Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6832
Revised: February 18, 2014
Accepted: March 4, 2014
Published online: June 14, 2014
Processing time: 198 Days and 20.5 Hours
AIM: To investigate the effect of the probiotic combination Lactibiane Tolerance® (LT) on epithelial barrier function in vitro and in vivo.
METHODS: The effect of the multispecies probiotic LT was assessed on several models of epithelial barrier function both in vitro (in basal and inflammatory conditions) and in vivo [visceral hypersensitivity induced by chronic stress or by colonic perfusion of a fecal supernatant (FSN) from patients with irritable bowel syndrome (IBS)]. In vitro, we measured the permeability of confluent T84 cell monolayers incubated with or without LT by evaluating the paracellular flux of macromolecules, in basal conditions and after stimulation with lipopolysaccharide (LPS) or with conditioned medium of colonic biopsies from IBS patients (IBS-CM). In vivo, male C57/Bl6 mice received orally NaCl or LT for 15 d and were submitted to water avoidance stress (WAS) before evaluating visceral sensitivity by measuring the myoelectrical activity of the abdominal muscle and the paracellular permeability with 51Cr-EDTA. Permeability and sensitivity were also measured after colonic instillation of FSN. Tight-junctions were assessed by immunoblotting and TLR-4 expression was evaluated by immunohistochemistry
RESULTS: Incubation of T84 cell monolayers with LT in basal conditions had no significant effect on permeability (P > 0.05 vs culture medium). By contrast, addition of LT bacterial bodies (LT) completely prevented the LPS-induced increase in paracellular permeability (P < 0.01 vs LPS 10 ng/mL (LPS 10); P < 0.01 vs LPS 100 ng/mL (LPS 100), P > 0.05 vs culture medium). The effect was dose dependent as addition of 109 LT bacterial bodies induced a stronger decrease in absorbance than 106 LT (109 LT + LPS 10: -20.1% ± 13.4, P < 0.01 vs LPS 10; 106 LT + LPS 10: -11.6% ± 6.2, P < 0.01 vs LPS 10; 109 LT + LPS 100: -14.4% ± 5.5, P < 0.01 vs LPS 100; 106 LT + LPS 100: -11.6% ± 7.3, P < 0.05 vs LPS 100). Moreover, the increase in paracellular permeability induced by culturing T84 cells with conditioned medium of colonic biopsies from IBS patients (IBS-CM) was completely inhibited in the presence of 109 LT (P < 0.01 vs IBS-CM). LT also significantly prevented the epithelial disruption induced by intracolonic infusion of fecal supernatant from IBS patients (P < 0.01 vs IBS FSN) or water avoidance stress P < 0.01 vs WAS) in C57/Bl6 mice and increased the expression of occludin in vitro and in vivo, as assessed by immnunoblotting. The WAS-induced effect on visceral sensitivity was prevented by LT treatment since values obtained for all steps of colorectal distension were significantly (P < 0.01) different from the WAS group. Finally, LT down-regulated the response mediated through TLR-4 in vitro (decrease in tumor necrosis factor α secretion in response to LPS: -65.8% for 109 LT and -52.5% for 106 LT, P < 0.01 vs LPS) and in vivo (inhibition of WAS induced an increase in TLR-4 expression in the LT treated mice colon, P < 0.01 vs WAS).
CONCLUSION: The probiotic LT mix prevented the disruption to the epithelial barrier induced by LPS, stress or colonic soluble factors from IBS patients and prevented visceral hypersensitivity.
Core tip: Some probiotics are effective in treatment of irritable bowel syndrome (IBS). The pathophysiology of IBS involves disruption of the epithelial barrier together with low grade inflammation, which represent peripheral targets for probiotics. This study aimed to elucidate the effect of a multispecies combination of bacterial strains on in vitro and in vivo models mimicking IBS. This commercialized combination was able to prevent visceral hypersensitivity and to repair the disruption to the epithelial barrier induced by lipopolysaccharide, stress or colonic soluble factors from IBS patients.
- Citation: Nébot-Vivinus M, Harkat C, Bzioueche H, Cartier C, Plichon-Dainese R, Moussa L, Eutamene H, Pishvaie D, Holowacz S, Seyrig C, Piche T, Theodorou V. Multispecies probiotic protects gut barrier function in experimental models. World J Gastroenterol 2014; 20(22): 6832-6843
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6832.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6832
Irritable bowel syndrome (IBS) is a common chronic functional disorder of the gastrointestinal tract characterized by abdominal pain and alterations in bowel habit. Its etiology remains poorly understood, but epithelial barrier (EB) impairment associated with low-grade inflammation and dysbiosis (increased Clostridium perfringens, decreased Bifidobacteria or Lactobacilli) have been incriminated, and are related, in some ways, to IBS severity. In IBS patients, as in stressed animals, an increase in gut permeability has been associated with altered expression or distribution of tight junction proteins such as occludin and ZO-1 and positively correlated with visceral pain or stress-induced visceral hypersensitivity. Increased intestinal permeability and the occurrence of dysbiosis in IBS may therefore participate in the generation of IBS-symptoms through Toll-like receptor (TLR) (recognizing microbial-associated molecular patterns) signaling. Accordingly, increased expression of TLR-4 in the colonic mucosa of IBS patients, according to the disease subtype, has been reported[1,2]. The evidence that dysbiosis explains, at least in part, the pathophysiology of IBS constitutes a strong argument for probiotic use, some of which have shown beneficial effects. However, the therapeutic gain over placebo was often low and the mechanism of action explaining their effect was often lacking[3,4].
Probiotics are defined as live organisms that exert a health benefit on the host through diverse mechanisms. They affect the composition or function of the commensal bacteria and the host epithelium, influence immunological responses[5], restrict bacterial and LPS translocation[6,7] and decrease visceral sensitivity[8,9]. In recent clinical trials, the use of probiotic combinations has been privileged over a single strain[10,11]. As enhancement of the intestinal barrier function was associated with a decrease in visceral sensitivity, we assumed that probiotic treatments targeting the maintenance of the EB integrity might be of particular interest to alleviate IBS symptoms and so tested different probiotic strains or combinations in vitro and in vivo. Central nervous system-directed stressors are one of the most common IBS-like animal models. Indeed, stress exacerbates IBS symptoms and increases intestinal permeability in animal models in which dysbiosis was reported.
This study aimed to provide preclinical data for a probiotic dietary supplement, Lactibiane Tolerance® (LT), which is composed of five different strains (Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus salivarius and two strains of Bifidobacterium lactis) and has already been characterized for its anti-inflammatory properties[12]. In vitro, we investigated the effect of the LT strain combination on the EB function of confluent human colonic T84 cell monolayers in basal and inflammatory conditions. In vivo, we studied the impact of LT strains on chronic stress-induced changes to the intestinal permeability and visceral sensitivity. Finally, a translational approach from humans to animals was assessed to evaluate the effect of LT on EB impairment and visceral hypersensitivity induced by IBS colonic fluid in C57/Bl6 mice.
All the research conducted for this work was performed in France. Colonic biopsies and fecal samples from healthy subjects and IBS patients fulfilling the Rome III criteria were collected in the Department of Gastroenterology of the University Hospital of Nice, France. The study was conducted with local approval from the Ethics Committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale N°07.047) and all patients gave their written informed consent. This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. All experimental protocols were approved by the Local Animal Care and Use Committee of the Institut National de la Recherche Agronomique and all effort was made to minimize suffering.
Cell culture: The human colon epithelial cell line T84 (ATCC, Rockville, Maryland, United States) was cultured at 37 °C with 5% CO2 in Dulbecco’s Modified Eagle Medium:Nutrient Mixture F-12 GlutaMax™ (DMEM⁄F-12) supplemented with 10% heat inactivated fetal calf serum, 1% penicillin, 1% streptomycin and 1% of MEM Non-essential Amino-Acids (all reagents from Gibco, Saint Aubin, France).
Bacterial culture: LT was provided by Pileje. This is a mix of 5 bacterial strains: 10% Lactobacillus acidophilus (LA201), 40% Lactobacillus plantarum (LA301), 20% Lactobacillus salivarius (LA302), 10% Bifidobacterium lactis (LA303) and 20% of Bifidobacterium lactis (LA304). Bacteria were grown on Man Rogoza Sharpe broth (Dominique Dutscher, Brumath, France) overnight at 37 °C in anaerobic conditions and used at the exponential phase of growth. Bacteria were separated from the culture supernatant by centrifugation (10 min at 3500 g), washed twice with PBS (Gibco, St Aubin, France) and resuspended in DMEM ⁄ F-12 without antibiotics. A suspension containing 10% LA201, 40% LA301, 20% LA302, 10% LA303 and 20% LA304 was prepared extemporaneously.
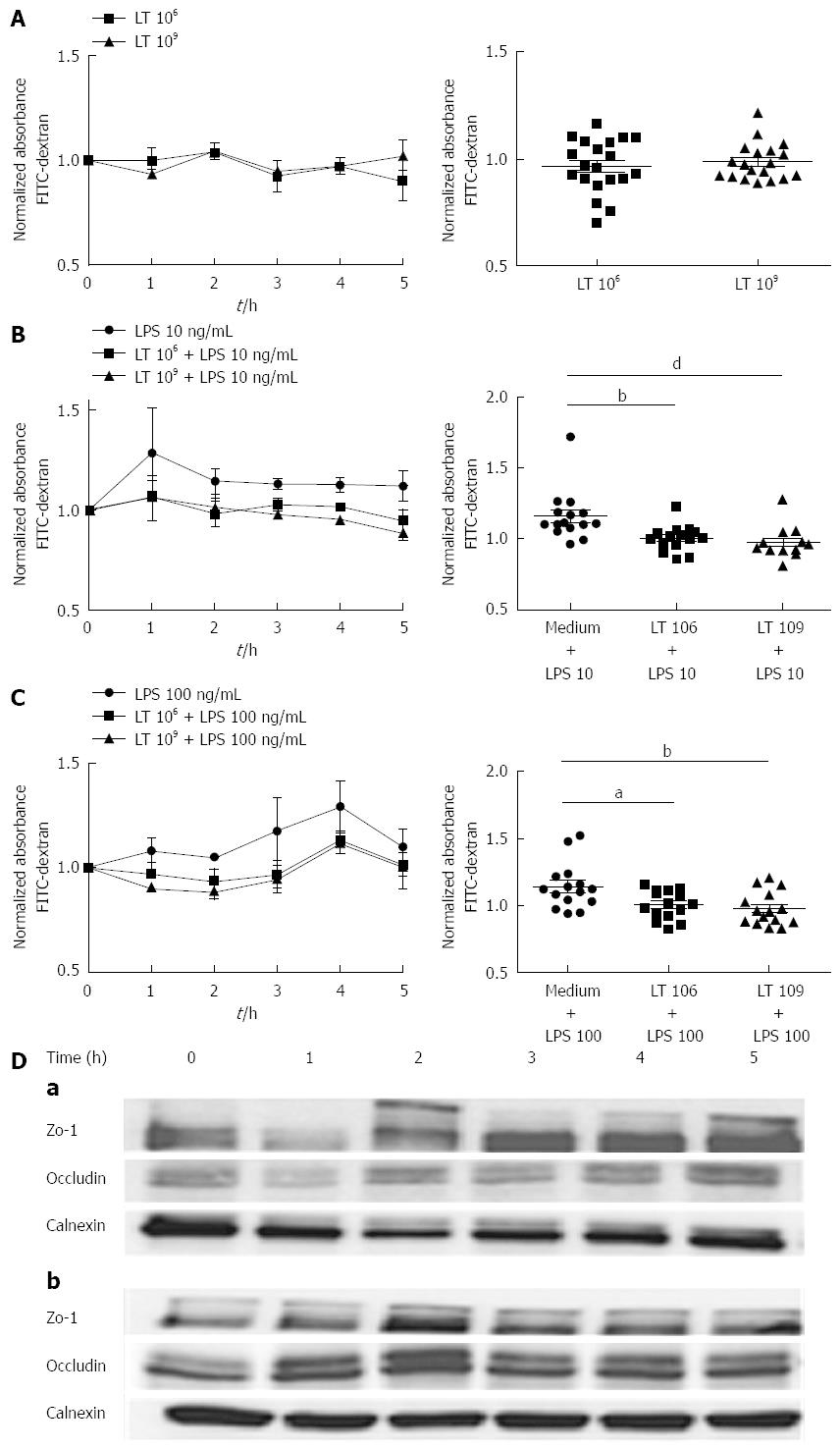
Permeability assays: Permeability assays were performed in Transwell chambers (Sigma, L’Isle D’Abeau Chesnes, St. Quentin Fallavier)[13]. T84 cells were grown on Transwell insert filters (0.4 μm pore size, 6.5-mm diameter) pre coated with 0.1% collagen (Sigma Aldrich, St Quentin Fallavier, France) until they reached confluence. Cell confluency in each well was examined with a microscope before adding 106 or 109 LT bacterial bodies (LT) or only culture medium to the inserts. Plates were incubated for 5 h at 37 °C, 5% CO2. When indicated, 10 ng/mL or 100 ng/mL LPS (Sigma Aldrich, St Quentin Fallavier, France) was added to the wells concomitantly to LT. 5 μL of 1 mg/mL FITC-dextran (478 Da, Invitrogen, CARLSBAD, Germany) were added to the apical side of T84 monolayers and the absorbance at 450 nm of the medium in the lower chamber was measured with a microplate reader (Tecan, Lyon, France) to assess dextran flux toward the lower chamber, before adding LT bodies and every hour for 5 h. Experiments were performed in duplicate. A one-way Anova analysis followed by the Bonferroni post-test were used for comparison of conditions with and without LT. Statistical significance was set at P < 0.05.
Determination of ZO-1 and occludin expression by immunoblotting: T84 cells were grown in 6-well microplates (Becton Dickinson, Pont de Claix, France) until they reached confluence, which was assessed by light microscopy, before incubating them with LT or culture medium only for 1 to 5 h. After washing, proteins were extracted with 1% Triton (Sigma Aldrich, Steinheim, Germany) buffer containing a protease and phosphatase inhibitor cocktail (Roche, Meylan, France). For analysis, 50 μg of total protein per lane was loaded onto a 7.5% SDS-polyacrylamide gel and transferred to 0.45 μm nitrocellulose membranes (Santa Cruz, Heidelberg, Germany). Membrane strips were cut, after staining the membrane with Ponceau red, according to the target, blocked for 1 h with 5% non-fat milk, 0.4% PBS-Tween 20 (Euromedex, Mundolsheim, France) buffer and incubated overnight at 4 °C with the primary antibodies (anti-ZO-1 mAb (clone1/ZO-1, 0.25 μg/mL, BD Bioscience, Le Pont de Claix, France); anti-occludin mAb (clone OC-3F10, 0.5 μg/mL, Invitrogen, Saint Aubin, France); (anti calnexin mAb (clone37, 0.25 μg/mL, BD Biosciences, Le Pont de Claix, France). After washing with 0.4% PBS-Tween 20, membranes were incubated with a secondary antibody. Proteins were detected with enhanced chemiluminescence reagents (ECL Advance Western Blotting Kit detection, GE Healthcare, United Kingdom), visualised with FUJIFILM LAS4000 (FUJIFILM) and analysed using FUJIFILM Multi Gauge V3.0 software. Calnexin was used as a loading control.
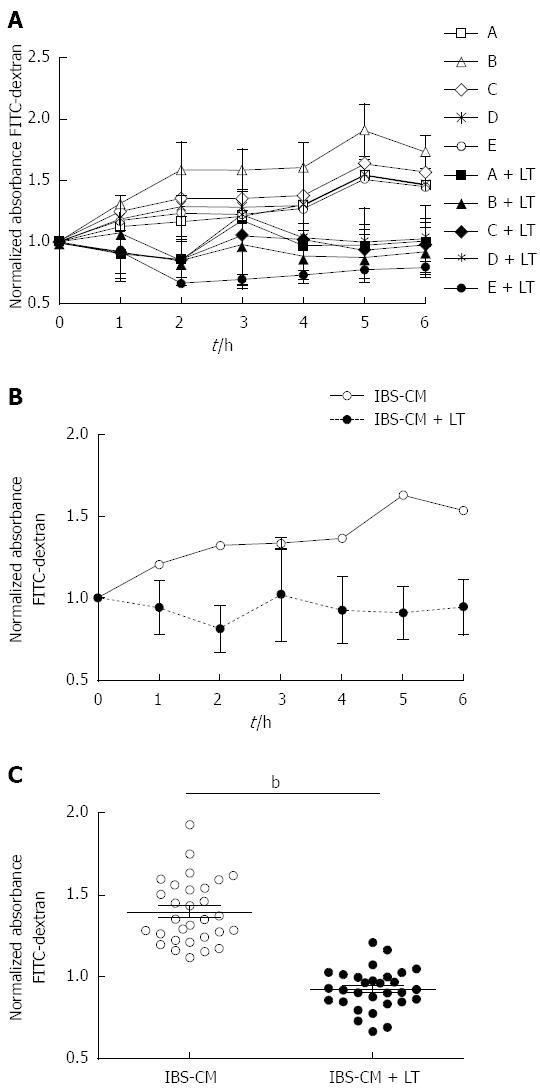
Preparation of IBS colonic biopsy conditioned medium: Colonic biopsies from 5 IBS patients classified according to Rome III criteria, 2 with constipation and 3 with predominance of diarrhea, were cultured as previously described[14]. Briefly, biopsies were cultured for 25 minutes at 37 °C under oxygenation in 10 mL/mg of DMEM F12. Conditioned medium was then collected, centrifuged, 0.2 μm filtered and frozen at -20 °C until use. When indicated, 150 μL of each IBS patient biopsy culture medium (IBS-CM) were added to the wells (100 μL into the lower chamber and 50 μL onto the insert), with or without addition of 109 LT. Cells were then cultured for 6 h at 37 °C, 5% CO2 and permeability assays performed as described above. Experiments were performed in duplicate. Statistical significance evaluated with the Student’s t test was set at P < 0.05.
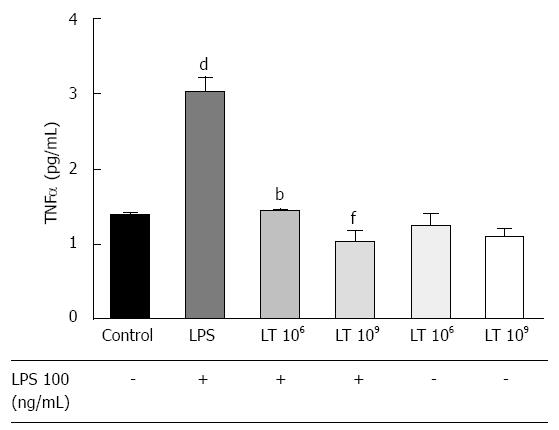
Determination of the tumor necrosis factorαresponse to LPS by ELISA: T84 cells were grown in 6-well microplates (Becton Dickinson, Pont de Claix, France) until they reached confluence, which was assessed by light microscopy. Cells were then cultured for 24 h with or without addition of 106 or 109 LT bacterial bodies (LT), in basal conditions or in the presence of 100 ng/mL LPS. Supernatants were then centrifuged, concentrated 30-fold in Corning Spin-X UF 6 mL concentrators 5 kDa (Sigma Aldrich, Schnelldorf, Germany) and tested for tumor necrosis factor α (TNFα) using a Human TNFα high sensitivity ELISA (eBioscience, Vienna, Austria) according to the manufacturer’s recommendations. Experiments were performed in duplicate. Statistical significance evaluated with the one-way Anova analysis followed by the Bonferroni post-test was set at P < 0.05.
Animals: Six to 8-week-old male C57/Bl6 mice (Janvier Le Genest St-Isle, France) were used. Mice were housed in propylene cages in a light and temperature-controlled room (12/12 h cycles, 21 °C ± 1 °C). They had free access to food (standard pellets; Harlan Tecklan, Oxon United Kingdom) and tap water.
Water avoidance stress: The water avoidance stress (WAS) protocol was adapted from Larauche et al[15]. Briefly mice were placed on a 3 cm × 3 cm platform in a 40 cm × 40 cm size pool filled with tap water for 1 hour during 4 consecutive days. The animals that fell into the water were gently dried with a towel and placed back onto the platform.
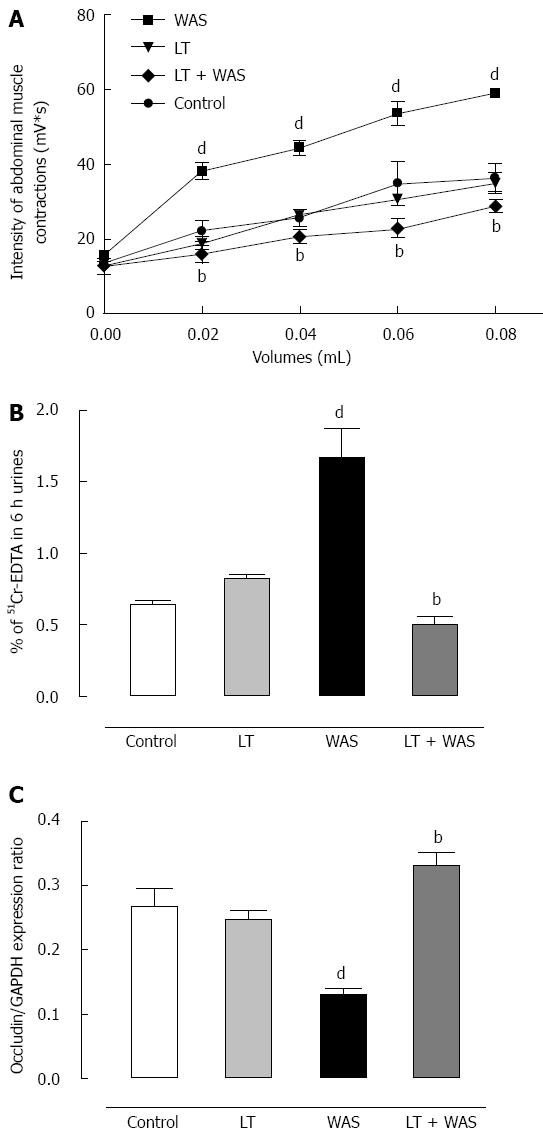
Visceral sensitivity measurement: A painful stimulus was given by colorectal distension, which evokes an abdominal response as a sign of visceral sensitivity, recorded by electromyography (EMG). Under anesthesia (xylasine 5 mg and ketamine 1 mg), administered subcutaneously (sc), animals were chronically fitted with two nickel-chrome electrodes implanted in the abdominal external oblique muscle and a third in the abdominal skin. The electrodes were drawn to the back of the neck sc and protected by a plastic tube. Four to 6 d post-surgery, mice were placed in a plastic tunnel 1 hour prior to the experiments to stimulate the emptying of bowel contents. Under light sodium pentobarbital anesthesia (1 mg, ip), distension catheters (Fogarty catheter for arterial embolectomy, 4F; Edwards Lifesciences, Nijmegen, The Netherlands) were inserted into the colon (2.5 cm from the rectum). The colorectal distension (CRD) was performed in conscious mice with progressively increasing volumes in 0.02 mL steps from 0 to 0.08 mL each step lasting 10 s with 5 min non distension periods in-between. During the distension periods, the abdominal muscle electrical activity was recorded and analyzed with a Powerlab Chart 5 program from AD instruments. Basal EMG activity was subtracted from the EMG activity registered during the periods of distension.
In vivo permeability studies: Mice received by gavage 200 mL of 51Cr-ethylenediamine tetra-acetic acid (51Cr-EDTA, 0.35 mCi diluted in water, Perkin Elmer Life Science, Courtaboeuf, France). 51Cr-EDTA was used as a marker of paracellular intestinal permeability[16]. Mice were then placed in metabolic cages and urine was collected for 6 h. The total radioactivity found in the urine was measured with a gammacounter (Cobra II; Packard, Meriden, CT). Permeability to 51Cr-EDTA was expressed as a percentage of the total radioactivity administered.
Determination of occludin expression: After sacrifice colonic samples were collected in dry ice and stored at -80 °C until processed. Proteins were extracted with RIPA buffer and protein concentrations assessed using the BCA-200 Protein Assay Kit (Pierce Rockford, IL). For analysis, 15 μg of protein was loaded onto a 10% SDS-polyacrylamide gel and transferred to 0.45 μm nitrocellulose membranes (Whatman, Dominique Deutcher, Brumath, France). The membranes were blocked 1 h with Odyssey blocking buffer (Rockland, Tebu-bio, France). Membranes were then incubated with rabbit anti-occludin 1/500 (Invitrogen, Life Technologies, CA) or rabbit anti-GAPDH 1/1000 antibodies (Cell Signaling Technology, Danvers, MA), overnight at 4 °C. After washing, membranes were incubated with fluorescent CF770 anti-rabbit 1/10000 antibody (Biotium, Hayward, CA). Finally, they were scanned on an infrared imaging system Odyssey (Li-Cor, Lincoln, NE). Occludin expression was assessed relative to GAPDH for each sample.
Immunohistochemistry of TLR-4: Colon specimens were fixed in 4% buffered paraformaldehyde and incubated 24 h in 30% sucrose at 4 °C. Samples were embedded in Neg 50 medium (Microm, Francheville, France) and frozen in isopentan at -45 °C. Cryostat sections (7 μm) were post-fixed with acetone (10 min, -20 °C) and hydrated in PBS-Tween. After incubation in blocking solution (PBS-Tween containing 1% bovine serum albumin and 2% donkey serum), sections were incubated (overnight, +4 °C) with rabbit anti-TLR-4 antibodies (1/200) (Abcam, Paris, France). Sections were then washed in PBS and incubated for 90 min at room temperature with Alexa fluor 488-conjugated IgG donkey anti-rabbit (1/2000) (Life Technologies, Saint-Aubin, France). Sections were mounted in Prolong gold antifade mounting medium (Life Technologies) and examined under a Nikon 90i fluorescence microscope. TLR-4 immunoreactivity was quantified employing the Nis-Elements-Ar software (Nikon, Champigny-sur-Marne, France). The total intensity per square micrometer of the epithelium apical region was measured on five fields of each group (n = 5).
Fecal sample collection and preparation: This series of experiments was carried out using fecal samples from healthy subjects and IBS patients who fulfilled the Rome III criteria. Samples were collected in the Department of Gastroenterology of the University Hospital of Nice, France. Fecal samples were kept at -20 °C and transported on dry ice to INRA, Toulouse, France, and then thawed at 4 °C. 0.5 g of each sample was diluted and homogenized in 4 mL of 0.9% NaCl solution. After centrifugation (10 min, 5000 g, 4 °C), pellets were discarded and fecal supernatants (FSN) were filtered on 0.8 μm syringe filters.
Intracolonic infusions: Mice were fasted for 24 h and then placed in a plastic tunnel as described for the visceral sensitivity measurement. Under light sodium pentobarbital anesthesia (1 mg, ip) a polyethylene perfusion catheter was inserted into the distal colon ending 3.5 cm from the anus. After recovery from anesthesia, animals were infused intracolonically with 0.3 mL of FSN from healthy or IBS patients.
Experimental design: The probiotic combination Lactibiane Tolerance® (LT) (freeze-dried bacteria provided by Pileje, Paris, France) was freshly prepared every day in 0.9% NaCl. Animals received orally LT at a concentration of 109 CFU/day/mouse or vehicle (0.9% NaCl) 15 d before and for 4 d during the WAS session. In a first series of experiments animals received LT or vehicle and were submitted to WAS or not. Animals not submitted to WAS were handled daily (controls). In 4 groups of 8 mice visceral sensitivity was evaluated 1 h after the WAS. Intestinal paracellular permeability was evaluated in another set of 4 groups of mice. Finally, in a last set of 4 groups of 5 mice submitted to WAS or not, colonic segments were collected after the WAS session to evaluate occludin expression by immunoblotting and TLR-4 expression by immunostaining. In a second series of experiments mice received, as previously described, LT or vehicle for 15 d. At the end of the treatment period they were infused intracolonically with 0.3 mL of FSN from healthy or IBS-D patients for 2 h (infusion rate 0.10 mL/h) and the CRD procedure and intestinal paracellular permeability measurement were performed 1 h after the end of the infusion. 4 FSN from healthy controls or IBS patients were tested. Each supernatant was tested in 5 mice.
All data are presented as means ± SEM. Statistical analyses were performed using Graph Pad Prism 4.0 (Graph Pad, San Diego, CA). A two-way Anova analysis followed by a Bonferroni post-test was used for comparison of LT treatment stress or FSN infusion-induced hypersensitivity. A one-way Anova analysis followed by a Bonferroni post-test was used for comparison of LT treatment stress or FSN infusion-induced hyperpermeability. Statistical significance was set at P < 0.05.
We compared the barrier function of human colonic T84 cell monolayers grown on 0.4 μm Transwell inserts and cultured with or without LT for 5 h by measuring the extravasation of FITC-dextran through the monolayers. In basal conditions, incubation with 109 or 106 LT had no significant effect on the monolayer permeability as shown by the absorbance levels in the lower chambers, which remained similar to control wells containing culture medium only (variation of the mean absorbance by comparison with the control (VAM/control) of -2.5% ± 4.3% for 109 LT and -3.2 % ± 5.5% for 106 LT; P > 0.05). By contrast, we observed a very significant (P < 0.01) difference in permeability between T84 monolayers treated or not with LT in the presence of 10 ng/mL LPS. The LPS-induced increase in absorbance in the lower chambers (VAM/control of +14.1% ± 9.1%; Figure 1B) was completely abolished in the presence of LT, as shown by the absorbance, which remained similar to control wells containing culture medium only (VAM/control of -2.5% ± 3.9% for LPS + 109 LT and -3.2% ± 3.6% for 106 LT-B; NS). The effect was dose-dependent, with stronger inhibition of FITC-dextran extravasation induced by addition of 109 LT than 106 LT when compared to wells containing LPS only (VAM/LPS of -20.1% ± 13.4% and -11.6% ± 6.2% respectively; P < 0.01). When LPS was used at a higher concentration (100 ng/mL), we obtained similar results (Figure 1C). Addition of 109 LT induced a significant decrease in FITC-dextran extravasation by comparison with wells containing LPS only (VAM/LPS: -14.4% ± 5.5%; P < 0.01) while addition of 106 LT induced a smaller but still significant decrease in absorbance (VAM/LPS: -11.6% ± 7.3%; P < 0.05). The protective effect on the barrier function was complete, as shown by the absence of a significant difference for FITC-dextran extravasation with controls wells containing culture medium only (VAM/control: -2.5% ± 8.9% for 109 LT and 0.4% ± 7.6% for 106 LT; NS).
ZO-1 expression was assessed by immunoblotting of samples of T84 monolayers incubated with LT. In basal conditions, incubation of T84 monolayers with LT induced a slight augmentation of ZO-1 expression, starting after 2 h, while the expression of occludin remained similar to controls (Figure 1D, a). In the presence of 10 ng/mL LPS, both ZO-1 and occludin expression increased after incubation of T84 with LT (Figure 1D, b). Calnexin was used as a loading control.
Incubation with LT strongly inhibited the TNFα response of T84 cells to LPS stimulation
Intestinal cell permeability can be modulated by inflammatory cytokines, in particular TNFα. Therefore, we investigated if incubation of T84 monolayers with LT altered TNFα secretion in response to LPS (Figure 2). Addition of 109 LT or 106 LT in basal conditions did not significantly alter TNFα secretion by the T84 monolayer. However, the augmentation in TNFα secretion induced by LPS stimulation (+119.6%, P < 0.001) was strongly inhibited in the presence of LT in the wells (-65.8%, P < 0.01 for 109 LT and -52.5%, P < 0.01 for 106 LT) with TNFα levels identical to the unstimulated control.
We cultured colonic biopsies from 5 patients with IBS, two with predominance of constipation (Figure 3A, patients A and B) and three with predominance of diarrhoea (Figure 3A, patients C, D, E), as previously described and collected the conditioned medium after centrifugation. As expected, incubation of confluent T84 monolayers with IBS biopsy conditioned medium (IBS-CM) for 6 h augmented the monolayer permeability as shown by the increased extravasation of FITC-dextran to lower chambers (Figure 3A and B). Addition of 109 LT to the inserts protected the monolayer from the IBS-CM-induced increase in permeability in all cases (Figure 3A). The inhibition of the IBS-CM-induced augmentation in absorbance was already visible after 1 hour of incubation with LT (-25.8% ± 5.0%) and maximal at 5 h (-72.2% ± 20.7%) with an average diminution of 47.2% ± 23.3% between wells containing IBS-CM + LT and IBS-CM only and was statistically significant (P < 0.01, Figure 3B and C).
WAS significantly (P < 0.01) increased the amplitude of the muscular EMG response to CRD from the 0.02 to 0.08 mL steps of distension applied in comparison to controls (Figure 4A). The WAS-induced effect on visceral sensitivity was prevented by LT treatment since values obtained for all steps of CRD were significantly (P < 0.01) different from the WAS group (Figure 4A). LT treatment in the absence of stress did not modify visceral sensitivity in response to CRD since the values obtained were not significantly different from control values (Figure 4A). Intestinal paracellular permeability was significantly increased in stressed animals compared to controls (0.65% ± 0.01% vs 1.67% ± 0.20% of 51Cr-EDTA recovery in 6 h urines) (Figure 4B). The increase in intestinal permeability induced by stress was associated with a sharp (P < 0.01) decrease in occludin expression by colonocytes (Figure 4C). LT treatment strongly (P < 0.01) prevented the WAS-induced effects on intestinal permeability and occludin expression, but did not modify intestinal permeability nor occludin expression in the absence of stress (Figures 4B and C).
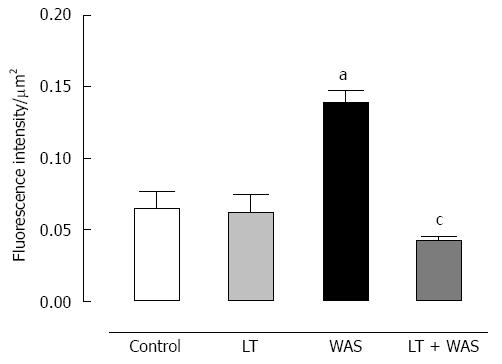
WAS strongly (P < 0.01) increased colonic TLR-4 expression in comparison with controls. LT treatment prevented this effect since the TLR-4 immunostaining intensity with LT treatment was similar to controls whereas LT treatment in the absence of stress did not modify colonic TLR-4 expression (Figure 5).
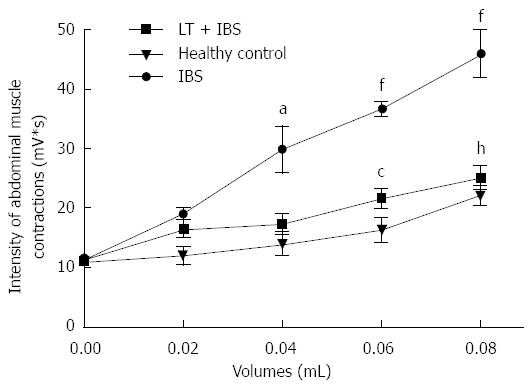
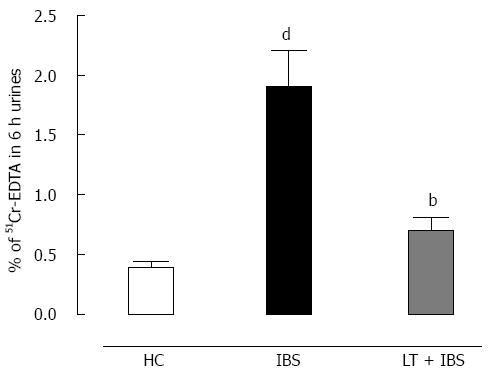
Intracolonic infusion of FSN from IBS patients performed prior to CRD significantly (P < 0.01) increased the muscular EMG response to CRD at 0.04 to 0.08 mL of distension when compared to values obtained after intracolonic infusion of healthy controls FSN (Figure 6). LT treatment blocked (P < 0.01) the IBS FSN-induced hypersensitivity at higher CRD volumes, namely 0.06 and 0.08 mL (Figure 6). A marked (P < 0.01) increase in intestinal paracellular permeability was also induced by IBS FSN infusion (Figure 7), which was totally blocked (P < 0.01) in animals treated with LT (Figure 7).
In the present study, we showed that the multispecies probiotic LT was able to prevent epithelial barrier disruption in in vitro and in vivo models mimicking IBS pathophysiology. The present findings have shown that LT prevented experimentally induced EB impairment both in vitro and in vivo.
In vitro, EB disruption of T84 monolayers was obtained by incubating cells with LPS or IBS colonic biopsy conditioned medium, which was completely prevented in the presence of LT. In vivo, increased gut paracellular permeability was induced by chronic stress or intracolonic infusion of IBS FSN in mice, which was prevented by a two week treatment with LT. Interestingly, LT live bacteria did not alter the basal EB function, which is in agreement with the generally admitted safety of probiotic treatments, whereas they prevented stress-induced gut hyperpermeability. Similarly, LT did not alter the T84 monolayers barrier function in basal conditions, but prevented LPS-induced hyperpermeability. Co-incubation of colonic biopsy conditioned medium (IBS-CM) with T84 monolayers resulted, as expected, in a significant increase in the T84 cells permeability and this effect was prevented in a significant way in the presence of LT.
In vivo, intra-colonic infusion of an IBS fecal supernatant in mice led to disruption of the epithelial integrity, suggesting that soluble mediators from both fluids are able to increase gut permeability, and this effect was prevented by LT treatment. These data are in line with results reported by Mangell et al[17] who showed that ingestion of Lactobacillus plantarum 299v by rats inhibited the Escherichia coli-induced intestinal permeability assessed in Ussing chambers, but had no effect in basal conditions. Enhancement of the intestinal EB function by probiotics is well recognized, modifying tight junction (TJ) dynamics[6] and decreasing bacterial translocation[6]. In agreement with these data, we have shown that LT treatment decreased WAS-induced occludin expression.
In vitro, incubation with LT also enhanced occludin expression in T84 monolayers in the presence of LPS, suggesting that LT could inhibit the degradation of occludin by enzymes that are activated in inflammatory conditions. Indeed, it was previously shown that occludin degradation could be induced by uPA, a protease activator[18] and is sensitive to proteasome inhibition[19]. Another possibility is that LT interacts with epithelial cell signaling proteins involved in TJ regulation. The observation that LT treatment increased ZO-1 expression is also of particular interest since IBS colonic biopsies reveal lower expression of ZO-1 mRNA associated with increased permeability[14], further supported by the marked internalization of ZO-1 observed in mouse colonocytes exposed to FSN from diarrhea-IBS[20].
Intestinal cell permeability can be modulated by inflammatory cytokines produced in response to various stimuli including TLR binding. In particular, TLR-4 is required for the recognition of LPS and its activation leads to EB impairment and pro-inflammatory mediator release[21]. LT prevention of WAS-induced EB integrity loss could possibly be linked to the ability of this treatment to decrease the WAS-induced TLR-4 up regulation in the colonic mucosa. We can therefore hypothesize that the effect of LT on permeability and TNFα secretion involves the inhibition of a pathway stimulated through TLR-4 activation.
Additionally, LT also prevented the visceral hypersensitivity (VHS) also induced by stress as by mucosal application of IBS FSN. These results highlight the ability of LT to enhance EB function and to improve the gut dysfunction associated with disruption of the intestinal EB integrity. Furthermore, mucosal application of IBS FSN to mice increased the abdominal response to colorectal distension. These findings are in agreement with previous studies showing that the addition of a IBS colonic supernatant increased Caco-2 cell permeability[14] and that mucosal exposure to IBS FSN increased gut permeability and visceral sensitivity in mice[20,22] .
Increased proteolytic activity in both fecal and colonic IBS supernatants has been described[14,20-23] and directly related to EB impairment, reflected by enhanced cytoskeleton contraction[24,25] and VHS, through a protease-activated receptor-2 pathway[20]. These findings strongly support the involvement of proteases. Interestingly, a positive correlation between increased gut permeability and visceral pain in IBS patients has been reported[26] suggesting that disruption of intestinal EB plays a pivotal role in emergence of IBS symptoms. It is noteworthy that stress-induced gut hyper-permeability has been identified as the cause of stress-induced VHS in rodents[27]. The leaky intestinal epithelium, by facilitating upload of luminal factors, leads to immune stimulation and release of pro-inflammatory mediators sensitizing afferent nerve terminals. Therefore one can hypothesize that targeting the reinforcement of the EB may contribute to improvement of IBS associated visceral pain. LT treatment prevented VHS induced by stress and IBS FSN intra-colonic infusion. Our data suggest that the anti-nociceptive effect of LT we observed resulted from the LT-induced decrease in gut permeability. These data are in agreement with a previous study showing that L. farciminis treatment prevented stress-induced VHS[8]. Concerning the effect of LT treatment on IBS colonic biopsy conditioned medium or fecal supernatant-induced hyperpermeability, we cannot exclude a possible inhibitory effect of LT-derived metabolites on soluble mediators present in these fluids. For example, lactobacilli are known to exert an anti-protease activity, suggesting a possible degradation or inhibition of proteases present in colonic and fecal IBS fluids. Interestingly, a decrease in the lactobacilli population has been observed in IBS fecal microbiota[28,29]. Moreover, Brint et al[1] reported an increase in TLR-4 receptors in the colonic mucosa of IBS patients.
Regarding the LT strain composition, the literature indicates that L. plantarum, which represents 40% of the LT, increases occludin, ZO-1 and cingulin gene expression when incubated with Caco-2 cells[30] while L. acidophilus and L. salivarius enhance EB function[31,32]. Interestingly, these strains exert their effect on gut permeability when given in combination with another Lactobacillus[32] or a Bifidobacterium[31] respectively, suggesting a synergistic action between strains. We can hypothesize that in the present study L. acidophilus and L salivarius contribute to the effect of LT on gut permeability through positive interaction with the other lactobacilli and bifidobacteria composing LT. Similarly, Bifidobacterium lactis was shown to prevent stress-induced EB disruption in the presence of other probiotic strains or bacterial products of fermentation[33]. Taken together, the in vitro and in vivo data suggest that live LT strains exert a beneficial effect on the intestinal barrier function and converge towards protection of the EB.
Finally, we also suggest that our experimental approaches including several models mimicking IBS epithelial disruption may help to better select strains with the highest therapeutic potential. Indeed, impairment of permeability has been associated with IBS severity[14,26]. In the present study, permeability was assessed in a subsequently validated model of stress-induced visceral hypersensitivity, intestinal hyperpermeability and translational effect of soluble mediators from IBS patients[14,27].
In conclusion, the LT multispecies combination tested in this study prevented EB impairment in vitro and in vivo. This effect was associated with (1) regulation of the TJ proteins leading to the normalization of EB integrity, possibly through an anti-protease activity; and (2) modulation of TLR-4 receptor expression through a direct action on TLR-4 expressing cells in the intestine or indirect action through a crosstalk between LT and the microbiota. Furthermore, LT prevented VHS probably by enhancing the EB function. Finally, the beneficial effects of LT on the EB integrity and on visceral sensitivity that were altered by colonic and fecal IBS fluids reinforced the rationale for evaluating its impact on IBS symptoms in a clinical trial.
The etiology of irritable bowel syndrome (IBS) is thought to be multifactorial, involving a complex interplay between increased intestinal permeability, low-grade immune activation and alterations in the gut flora, thus leading to activation in the enteric nervous system and increased visceral hypersensitivity. Some of these postulated factors may be potent attractive molecular and/or cellular targets for probiotic therapy.
This study aimed to provide preclinical data for a probiotic dietary supplement composed of five different strains (Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus salivarius and two strains of Bifidobacterium lactis). In this study, the authors demonstrated that these strains when delivered in combination are able to prevent visceral hypersensitivity and to repair disruption to the epithelial barrier that was induced by different inflammatory and stress conditions that mimick IBS.
There is a limited amount of convincing evidence to support the empiric use of probiotics in IBS. The randomized controlled trials that have been performed are typically small and methodologically biased. The study design reported here is of particular interest since the effect of probiotics was evaluated by combining several in vitro and in vivo experimental approaches that are likely to cover the spectrum of IBS pathophysiology. The authors clearly need to increase the number of studies using designed integrative models and techniques to assess the complexity of probiotic effects in IBS.
This study reinforces previous findings obtained in a randomized trial that used these strains and showed a clinical benefit to IBS symptoms. Moreover, the translational experimental approach using several models such as stress, low-grade inflammation, mucosal barrier defects and hypersensitivity should be encouraged to obtain a thorough comprehension of the effect of probiotics in humans.
Compromised intestinal barrier function has been reported to be associated with low-grade inflammation in the gut mucosa of IBS patients. Intestinal barrier function is believed to play a crucial role, at least in part, in some of the IBS symptoms, mainly abdominal pain and discomfort.
The role of probiotics in IBS in humans has already been reviewed in many articles. High quality studies in humans are limited. Nebot-Vivinus and coworkers present a study about the effect of the multispecies probiotics Lactibiane Tolerance® (LT) on various models of epithelial barrier function both in vitro and in vivo. LT is a bacterial composition of five different strains (Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus salivarius and two strains of Bifidobacterium lactis). In this interesting study the authors show that LT helps to prevent visceral hypersensitivity and to repair disruption to the epithelial barrier. Additionally in this study LT down-regulated the response mediated through TLR-4 in vitro and in vivo.
P- Reviewers: Basilisco G, Lenze F S- Editor: Wen LL L- Editor: O’Neill M E- Editor: Ma S
| 1. | Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Belmonte L, Beutheu Youmba S, Bertiaux-Vandaële N, Antonietti M, Lecleire S, Zalar A, Gourcerol G, Leroi AM, Déchelotte P, Coëffier M. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS One. 2012;7:e42777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Dai C, Zheng CQ, Jiang M, Ma XY, Jiang LJ. Probiotics and irritable bowel syndrome. World J Gastroenterol. 2013;19:5973-5980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 5. | Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 6. | Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 449] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 8. | Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthésy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901-1907. [PubMed] |
| 10. | Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Lyra A, Krogius-Kurikka L, Nikkilä J, Malinen E, Kajander K, Kurikka K, Korpela R, Palva A. Effect of a multispecies probiotic supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol. 2010;10:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Drouault-Holowacz S, Foligné B, Dennin V, Goudercourt D, Terpend K, Burckel A, Pot B. Anti-inflammatory potential of the probiotic dietary supplement Lactibiane Tolérance: in vitro and in vivo considerations. Clin Nutr. 2006;25:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Hayasaka D, Maeda K, Ennis FA, Terajima M. Increased permeability of human endothelial cell line EA.hy926 induced by hantavirus-specific cytotoxic T lymphocytes. Virus Res. 2007;123:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Larauche M, Bradesi S, Million M, McLean P, Taché Y, Mayer EA, McRoberts JA. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1033-G1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Bjarnason I, Peters TJ, Veall N. 51Cr-EDTA test for intestinal permeability. Lancet. 1984;2:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Mangell P, Nejdfors P, Wang M, Ahrné S, Weström B, Thorlacius H, Jeppsson B. Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci. 2002;47:511-516. [PubMed] |
| 18. | Behzadian MA, Windsor LJ, Ghaly N, Liou G, Tsai NT, Caldwell RB. VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. FASEB J. 2003;17:752-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J Biol Chem. 2002;277:10201-10208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Silva MA, Porras M, Jury J, Vergara P, Perdue MH. Characterization of ileal dendritic cell distribution in a rat model of acute and chronic inflammation. Inflamm Bowel Dis. 2006;12:457-470. [PubMed] |
| 22. | Annaházi A, Gecse K, Dabek M, Ait-Belgnaoui A, Rosztóczy A, Róka R, Molnár T, Theodorou V, Wittmann T, Bueno L. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain. 2009;144:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 454] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 24. | Cenac N, Chin AC, Garcia-Villar R, Salvador-Cartier C, Ferrier L, Vergnolle N, Buret AG, Fioramonti J, Bueno L. PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol. 2004;558:913-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Róka R, Demaude J, Cenac N, Ferrier L, Salvador-Cartier C, Garcia-Villar R, Fioramonti J, Bueno L. Colonic luminal proteases activate colonocyte proteinase-activated receptor-2 and regulate paracellular permeability in mice. Neurogastroenterol Motil. 2007;19:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 27. | Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 773] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 29. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 30. | Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 31. | Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, Clerici M, Ricci E, Fusi A, De Vecchi E. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012;46 Suppl:S33-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Paturi G, Phillips M, Kailasapathy K. Effect of probiotic strains Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 on systemic immune functions and bacterial translocation in mice. J Food Prot. 2008;71:796-801. [PubMed] |
| 33. | Agostini S, Goubern M, Tondereau V, Salvador-Cartier C, Bezirard V, Lévèque M, Keränen H, Theodorou V, Bourdu-Naturel S, Goupil-Feuillerat N. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil. 2012;24:376-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |