes.
To better evaluate the CFBB, the exchanged plasma from patients with severe hepatitis was used to simulate patient treatment. The plasma circulated through the CFBB had 5 × 109 cells, which met the preliminary requirements of a BAL. In addition, we studied the effect of encapsulated hepatocytes under fluidization conditions on exchanged plasma from patients with severe hepatitis. We tried previously to use the concentrated “toxic” plasma (not diluted) in our research model. However, after the 6-h BAL session, SEM data indicated that the hepatocytes were necrotic and cell viability was markedly decreased (data not shown). This might be related to the toxic effect induced by the high toxin levels present in the plasma of patients with severe hepatitis. Therefore, we diluted the concentrated plasma twofold for these experiments. The measured parameters were related to the structural integrity of the hepatocytes, including their viability, because ALT is a cytoplasmic enzyme that is released after damage to the hepatocytes. In addition, the metabolic and synthetic functions of hepatocytes were indicated by the TBil and albumin measurements. In this study, a statistically significance difference in cell viability and ALT levels between the groups was observed after the 6-h BAL session, which indicated decreased damage to the hepatocytes in the CFBB group. The plasma levels of enzymes and albumin in the CFBB group that contained encapsulated hepatocytes were likely related to the protective effect of fluidization compared to the static conditions. Moreover, the TBil concentration was slightly higher after plasma circulation because of the lack of bilirubin excretion mechanism in our system, which suggests that an absorber of bilirubin is necessary in BAL circulation.
The ultrastructure of the microencapsulated hepatocytes was also observed after the plasma experiments. We found that the hepatocytes remained viable and many organelles were healthy, with only slight necrosis in the CFBB group. In contrast, after 6 h of static culture, the static encapsulated hepatocytes presented obvious necrosis and vacuolization. Therefore, we concluded that the CFBB bioreactor system can maintain functional hepatocytes that are protected from potential toxins.
This study was only performed in vitro; we did not use a BAL in vivo in our study. Therefore, these factors must be considered in a large-animal model of severe liver failure or preclinical application in future research. By creating a hybrid of the CFBB with a BAL, more toxic elements would be removed, and thus the BAL could exert longer-lasting effects, which will be investigated in the future.
The technology described in this experiment is simple to operate, although a systematic evaluation has yet to be demonstrated. Previously, the curative effect of BALs has generally been observed through animal liver failure induced by drug poisoning and small liver remnant volume or anhepatic state postoperatively[25-27]. Currently, there are no systematic data describing the exchange plasma of patients with severe hepatitis as an alternative to animal models for the curative effect of BAL. The merits of this method are as follows: (1) a rich source of plasma with aseptic collection; (2) this technique precludes the effect of multiple factors on the curative effect of BAL, which is favorable for the BAL mechanism; (3) the plasma was isolated from patients with severe hepatitis; therefore, the surrogate model meets the clinical requirements of severe hepatitis with more practical significance; and (4) it is a low-cost method with good repeatability, and it is easy to operate. Inevitably, some limitations exist. This technique has only been conducted in vitro; thus, it may not be extrapolated to animal or human studies because there are differences between the two systems. These considerations should be addressed in the future. The CFBB can function as a bioartificial liver based on a bioreactor. The efficacy of this novel bioreactor is promising for the study of liver failure.
A bioartificial liver (BAL) support system represents a new tool for terminal-stage liver failure. A bioreactor is the key device for a BAL system. The most widely investigated bioreactor at present is based on a hollow fiber membrane. However, this model is difficult to scale-up. Moreover, the semi-permeable membrane can act as a diffusion barrier between hepatocytes and patients’ plasma; therefore, the xenogenic hepatocytes are placed directly in contact with patient plasma without an impermeable immunological barrier. As such, hepatocytes immobilized into alginate/chitosan microcapsules might be an interesting alternative. Within a bioreactor, the microcapsules are usually hosted either in a fixed or fluidized bed. In a fixed bed, the beads cannot withstand the extreme stresses caused by high perfusion velocity. The fluid often mobilizes through channels in the space that is devoid of beads, which results in formation of a void volume or dead space in the traditional fluidized bed bioreactors.
A fluidized bed bioreactor is fit for encapsulated hepatocytes. In the area of a fixed bed bioreactor, the beads cannot withstand the extreme stresses caused by high perfusion velocity and the BAL based on fixed fluidized bed has inefficient mass exchange; much research is being devoted to determining how to overcome the extreme stress and decrease the void volume or dead space in the traditional fixed bed bioreactors.
In this study, the authors constructed a novel choanoid fluidized bed bioreactor (CFBB) and received a national invention patent. Traditional fluidized bed bioreactors have some shortcomings, such as a void volume or dead space, which directly affect the stability and mass exchange of a BAL. To address these problems, the authors constructed this bioreactor, which is characterized by a perfect combination of the “choanoid” form and fluidized bed. The BAL based on a CFBB with microencapsulated immortalized human hepatocytes appeared to be effective in treatment of severe hepatitis plasma associated with protection of hepatocytes. The efficacy of this novel bioreactor is promising for the study of liver failure.
The study results suggest that the CFBB is a promising key BAL device for the study of liver failure.
A Bioreactor, as a key device in bioartificial liver system, can provide a favorable growth and metabolic environment, mass exchange and immunological isolation as a platform. The fluidized bed is formed when a quantity of a solid is placed under appropriate conditions to cause the solid/fluid mixture to behave as a fluid. This is usually achieved by the introduction of pressurized fluid through the particulate medium. The resulting phenomenon is called fluidization. Fluidized beds are used for several purposes, such as fluidized bed reactors (types of chemical reactors) or mass transfer.
This is a good descriptive study in which the authors constructed and evaluated a novel CFBB based on extracorporeal circulation. The results are interesting and suggest that the CFBB can function as a bioartificial liver based on a bioreactor and the efficacy of this novel bioreactor is promising for the study of liver failure.
P- Reviewer: Liu ZH S- Editor: Wen LL L- Editor: Stewart GJ E- Editor: Ma S
| 1. | Lee WM, Squires RH, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 2. | Frühauf NR, Oldhafer KJ, Höltje M, Kaiser GM, Frühauf JH, Stavrou GA, Bader A, Broelsch CE. A bioartificial liver support system using primary hepatocytes: a preclinical study in a new porcine hepatectomy model. Surgery. 2004;136:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Tilles AW, Berthiaume F, Yarmush ML, Toner M. Critical issues in bioartificial liver development. Technol Health Care. 2002;10:177-186. [PubMed] |
| 4. | Gerlach JC. Development of a hybrid liver support system: a review. Int J Artif Organs. 1996;19:645-654. [PubMed] |
| 5. | Tilles AW, Berthiaume F, Yarmush ML, Tompkins RG, Toner M. Bioengineering of liver assist devices. J Hepatobiliary Pancreat Surg. 2002;9:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Shito M, Kim NH, Baskaran H, Tilles AW, Tompkins RG, Yarmush ML, Toner M. In vitro and in vivo evaluation of albumin synthesis rate of porcine hepatocytes in a flat-plate bioreactor. Artif Organs. 2001;25:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Shito M, Tilles AW, Tompkins RG, Yarmush ML, Toner M. Efficacy of an extracorporeal flat-plate bioartificial liver in treating fulminant hepatic failure. J Surg Res. 2003;111:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Lu HF, Lim WS, Zhang PC, Chia SM, Yu H, Mao HQ, Leong KW. Galactosylated poly(vinylidene difluoride) hollow fiber bioreactor for hepatocyte culture. Tissue Eng. 2005;11:1667-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Ho Ye S, Watanabe J, Takai M, Iwasaki Y, Ishihara K. High functional hollow fiber membrane modified with phospholipid polymers for a liver assist bioreactor. Biomaterials. 2006;27:1955-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Wen F, Chang S, Toh YC, Arooz T, Zhuo L, Teoh SH, Yu H. Development of dual-compartment perfusion bioreactor for serial coculture of hepatocytes and stellate cells in poly(lactic-co-glycolic acid)-collagen scaffolds. J Biomed Mater Res B Appl Biomater. 2008;87:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Chen JP, Lin CT. Dynamic seeding and perfusion culture of hepatocytes with galactosylated vegetable sponge in packed-bed bioreactor. J Biosci Bioeng. 2006;102:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Török E, Vogel C, Lütgehetmann M, Ma PX, Dandri M, Petersen J, Burda MR, Siebert K, Düllmann J, Rogiers X. Morphological and functional analysis of rat hepatocyte spheroids generated on poly(L-lactic acid) polymer in a pulsatile flow bioreactor. Tissue Eng. 2006;12:1881-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Kinasiewicz A, Gautier A, Lewiska D, Smietanka A, Legallais C, Weryński A. Three-dimensional growth of human hepatoma C3A cells within alginate beads for fluidized bioartificial liver. Int J Artif Organs. 2008;31:340-347. [PubMed] |
| 14. | Murtas S, Capuani G, Dentini M, Manetti C, Masci G, Massimi M, Miccheli A, Crescenzi V. Alginate beads as immobilization matrix for hepatocytes perfused in a bioreactor: a physico-chemical characterization. J Biomater Sci Polym Ed. 2005;16:829-846. [PubMed] |
| 15. | Gerlach JC, Encke J, Hole O, Müller C, Ryan CJ, Neuhaus P. Bioreactor for a larger scale hepatocyte in vitro perfusion. Transplantation. 1994;58:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 115] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Vournakis JN, Runstadler PW. Optimization of the microenvironment for mammalian cell culture in flexible collagen microspheres in a fluidized-bed bioreactor. Biotechnology. 1991;17:305-326. [PubMed] |
| 17. | Gåserød O, Sannes A, Skjåk-Braek G. Microcapsules of alginate-chitosan. II. A study of capsule stability and permeability. Biomaterials. 1999;20:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 196] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Coward SM, Legallais C, David B, Thomas M, Foo Y, Mavri-Damelin D, Hodgson HJ, Selden C. Alginate-encapsulated HepG2 cells in a fluidized bed bioreactor maintain function in human liver failure plasma. Artif Organs. 2009;33:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Nagaki M, Naito T, Ohnishi H, Akaike T, Muto Y, Moriwaki H. Effects of plasma from patients with fulminant hepatic failure on function of primary rat hepatocytes in three-dimensional culture. Liver Int. 2005;25:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Chen Z, Ding Y, Li G. Configuration of a new bioartificial liver support system and in vitro evaluation of its functions. Ann Clin Lab Sci. 2005;35:7-14. [PubMed] |
| 21. | Pan X, Li J, Du W, Yu X, Zhu C, Yu C, Cao H, Zhang Y, Chen Y, Li L. Establishment and characterization of immortalized human hepatocyte cell line for applications in bioartificial livers. Biotechnol Lett. 2012;34:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Yu CB, Lv GL, Pan XP, Chen YS, Cao HC, Zhang YM, Du WB, Yang SG, Li LJ. In vitro large-scale cultivation and evaluation of microencapsulated immortalized human hepatocytes (HepLL) in roller bottles. Int J Artif Organs. 2009;32:272-281. [PubMed] |
| 23. | Watanabe FD, Mullon CJ, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, Khalili T, Arnaout W, Shackleton CR, Rozga J. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg. 1997;225:484-91; discussion 491-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 320] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Xu X, Liu X, Ling Q, Wei Q, Liu Z, Xu X, Zhou L, Zhang M, Wu J, Huang J. Artificial liver support system combined with liver transplantation in the treatment of patients with acute-on-chronic liver failure. PLoS One. 2013;8:e58738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 542] [Reference Citation Analysis (0)] |
| 25. | Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991;36:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Donato MT, Castell JV, Gómez-Lechón MJ. Characterization of drug metabolizing activities in pig hepatocytes for use in bioartificial liver devices: comparison with other hepatic cellular models. J Hepatol. 1999;31:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Naka S, Takeshita K, Yamamoto T, Tani T, Kodama M. Bioartificial liver support system using porcine hepatocytes entrapped in a three-dimensional hollow fiber module with collagen gel: An evaluation in the swine acute liver failure model. Artif Organs. 1999;23:822-828. [PubMed] |