Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6786
Revised: January 16, 2014
Accepted: March 6, 2014
Published online: June 14, 2014
Processing time: 261 Days and 15.4 Hours
Colorectal cancer (CRC) is a very heterogeneous disease that is caused by the interaction of genetic and environmental factors. CRC develops through a gradual accumulation of genetic and epigenetic changes, leading to the transformation of normal colonic mucosa into invasive cancer. CRC is one of the most prevalent and incident cancers worldwide, as well as one of the most deadly. Approximately 1235108 people are diagnosed annually with CRC, and 609051 die from CRC annually. The World Health Organization estimates an increase of 77% in the number of newly diagnosed cases of CRC and an increase of 80% in deaths from CRC by 2030. The incidence of CRC can benefit from different strategies depending on its stage: health promotion through health education campaigns (when the disease is not yet present), the implementation of screening programs (for detection of the disease in its early stages), and the development of nearly personalized treatments according to both patient characteristics (age, sex) and the cancer itself (gene expression). Although there are different strategies for screening and although the number of such strategies is increasing due to the potential of emerging technologies in molecular marker application, not all strategies meet the criteria required for screening tests in population programs; the three most accepted tests are the fecal occult blood test (FOBT), colonoscopy and sigmoidoscopy. FOBT is the most used method for CRC screening worldwide and is also the primary choice in most population-based screening programs in Europe. Due to its non-invasive nature and low cost, it is one of the most accepted techniques by population. CRC is a very heterogeneous disease, and with a few exceptions (APC, p53, KRAS), most of the genes involved in CRC are observed in a small percentage of cases. The design of genetic and epigenetic marker panels that are able to provide maximum coverage in the diagnosis of colorectal neoplasia seems a reasonable strategy. In recent years, the use of DNA, RNA and protein markers in different biological samples has been explored as strategies for CRC diagnosis. Although there is not yet sufficient evidence to recommend the analysis of biomarkers such as DNA, RNA or proteins in the blood or stool, it is likely that given the quick progression of technology tools in molecular biology, increasingly sensitive and less expensive, these tools will gradually be employed in clinical practice and will likely be developed in mass.
Core tip: Although there are different strategies for screening, the number of which is increasing due to the potential of emerging technologies in molecular marker application, not all strategies meet the criteria required for screening tests in population programs; the three most accepted tests are fecal occult blood test, colonoscopy and sigmoidoscopy.
- Citation: Binefa G, Rodríguez-Moranta F, Teule &, Medina-Hayas M. Colorectal cancer: From prevention to personalized medicine. World J Gastroenterol 2014; 20(22): 6786-6808
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6786.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6786
Colorectal cancer (CRC) is a very heterogeneous disease that is caused by the interaction of genetic and environmental factors and can be classified based on the importance of each of these factors. The majority of CRCs are sporadic (70%-80%), with age being the most important risk factor. Only a small proportion of cases are due to inherited forms, either familial adenomatous polyposis (less than 1%), non-polyposis hereditary CRC or Lynch syndrome (2%-5%) or MYH-gene associated polyposis (< 1%)[1]. An additional 20%-25% of cases are estimated to have an associated hereditary component, which has not yet been well established and is known as familial CRC[2].
CRC develops through a gradual accumulation of genetic and epigenetic changes, leading to the transformation of normal colonic mucosa into invasive cancer. Most CRC develops from adenomas (adenoma-carcinoma sequence), and the neoplastic transformation time is considered approximately 10-15 years, which represents the available time to detect and remove these adenomas before their progression to invasive carcinoma.
Three main routes of CRC carcinogenesis are currently considered. The first known was proposed by Fearon et al[3] and is called the suppressor pathway or pathway of chromosomal instability. This route involves the accumulation of mutations that leads to oncogene activation (KRAS) and suppressor gene inactivation (DCC, APC, SMAD4, TP53). The accumulation of these molecular alterations, regardless of the order in which they are acquired, is responsible for neoplastic transformation[4]. A second mechanism involves the accumulation of errors during DNA replication due to the presence of mutations in genes responsible for its repair (MSH2, MLH1, MSH6, PMS2, MLH3, MSH3, PMS1 and Exo1)[5]. These errors accumulate predominantly in repetitive DNA fragments (microsatellites) scattered throughout the genome, resulting in mutations in various target genes. This mutator pathway or microsatellite instability is involved in Lynch syndrome and in 15%-20% of sporadic CRCs[4]. The mutator pathway tumors occur more frequently in older women and locations proximal to the splenic angle[6]. These tumors are histologically characterized by an increased lymphocyte infiltration (Crohn-like) and as being mucinous and poorly differentiated tumors[7].
Finally, a third route of carcinogenesis has been recently identified in the field of epigenetics: aberrant hypermethylation, a mechanism to silence gene function. Dinucleotide methylation in the promoter region of many genes has been referred to as the CpG island methylator phenotype (CIMP). The CIMP is responsible for 15%-20% of sporadic CRC. A tumor is considered to be CIMP-positive if it exhibits methylation of at least 3 of the following markers: CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1[8]. Methylating pathway tumors occur more frequently in women and elderly people, are preferably located in the right colon and do not benefit from treatment with 5-fluorouracil (5-FU). These tumors are histologically poorly differentiated tumors with mucinous differentiation or signet rings, exhibit microsatellite instability and are BRAF mutation carriers. The precursor lesions of these CIMP tumors are sessile serrated adenomas[9]. A better understanding of carcinogenesis pathways has allowed the development of diagnostic and prognostic markers as well as the investigation of new therapeutic targets and predictors of response to cancer treatments.
CRC is one of the most prevalent and incident cancers worldwide, along with lung and breast cancers, and is one of the most deadly. Approximately 1235108 people are diagnosed annually with CRC, and approximately 609051 die from CRC annually[10].
CRC is more frequent and causes more deaths in men than in women worldwide, except in the Caribbean. CRC is the third most common cancer in men (663000 cases/year) and the second most common cancer in women, after breast cancer, with 571000 cases a year.
Approximately 60% of CRC cases are diagnosed in developed countries, and after Japan, Europe represents one of the regions with the highest rates both in incidence and mortality.
Japan is one of the countries with the highest incidence rate, especially in men (41.7 cases per 100000); despite this fact, CRC mortality rates are below those of Europe[10]. This low mortality rate is due, in part, to the effect of the screening program implemented since 1992, one of the first in the world, along with Italy and Israel[11].
In Europe, CRC is the third most common cancer and is one of the leading causes of cancer death. An estimated 432414 new cases and 212219 deaths occur each year due to CRC, which represents an age-standardized rate of 29.6 and 13.3 per 100000, respectively[12].
Although historically the incidence and mortality rates in the US have remained above those in Europe, this relationship has recently changed. According to the latest GLOBOCAN data[10], the standardized incidence rate by age in the US stands at 29.2 cases per 100000, with a mortality rate of 8.8. It has been estimated that Europe is undergoing a minimum annual increase of 0.5% in CRC incidence.
The European countries with the highest incidence rates of CRC in men are Slovakia, Hungary and Czech Republic, all with results greater than 50 cases per 100000. In women, the highest rates (> 30 cases per 100000) are observed in Norway, Denmark and the Netherlands[12,13].
Within Europe, Spain is positioned slightly above the European average in terms of incidence rate (30.4 cases per 100000)[12,14], although the mortality rate is average (13.3 per 100000). CRC is the most common tumor in Spain when considering both sexes together and is the second leading cause of cancer death in both men and women. Estimations for next year predict a pattern similar to the present one, with increased mortality in men and a stabilization in women[15]. There is a marked geographic variation in CRC rates, with Catalonia presenting the highest incidence of this tumor, with an adjusted rate above the European average in men[16].
Based on current incidence and mortality rates as well as on projected demographic changes in the world population for the coming decades, the World Health Organization (WHO) estimates an increase of 77% in the number of newly diagnosed CRC cases and an increase of 80% in deaths from CRC by 2030[13,17]. Most of the additional incidence and mortality would occur in the world’s less developed regions. This estimation could be higher if developing countries continue with an increasingly Westernized lifestyle[18].
Since the 1990s, there has been an improvement in CRC relative survival at 5 years in both sexes that can be explained by an early diagnosis at initial stages, a breakthrough in the treatment of stage II and III disease and also a decrease in postoperative mortality. Different theories have been proposed to explain the difference in the CRC mortality rate between men and women, one of which is that the use of hormone replacement treatment in women may be a protective factor[19]. Other factors that could explain the different patterns of mortality are an increased access to health care and the adoption of healthier lifestyles by women.
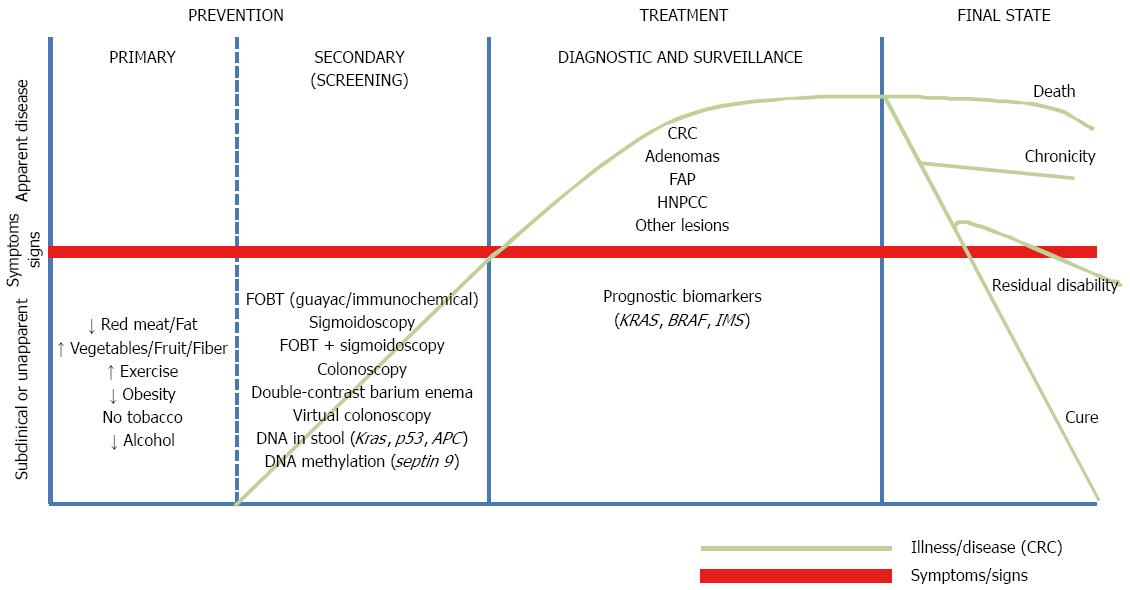
Current data confirm the need to urgently incorporate measures to improve the situation, taking into account both the accelerated aging process and estimates of an increase in incidence rates. As shown in Figure 1, CRC can benefit from different strategies depending on its stage: health promotion through health education campaigns (when the disease is not yet present), the implementation of screening programs (for the detection of the disease in its early stages), and the development of nearly personalized treatments according to both patient characteristics (age, sex) and the cancer itself (gene expression).
As previously mentioned, over 70% of CRC cases are sporadic and thus related to lifestyle. Despite being one of the most common cancers, CRC is also one that could benefit most from prevention through primary and secondary prevention strategies, and it is estimated that between 66% and 75% of CRC cases could be avoided with a healthy lifestyle[20].
The known risk factors for CRC are as follows: a diet low in fruit and vegetables, excessive intake of red meat and saturated fat, alcohol intake, a sedentary lifestyle, tobacco and being overweight[21]. However, the main risk factor is age. From 50 onwards, CRC is much more frequent, and the incidence increases exponentially with age.
Primary prevention is the best strategy to avoid CRC, but health promotion programs, aimed at changing dietary and hygiene habits, have long-term results and should therefore be complemented by other more immediate impact strategies such as secondary prevention.
Among the different CRC prevention options, secondary prevention is considered one of the most appropriate, as it can detect cancer precursor lesions (reducing incidence and mortality) and/or early-stage disease, when treatment is more effective (also reducing mortality).
Due to its high morbidity and mortality, its well-known natural history, the diagnostic methods to detect the disease in early stages or as even precursor lesions, and treatments that can improve survival if implemented in early stages, CRC meets the main requirements established by the WHO to be screened[22].
It is well known that the main CRC prognostic factor is the stage at diagnosis. CRC survival at 5 years is between 50% and 60%[23] and is higher in the initial stages (75%-90%) than in advanced stages (< 15%)[24]. Many CRCs are detected from the presence of signs or symptoms, which typically appear in advanced phases; in these cases, a quick diagnosis does not ensure a better prognosis, as the presentation of any symptoms may indicate the presence of advanced CRC. Thus, early detection and implementation, ideally, of CRC population-screening programs is of paramount importance. However, not everyone is likely to benefit from the programs; target population are men and women over 50 years (as this is the age from which developing CRC is more common) who are asymptomatic and lack a personal or family history of CRC, the so called average risk population. There are rapid diagnostic circuits for cases with symptoms or a high suspicion diagnosis and genetic counseling units or high risk clinics for cases with a family history.
In 2000, the Advisory Committee on Cancer Prevention recommended to European Union member states the use of CRC screening in the asymptomatic population from 50 years of age[25].
Although there are different strategies for screening, the number of which is increasing due to the potential of emerging technologies in molecular marker application, not all strategies meet the criteria required for screening tests in population programs: (1) acceptable sensitivity and specificity; (2) a demonstrated reduction in CRC mortality; (3) acceptable by the target population; and (4) low cost. In this sense, the three most accepted tests are the fecal occult blood test (FOBT), colonoscopy and sigmoidoscopy.
European population screening programs typically use the biennial FOBT as a screening test and colonoscopy as a confirmatory exploration (in positive FOBT cases)[26]. In contrast, in Japan, the interval is an annual FOBT. In the US, recommendations are either of one annual FOBT, sigmoidoscopy, double-contrast barium enema (DCBE) or CT colonography every 5 years or colonoscopy every 10 years. Among the various implemented programs, there are other differences in addition to the screening intervals, such as the target population to which they are addressed or the type of test used[27,28] (Table 1).
| Country | Test | Periodicity | Target population (age) | Year2 |
| Germany1 | FOBT | Annual | 50-54 | 1971 |
| FOBT or CS | Biennial/Every 10 yr | ≥ 55 | ||
| Italy | FOBT | Biennial | 50-69/74 | 1982 |
| FS | Once-only | 58-60 | ||
| Israel | FOBT | Annual | 50-74 | early 1990s |
| Japan | FOBT | Annual | ≥ 40 | 1992 |
| United States1 | FOBT | Annual | 50-75 | 1994 |
| FS/FOBT | Every 5/Every 3 yr | |||
| CS | Every 10 yr | |||
| Taiwan | FOBT | Biennial | 50-69 | 1995 |
| Spain | FOBT | Biennial | 50-69 | 2000 |
| Poland1 | CS | Periodic | 50-66 | 2000 |
| Czech Republic1 | FOBT | Annual | 50-54 | 2000 |
| FOBT/CS | Biennial/Every 10 yr | ≥ 55 | ||
| France | FOBT | Biennial | 50-74 | 2002 |
| Finland | FOBT | Biennial | 60-69 | 2004 |
| South Korea | FOBT | Annual | ≥ 50 | 2004 |
| Latvia1 | FOBT | Annual | ≥ 50 | 2005 |
| Australia | FOBT | Biennial | 55-74 | 2002 |
| England | FOBT | Biennial | 50-74 | 2006 |
| The Netherlands | FOBT | Biennial | 60-69 | 2006 |
| Canada | 2007 | |||
| Ontario | FOBT | Biennial | ≥ 50 | |
| Manitoba | FOBT | Biennial | 50-74 | |
| Croatia | FOBT | Biennial | 50-74 | 2007 |
| Scotland | FOBT | Biennial | 50-74 | 2007 |
| Sweden | FOBT | Biennial | 60-69 | 2008 |
However, there are many other techniques that, although far from being incorporated into screening programs, are extremely useful for the early diagnosis of CRC and for determining the course of treatment.
All tests or techniques (screening or not) can be divided into two groups as detailed below: (1) explorations to visualize the large intestine; and (2) analyses of biological samples.
Colonoscopy is considered the gold standard of excellence for the diagnosis of colorectal pathologies.
Although its use is clearly associated with a reduction in CRC-related deaths, there are no results from randomized controlled clinical trials demonstrating its effectiveness. However, other studies (prospective observational, case-control) have reported a significant reduction in CRC mortality and incidence, reaching, in some cases, up to 65% and 67%, respectively[29,30], some of them highlighting the differences according to the lesion location, being more favorable for the left colon[31,32].
Two major multicenter randomized clinical trials are currently being conducted in Europe to assess colonoscopy effectiveness. The NordICC study is being conducted in the Nordic countries, the Netherlands and Poland and compares colonoscopy (22000 people between 55 and 64 years old are invited to undergo once-only colonoscopy) with no screening (44000 people of the same age)[33]. The COLONPREV is a Spanish study in which over 8 regions collaborate and in which the performance of a once-only colonoscopy (group of 26703 subjects from 50 to 69 years old) is compared with a biennial immunological fecal occult blood test (FIT) (26599 subjects)[34]. The final results of both studies are expected in 2026 and 2021, respectively.
However, colonoscopy has some disadvantages. Because it is an invasive test, the procedure is not exempt of complications, with perforation and post-polypectomy bleeding being the most serious. According to the European Guidelines for quality assurance in CRC screening and diagnosis[28], major complications occur in 3‰ to 16‰ of examinations, depending on whether the colonoscopy is chosen as a screening test or as a confirmatory test after a positive FOBT.
Furthermore, a lesion miss rate ranging between 6% and 12% for large polyps and 5% for cancers has been described[35-38]. Thus, it is important to ensure good colonic cleansing and to use experienced endoscopists with an extensive history of examinations performed over his or her career and a minimum of annual procedures[28,39,40].
Other limitations of the use of colonoscopy as a screening test are its cost and its lower acceptance by the population (because of the requirement of a specific diet and the intake of a bowel cleansing preparation, the fear of anesthesia and the exploration itself or shame)[41-44]. In the COLONPREV study previously mentioned[34], the participation rate was higher in the FIT group than in the colonoscopy group (34.2% vs 24.6%). In an Italian trial in 2007, the results were very similar, with an attendance rate of 32.3% for FIT and 26.5% for colonoscopy[44]. These data demonstrate the clear preference of the population.
As the gold standard test for the detection of colorectal pathology, colonoscopy exhibits the best results, exceeding 98% sensitivity and 99% specificity for lesions > 6 mm[45-47].
Assuming an exploration under perfect conditions (excellent or good colonic cleansing that allows a view of > 90% of the mucosa and cecal intubation) and taking into account the natural history of CRC, the different guidelines currently available recommend a colonoscopy every 10 years for an average-risk population starting at an age of 50 years[28,48,49]. The cases with family or personal history with a high risk of developing CRC must follow different controls to the average-risk population, usually by reducing the interval between surveillance colonoscopies.
With respect to colonoscopy, sigmoidoscopy has the advantage of requiring less preparation and is typically performed without sedation. However, sigmoidoscopy has the great disadvantage of only detecting distal colon neoplasms.
The decision to perform a colonoscopy if a neoplasia is detected with sigmoidoscopy is controversial and must be individualized. Factors associated with an increased risk of proximal neoplasia include age > 65 years, villous histology in the distal lesion, distal adenoma ≥ 1 cm or multiple adenomas in the distal colon, and family history of CRC[50,51]. Several studies demonstrate that the prevalence of advanced adenomas in the proximal colon in patients without distal lesions is only 2%-5%. Moreover, evidence also suggests that the risk of proximal advanced neoplasia in individuals with only hyperplastic polyps in the distal colon is comparable to the risk of people without distal polyps[52].
Unlike colonoscopy, there are results from randomized clinical trials assessing sigmoidoscopy. The most favorable results are those of a US study[53], in which a 33% reduction in CRC incidence and a 43% reduction in mortality were obtained. United Kingdom[54] and Italy (SCORE)[55] trials also achieved satisfactory results (decreased incidence and mortality of 21% and 26%). In contrast, in the Norwegian study, there was no benefit after 7 years of follow-up[56].
The sensitivity for detecting advanced neoplasia is lower than that of colonoscopy. Results between 78% and 83% have been reported[50-52].
A sigmoidoscopy is recommended every five years in an average-risk population. Sigmoidoscopy can be performed alone or combined with an FOBT annually or biennially[28,48,49].
There is little evidence regarding DCBE, and the existing few results do not belong to randomized trials. In a case-control study, a reduction in CRC mortality of 33% was detected[57]. In a substudy of the popular National Polyp Study, DCBE detected 53% of polyps between 6 and 10 mm, 48% of polyps larger than 10 mm and 32% of those under 6 mm[58]. In a non-randomized study conducted in general clinical practice, the sensitivity for detecting adenomas > 7 mm and CRC with DCBE was 73% and 85%, respectively[59].
DCBE is not a very frequently used first choice test for screening due to its cost and its low acceptance by the population. DCBE is often used as a complementary test to endoscopic techniques.
Although CT colonography exhibits superior patient acceptability than colonoscopy in symptomatic patients[60], this method is very recent and remains little explored for screening. No sedation is required, but as in conventional colonoscopy, preparation is needed, and in the case of finding lesions, an additional exploration has to be performed to resect or biopsy the lesions.
Two meta-analyses[61,62] have reached the same conclusion regarding the use of CT colonography: its sensitivity and specificity are high for the identification of polyps > 10 mm (82%) but not for smaller polyps (56% for polyps < 5 mm and 63% for lesions between 6 and 10 mm).
One of the disadvantages described is the high percentage of extracolonic lesions detected (to 66%), nearly a third of them requiring further testing and monitoring, generating an unexpected additional cost[63,64].
Although its use as a first choice for CRC screening is unclear, the American Association of Gastroenterologists recommends CT colonography every five years[48].
There are some new endoscopic techniques (wide angle colonoscopy, endoscopy capsule, narrow band imaging, autofluorescence imaging system, etc.), still under development but very promising for CRC screening, with the goal of increasing population acceptance as well as the detection rate of neoplasia, especially in the proximal colon. It will be necessary to wait a few years to understand the results of the different ongoing studies[65,66].
Fecal hemoglobin: Most cases of CRCs develop in advanced adenomatous polyps (AA) (greater than 10 mm in diameter, with high-grade dysplasia or with more than 20% villous component). These preneoplastic lesions and CRC are characterized by presenting intermittently inappreciable blood loss in stool, which can be detected by FOBT before becoming clinically visible.
FOBT is the most used method for CRC screening worldwide and it is also the choice in most population-based screening programs in Europe. Due to its non-invasive nature and low cost, FOBT is one of the most accepted techniques by the population.
There are two basic types of FOBT: those based on guaiac resin (mostly biochemical qualitative) and those that are immunologically based (qualitative or quantitative).
The guaiac test (gFOBT) is the most studied by randomized clinical trials, demonstrating its effectiveness in reducing both CRC incidence and mortality[67-71]. These studies demonstrate a reduction in mortality between 15% and 33%, primarily due to the higher proportion of early-stage diagnoses; a decreased incidence is also confirmed thanks to preneoplastic lesion (adenomas) detection and their removal to avoid CRC progression. The largest reduction is observed when offering the test annually (33% after 13-years follow-up). A biennial test obtained a reduction between 15%-21% after 8-years follow-up and between 18%-21% after 10 years[67-71].
In gFOBT, peroxidase activity in the hemoglobin heme subunit is detected. These tests are not specific to human hemoglobin and can react with blood in the diet (e.g., from red meat) and with the presence of peroxidase in some vegetables. Therefore, and to avoid false positives, dietary restriction few days before the test is recommended[72]. In addition, gFOBT are not specific for lower gastrointestinal bleeding and may give false positives secondary to a bleeding pathology in the upper digestive tract. The test is performed by collecting two small stool samples from three separate bowel movements.
The two main types of gFOBT are the standard and the sensitive methods, differing by their ability to detect peroxidase activity (being better for the sensitive test).
The tests are qualitative tests, whose results are obtained using a reagent that changes the color of the stool sample; therefore, the result is exposed to a subjective assessment by the lab technician.
gFOBT sensitivity for neoplasia is very wide, from 6.2% to 83.3%, depending on the test used. Specificity is more constant, exceeding 80% in all cases and reaching 98.4% in some cases[73-75].
FITs are based on the use of monoclonal or polyclonal antibodies specific for human hemoglobin. The tests detect the presence of globin through immunochemical reactions[76]. The most commonly used methods are latex agglutination (turbidimetry), enzyme immunoassay (ELISA) and immunochromatography.
FITs do not require dietary restriction, as they are specific for human hemoglobin. The tests are also specific for lower gastrointestinal bleeding. The tests can be qualitative and semi-quantitative, being able to set the cutoff point at convenience. These features make it increasingly often the chosen test in screening programs across Europe (such as Italy, France, Holland, Spain, Slovenia), Japan, New Zealand and Australia. The United Kingdom, a faithful user of gFOBT, is considering changing to FIT shortly.
FIT is better accepted by the population because it does not require a special diet, is easier to perform and requires fewer samples than gFOBT. However, it has the disadvantage that samples should be stored in a refrigerator, as high temperatures can alter the outcome, increasing the number of false negatives[77].
Results from the literature regarding FIT sensitivity are highly variable, including tests with very little sensitivity (5.4%) and those that reach nearly 98%. Specificity ranges from 77% to 99%[73,78]. Significant differences were reported according to the lesion location, with higher sensitivity for distal colon neoplasms[79].
Several studies have demonstrated the superiority of FIT to gFOBT in AA and CRC detection rates as well as a higher participation rate[80-83].
Regardless of the FOBT used (gFOBT, FIT), a colonoscopy should be performed in all patients with a positive FOBT.
One of the main disadvantages of FOBT is its false positive and negative results. False positives lead to unnecessary explorations (with their associated complications), and false negatives give a false tranquility[84-86]. Currently, a test can be chosen from a vast variety in the market (over 70 tests)[87].
Thus far, we have seen CRC early detection techniques most frequently used in the context of population programs. Table 2 summarizes their main characteristics.
| Test | Sensitivity | Specificity | Considerations |
| Colonoscopy | 90% | It requires a bowel cleansing preparation and sedation. | |
| Adenoma ≤ 5 mm | 70%-79% | Risk of severe complications. | |
| Adenoma 6-9 mm | 80%-92% | Not well accepted by population. | |
| Adenoma ≥ 10 mm | 92%-99% | ||
| CRC | 92%-99% | ||
| Sigmoidoscopy1 | 92% | It requires less preparation. | |
| Adenoma ≤ 5 mm | 70%-79% | Sedation it is not necessary. | |
| Adenoma 6-9 mm | 80%-92% | Proximal lesions are not detected | |
| Adenoma ≥ 10 mm | 92%-99% | ||
| CRC | 90%-92% | ||
| gFOBT standard | 95%-99% | Dietary and pharmacological interactions. | |
| Adenoma ≤ 5 mm | 1%-5% | 3 samples | |
| Adenoma 6-9 mm | 5%-13.7% | ||
| Adenoma ≥ 10 mm | 8.9%-27.5% | ||
| CRC | 25%-50% | ||
| gFOBT sensitive | 90%-95% | Dietary and pharmacological interactions. | |
| Adenoma ≤ 5 mm | 5%-10% | 3 samples | |
| Adenoma 6-9 mm | 10%-26.2% | ||
| Adenoma ≥ 10 mm | 17.7%-49.4% | ||
| CRC | 50%-87% | ||
| FIT | 92.5%-98% | The sample needs to be refrigerated. | |
| Adenoma ≤ 5 mm | 2%-7.5% | ||
| Adenoma 6-9 mm | 7.5%-24.0% | ||
| Adenoma ≥ 10 mm | 16%-48% | ||
| CRC | 50%-87% |
Fecal DNA and RNA: The spectacular improvement in the knowledge of the genome[88-90], methylome[91], transcriptome[92,93] and proteonome[94] has led to the exploration of new methods for CRC diagnosis.
CRC is a very heterogeneous disease, and with a few exceptions (APC, p53, KRAS) most genes involved are observed in a small percentage of cases[89,90]. Furthermore, hypermethylation of suppressor gene promoters is an early event in carcinogenesis[95] detectable in the majority of CRCs, although this phenomenon is not universal[96]. Therefore, the design of genetic and epigenetic marker panels able to give maximum coverage in the diagnosis of colorectal neoplasia seems a reasonable strategy. In recent years, the use of DNA, RNA and protein markers in different biological samples has been explored[92,94] as strategies for CRC diagnosis.
These tests have been the non-invasive molecular tests most extensively evaluated. CRC cells exhibit a high mitotic index and a low adhesion to the basal membrane, which facilitates their continuous exfoliation into the intestinal lumen[97], unlike intermittent blood loss detected by FOBT. This constant exfoliation may be used for molecular analysis.
Only 0.01% of total fecal DNA tests (sDNA) is human; the rest comes from diet and bacterial flora[98]. Furthermore, the tumor sDNA is a small percentage of all human sDNA[99,100] and is even smaller in the case of AA. This implies that marker detection techniques must exhibit high sensitivity (Table 3).
| Marker | Study | Sensitivity | Specificity | |
| CRC | Adenoma > 1 cm | |||
| Meth vimentin | Chen et al[166] | 43 (46) | - | 178 (90) |
| Itzkowitz et al[167] | 29 (73) | - | 106 (89) | |
| Li et al[168] | 9 (41) | 9 (45) | 63 (95) | |
| Meth SFRP2 | Huang et al[169] | 49 (94) | 11 (53) | 23 (96) |
| Wang et al[170] | 60 (87) | 21 (62) | 28 (93) | |
| Meth TFPI2 | Glöckner et al[171] | 36 (76) | 4 (21) | - |
| Meth ITGH4 | Ausch et al[172] | - | 9 (69) | 22 (79) |
| Meth Spastic paraplegia-20 | Zhang et al[173] | 77 (80) | - | 30 (100) |
| Meth PHACTR3 | Bosch et al[174] | (50-60) | (17-29) | (92-98) |
| Meth TFPI2, long DNA | Zhang et al[175] | 52 (87) | 4 (44) | 25 (83) |
| APC, KRAS, p53, long DNA | Imperiale et al[102] | 16 (52) | 84 (12) | 1344 (94) |
| APC, KRAS, p53, long DNA | Alhquist et al[103] | 3 (25) | 47 (8) | 2246 (96) |
| APC, KRAS, Meth vimentin | Alhquist et al[103] | 11 (58) | 55 (45) | 63 (84) |
| Meth SFRP2, HPPI, MGMT | Huang et al[169] | 50 (96) | 15 (71) | 23 (96) |
| Meth APC, ATM, hMLH1, sFRP2, HLTF, MGMT, and GSTP1 | Leung et al[176] | 15 (75) | 17 (68) | 27 (90) |
| Meth vimentin, long DNA | Itzkowitz et al[167] | 68 (83) | 6 (86) | 298 (82) |
| Meth RASSF2 or SFRP2 | Nagasaka et al[177] | 63 (75) | 25 (44) | 101 (89) |
| Meth BMP3, hDNA, KRAS, APC | Zou et al[100] | 67 (91) | 21 (78) | 85 (85) |
| Meth vimentin, MLH1, MGMT | Baek et al[178] | 45 (75) | 31 (60) | 32 (87) |
| Meth RARB2, p16INK4a, MGMT, APC | Azuara et al[179] | 16 (62) | 8 (40) | 20 (100) |
| KRAS, a actina Meth NDRG4, BMP3, vimentin, TFPI2 | Ahlquist et al[107] | 214 (85) | 72 (54) | 264 (90) |
| β-actin, KRAS, meth BMP3 and NDRG4, fecal hemoglobin | Lidgard et al[105] | 91 (98) | 48 (57) | 139 (90) |
The first sDNA tests investigated mutations in KRAS[101] in CRC patients. Imperiale et al[102] assessed a population-based cohort comparing a gFOBT with an sDNA panel that analyzed 21 mutations. The sensitivity for CRC was 13% and 53%, respectively (P = 0.003). First-generation sDNA tests did not incorporate stabilizing buffers, and so studies using stool samples sent by mail[102,103] exhibited a low sensitivity but one far superior to gFOBT. Stabilizing buffers were subsequently incorporated[100,104], avoiding marker degradation during transport and storage, as well as new, more sensitive detection techniques such as the digital melt curve method[100] and beads, emulsion, amplification, and magnetics (BEAMing)[99], enabling the detection of < 0.1% of mutated copies (the first generation detection threshold was > 1%). A technique called allele-specific quantitative real-time target and signal amplification (QuARTS) detected less frequent mutations significantly improving sensitivity for AA compared with previous sDNA techniques[100]. A trial comparing sDNA testing with gFOBT[102] obtained a sensitivity for AA of 46% and 16%, respectively.
Although APC and p53 are mutated in the majority of CRCs, mutations are distributed through hundreds of positions, making mutational analysis non-viable for trials in clinical practice. Abnormal methylation of specific suppressor gene promoters is an early event in CRC carcinogenesis[95], thus making it an attractive target for the identification of biomarkers. The methylation status of various genes has exhibited remarkable diagnostic accuracy for CRC and AA, which has been improved by analyzing multimarker panels (Table 3). Sensitivity for adenoma detection increases with the size of the lesion[104]. Serrated lesions may also be detected by these sDNA marker panels[105].
One limitation of FOBT is its lower sensitivity for proximal lesions[78]. In addition, colonoscopy has been recently questioned in the detection of proximal lesions[106]. sDNA tests may not be affected by the neoplasia location[106]. Ahlquist et al[107] evaluated a panel of markers that identified 85% of patients with CRC and 54% with AA, without sensitivity differences depending on the location.
A recent technique is fluorescent long DNA (FL-DNA), which allows the identification of tumor DNA fragments > 150-200 base pairs. Neoplastic cells are characterized by not undergoing apoptosis, unlike normal cells that typically initiate cleavage and degradation of DNA, producing small identifiable fragments. FL-DNA has exhibited a sensitivity of 80% in the detection of CRC[108].
The major disadvantage of sDNA tests is their cost. Song et al[109] concluded, from a Markov model, that FOBT and colonoscopy were more cost-effective than fecal sDNA analysis. In the future, the use of new generation sDNA platforms with fewer markers could reduce costs and improve their viability in clinical practice[110].
RNA expression levels in stool can also be quantified to identify CRC patients[111]. An mRNA analysis of COX-2 in feces yielded a sensitivity of 87% for CRC with a specificity of 100%. mRNA of matrix-7 metalloproteinase detected 65% of CRC cases. The combination of these two markers reached a sensitivity of 90%. However, mRNA levels in stool exhibited a very low sensitivity for AA (4%)[111] (Table 4).
| Marker | Sample | Study | Sensitivity | Specificity | |
| CRC | Adenoma | ||||
| CDA, MGC20553, BANK1, BCNP1, MS4A1 | Blood | Han et al[138] | 30 (88) | - | 27 (64) |
| ANXA3, CLEC4D, LMNB1, PRRG4, TNFAIP6, VNN1, IL2RB | Plasma | Marshall et al[180] | 145 (72) | - | 146 (70) |
| ANXA3, CLEC4D, TNFAIP6, LMNB1, PRRG4, VNN1, IL2RB | Plasma | Yip et al[181] | 60 (61) | - | 85 (77) |
| MicroRNA (miRNA-21, miRNA-106a) | Fecal | Link et al[113] | (74) | - | (79) |
| MicroRNA-L6 | Plasma | Schiedeck et al[182] | 145 (79) | - | 45 (100) |
| MicroRNA (miRNA-92) | Plasma | Ng et al[142] | 80 (89) | - | 35 (70) |
| MicroRNA (miRNA-92a, miRNA-21) | Fecal | Wu et al[183] | 63 (72) | 32 (56) | 74 (73) |
| ANXA3, CLEC4D, LMNB1, PRRG4, TNFAIP6, VNN1, IL2RB | Plasma | Chao et al[184] | 215 (78) | - | 215 (66) |
| COX2, matrix metalloproteinase 7 | Fecal | Takai et al[111] | 56 (90) | - | 29 (100) |
MicroRNAs (miRNAs or miR) are small non-coding RNA molecules of 18-22 nucleotides that regulate gene expression at a posttranscriptional level. CRC exhibits a unique and identifiable pattern of miRNA expression[112] that can be detected in feces. A pilot study assessed an miRNA expression profile, detecting overexpression of miR21 and miR106 with a sensitivity and specificity of 74.1% and 79.0%, respectively[113]. Koga et al[114] observed clusters miR17-92 and miR-135 overexpressed but could not confirm miR21 overexpression. Kanaoka et al[115] evaluated prostaglandin-synthase 2 in a small group of patients and reported a sensitivity between 50% to 90% and a specificity of 93% in the CRC diagnosis.
Protein in feces: Some plasma protein markers (albumin, lactoferrin, calprotectin, haptoglobin)[116] are detectable in feces, although with a very low sensitivity for AA. However, detection of proteins derived from CRC and AA could have a greater discriminant value. A pilot study employing magnetic resonance spectroscopy used feces from patients with CRC exhibiting a sensitivity and specificity of 92%[117].
Pyruvate kinase isoenzyme type M2 (M2-PK) is the dimeric form of pyruvate kinase isoenzyme glycolysis. M2-PK is overexpressed in all proliferating cells[118,119] and is measurable in feces. A meta-analysis evaluated the concentration of M2-PK in stool samples from 704 patients with CRC and 11412 healthy controls from 17 independent studies. The sensitivity for CRC and adenomas > 1 cm was 80% and 44%, respectively, with a specificity of 95%.
Other mutated proteins detected in feces include S100 calcium binding protein A12 and metallopeptidase inhibitor 1. The latter exhibits a sensitivity for CRC of 85% and a specificity of 95% compared with controls[120].
Unlike stool, all of the blood/plasma DNA proceeds from the host, but the number of altered DNA copies in early CRC or preneoplastic lesions may be absent or at levels below the detection limit[121]. Some plasma enzymes could affect the marker stability[122]. Therefore, a processing optimization of the sample to remove PCR inhibitors will be required to improve its sensitivity[121].
Tumor cells can get into the blood through blood vessel invasion[123,124], where they then circulate in the blood and release detectable markers in plasma samples or circulating phagocytes. However, such vessel invasion occurs more frequently in advanced stages of the disease and is absent in AA[125-127]; thus, the detection of circulating tumor cells could be of prognostic value but would not be useful in the context of CRC screening. Moreover, the presence of free nucleic acids of tumor origin in plasma has been documented, and although these nucleic acids exhibit a small representation in plasma[128,129], their use as biomarkers could be possible[130]. However, certain tumor markers could be phagocytosed by inflammatory cells, allowing their detection in blood at any disease stage.
DNA in plasma: A variety of DNA markers have been assessed in plasma (Table 5). With respect to the detection of mutations, by BEAMing assay (Beads, Emulsification, Amplification, and Magnetics), APC mutations in tumor tissue and plasma samples were detected with a sensitivity of 73%, but the sensitivity was only 9% in the case of AA[121].
| Marker | Study | Sensitivity | Specificity | |
| CRC | Adenoma | |||
| APC, KRAS, p53 | Wang et al[185] | 36 (46) | - | 50 (100) |
| APC mutation | Diehl et al[121] | 16 (73) | 1 (9) | 33 (100) |
| APC, MLH1, HLTF | Leung et al[186] | 3-28 (6-57) | - | 37-41 (90-100) |
| Meth SEPT9 | Lofton-Day et al[134] | 92 (69) | - | 154 (86) |
| Grützmann et al[133] | 73 (58) | 3 (18) | 165 (90) | |
| deVos et al[131] | 62 (69) | - | 132 (89) | |
| Tóth et al[187] | 88 (96) | - | 78 (85) | |
| TMEF2, NGFR, SEPT9 | Lofton-Day et al[134] | 40-69 (30-52) | - | 170 (95) |
| Meth on 10 genes | Lee et al[188] | 210 (87) | 48 (75) | 254 (92) |
| Meth Vimentin | Li et al[168] | 48 (59) | - | 102 (93) |
As an alternative to mutation analysis, the accuracy of detecting epigenetic changes in plasma was assessed. Specifically, hypermethylation of Septine 9 (a gene belonging to a guanosine triphosphatase class) is associated with CRC[131,132]. Methylation analysis of Septin 9 in plasma detected between 58% and 96% of CRC patients and 18% of AA, with specificities of 86%-100%[131-134]. Its low sensitivity for AA would force patients to take the test annually or biennially. A multicenter trial included 7941 individuals ≥ 50 years with an average CRC risk. A sensitivity of 48% and a specificity of 91% were observed. However, the sensitivity for AA was low (11%)[135]. There is currently an automated test available on the market (Epi proColon Early Detection Assay Kit®)[132]. The addition of new markers to this detection blood test could optimize its performance in the future[136]. Nevertheless, its high cost compared with FOBT could limit its use.
RNA in plasma: The transcriptome of peripheral blood and plasma provides a source of potential diagnostic markers[137]. Han et al[138] designed a plasmatic marker panel (BANK1, BCNP1, CDA, MGC20553 and MS4A1) as a result of mRNA leukocyte microarray expression profiling, capable of discriminating a CRC patient from a healthy individual. An independent analysis revealed a sensitivity and specificity of 88% and 64%, respectively.
miRNAs have been demonstrated to be stable plasma markers, reproducible and consistent[139-141]. High miR92 levels have been identified in CRC patient plasma compared with healthy control samples[142], and significantly elevated miR92a and miR29a levels were detected in AA and CRC patients compared with controls[143].
Proteins in plasma: Carcinoembryonic antigen (CEA) is the most investigated protein but is not useful in CRC screening due to its low sensitivity (43% to 69%) in early CRC. Other antigens, such as CA 19.9, CA 50, CA 72.4, CO 29.11, have been studied without demonstrating acceptable diagnostic performance[144]. Furthermore, the performance of some proteins analyzed as single markers has demonstrating promising results in a small group of patients, thus they must be validated. Among these proteins, the most prominent include CD26 (sensitivity 90%, specificity 90%)[145], alpha-defensin 1 (sensitivity 69%, specificity 100%)[146], colon cancer-specific antigen (CCSA)-3 and CCSA-4 (for CRC sensitivity 100%, specificity 96%; for AA sensitivity 78%)[147], CCSA-2 (for CRC sensitivity 89%, specificity 84%; for AA sensitivity 20%)[148]; and TIMP-1 (sensitivity 60%, specificity 98%)[149] (Table 5).
The incorporation of new technologies in proteomics allows the analysis of large-scale protein patterns, including chromatographic techniques based on mass spectrometry (MS) assays, surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF)-MS and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS, that identify peptide patterns that discriminate CRC patients from healthy individuals with a sensitivity and specificity of over 90%[150]. The application of these profiles has to be defined, as most of these studies are conducted in a small number of cases, and in some of them, the identified biomarkers are not CRC-specific[151,152] but part of larger serum protein degradation products.
Circulating lymphocytes may contain traces of dysplastic lesions[153]. A pilot study examined the possibility of detecting CD24 in circulating leukocytes, demonstrating the ability to detect CRC and AA[154].
Another area of research is the identification of tumor-associated autoantibodies (epithelial cell adhesion molecule, p53, p62, CEA, HER-2/neu, Ras, topoisomerase II-alpha, histone deacetylase 3 and 5, ubiquitin L3, tyrosinase, tropomyosin, cyclin B1) as CRC markers[155-157]. These markers are absent in healthy individuals, and because they are very stable molecules and can be detected by immunoassays, they suggest a promising avenue for research.
Babel et al[158] used a microarray platform evaluating 8000 proteins. The authors identified 43 proteins capable of discriminating tumor serum from normal serum. The combined ELISA analysis of two of the markers (MAPKAPK3 and ACVR2B) in a CRC population and in a healthy population obtained a sensitivity of 83% and a specificity of 74%[156]. The accuracy of these proteins was enhanced through the incorporation of two new proteins to the panel (MST1/STK4 and SULF1)[159].
This research field, still in the experimental stage, is very interesting because it uses immunoassay techniques characterized by low cost, robustness and applicability.
Tumor markers can access urine through blood circulation. The markers are metabolized in small fragments and cross glomerular filtration[160]. Urine sample collection is easy and non-invasive, and DNA isolation is easier than in the feces, due to its low content in foreign proteins. Urine contains products that have been excreted over a period of time; therefore, urine may be less vulnerable to the intermittent release of markers in the blood[160,161].
Nucleosides that can accurately discriminate CRC patients from healthy controls have been identified in urine[162]. One study evaluated mutated KRAS sequences in the tissue and urine of a small number of CRC patients and healthy controls, reporting an 83% concordance between the mutated DNA in tissue and the corresponding urine sample[161]. This concordance of results between urine and tissue could be higher than that observed between plasma and tumor tissue[163].
Hypermethylation detection of the vimentin gene in urine samples was significantly associated with CRC compared with controls[164]. The use of SELDI-TOF MS and MALDI-TOF MS platforms to identify protein profiles in the urine of CRC patients have been evaluated by Ward et al[165], with a sensitivity of 78% and a specificity of 87%.
Surgery: Surgical treatment for non-metastatic rectal cancer should include total mesorectal excision (TME), which consists of the total excision of the rectum vascular pedicle along fascia anatomic planes[189] with adequate distal and circumferential margins and inferior mesenteric lymphadenectomy[190]. Sphincter-sparing surgery is possible in most cases of middle or lower rectal tumors as long as there is a distal margin at least of 1 cm. In very low tumors, an abdominoperineal resection may be required[191].
Local excision by transanal endoscopic microsurgery is also an option in tumors that do not exceed the upper third of the submucosa if a complete excision with adequate margins is obtained[192].
Likewise, it has been postulated that in colon cancer, surgery should be based on colectomy and bloc resection of lymph nodes[193]. In most cases, a laparoscopic intervention is possible[194]. The evaluation of a minimum of 12 lymph nodes is considered a measure of surgery quality[195,196]. Resection must be complete to be curative, so other atypical nodes or ganglions outside the resection field should be biopsied or resected whenever possible.
Adjuvant chemoradiotherapy: The anatomic complexity of the pelvis adds complication to rectal cancer treatment. The locoregional recurrence risk is higher in rectal than in colon cancer and is associated with a worse prognosis. This risk is due to the absence of serosa in the rectum and its proximity to other pelvic organs and structures, making it difficult to obtain wide surgical margins[197]. The treatment of choice for stage II and III rectal cancer, clinically T3, T4 or ganglionar affectation (Table 6)[198], includes preoperative chemoradiotherapy with conventional radiotherapy fractionation of 50.4 Gy in 28 fractions. Performing this treatment preoperatively reduces the incidence of local recurrence and toxicity, also allowing greater sphincter preservation, compared with its postoperative administration[199]. In those patients not treated with preoperative chemoradiotherapy, postoperative administration is recommended, exhibiting no differences in 10-year overall survival (OS), in 10-year cumulative incidence for distant metastasis, or in 10-year progression-free survival (PFS)[200].
| T = Primary tumor |
| TX = Primary tumor cannot be assessed |
| T0 = No evidence of primary tumor |
| Tis = Carcinoma in situ: intraepithelial or invasion of lamina propria |
| T1 = Tumor invades submucosa |
| T2 = Tumor invades muscularis propria |
| T3 = Tumor invades through the muscularis propia into subserosa or into nonperitonealized pericolic or perirectal tissues |
| T4a = Tumor penetrates to the surface of the visceral peritoneum |
| T4b = Tumor directly invades or is adherent to other organs or structures |
| N = Regional lymph nodes |
| NX = Regional lymph nodes cannot be assessed |
| N0 = No regional lymph node metastasis |
| N1a = Metastasis in one regional lymph node |
| N1b = Metastasis in two to three regional lymph nodes |
| N1c = Tumor deposit(s) in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis |
| N2a = Metastasis in four to six regional lymph nodes |
| N2b = Metastasis in seven or more regional lymph nodes |
| M = Distant metastasis |
| MX = Distant metastasis cannot be assessed |
| M0 = No distant metastasis |
| M1a = Distant metastasis to one organ or site |
| M1b = Distant metastasis to more than one organ/site or the peritoneum |
| Staging |
| Stage I (T1-T2, N0, M0) |
| Stage IIA (T3, N0, M0) |
| Stage IIB (T4a, N0, M0) |
| Stage IIC (T4b, N0, M0) |
| Stage IIIA (T1-T2, N1, M0 and T1, N2a, M0) |
| Stage IIIB (T1-T2, N2b, M0; T2-T3, N2a, M0 and T3-T4a, N1, M0) |
| Stage IIIC (T3-T4a, N2b, M0 and T4b, N1-N2, M0 and T4a, N2a, M0) |
| Stage IVA (any T, any N and M1a) |
| Stage IVB (any T, any N and M1b) |
The addition of concomitant chemotherapy to radiotherapy in locoregional rectal cancer treatment, both preoperatively and postoperatively, is due to its sensitizing effect in irradiated tissues, increasing the complete pathological responses percentage, along with its micrometastases potential eradication, improving disease systemic control[201]. To date, conventional chemotherapeutic treatment concomitant with radiation includes 5-FU continuous infusion or, more recently, capecitabine (one fluorouracil oral prodrug), with both drugs being equivalent[202]. The use of short hypofractionated radiotherapy (25 Gy for 5 d) has also exhibited a decreased local recurrence rate and improved survival compared with surgery alone[203,204] and is currently considered an alternative to conventional chemoradiotherapy treatment in elderly patients or with higher comorbidity.
Unlike rectal cancer, adjuvant treatment of CRC is more aimed at the prevention of distant metastases because the local recurrence rates are lower. Therefore, treatment with perioperative chemoradiotherapy is administered only in selected cases of T4 tumors with fixed structures impairment or recurrent disease[205].
Adjuvant chemotherapy: Surgery is the main treatment for CRC cure, but in cases with ganglionar involvement (stage III), the administration of adjuvant chemotherapy is the treatment of choice for optimizing the chances of healing. The administration of 5-FU and folinic acid (Leucovorin) for 6 mo improves survival by approximately 10%-15%[206]. The oral capecitabine monotherapy scheme has been shown to be equivalent to that of 5-FU with a lower percentage of adverse effects[207]. Despite this, the treatment of choice in stage III after MOSAIC results[208] is the scheme that uses oxaliplatin with 5-FU and Leucovorin (FOLFOX), adding a 7% 3-year disease-free survival (DFS) with respect to the scheme without oxaliplatin. Similarly, the scheme combining capecitabine with oxaliplatin (CapeOx) also proved to be superior to the traditional scheme of 5-FU and Leucovorin, increasing the 3-year DFS in 4.4%[209]. Both schemes with oxaliplatin are considered of choice in stage III, reserving schemes without oxaliplatin for patients where this drug is considered contraindicated.
The use of adjuvant chemotherapy in stage II colon cancer is more controversial. There is a subgroup of patients at this stage with increased risk of recurrence [T4, inadequate nodal surgery (< 12 nodes removed), lymphovascular invasion, visceral peritoneum affectation, bowel obstruction or poorly differentiated histology] in which the use of adjuvant chemotherapy with the previous schemes discussed can be considered[210]. In these cases, it is recommended to discuss with the patient the pros and cons of using chemotherapy, as the survival benefit is unclear[211,212]. However, there are good prognostic markers, such as high microsatellite instability (H-MSI), to be taken into account when assessing the use of adjuvant chemotherapy in stage II disease because there are studies reporting little benefit or even a negative impact of 5-FU adjuvant chemotherapy without oxaliplatin in these cases[213]. On this basis, the generalized use of adjuvant chemotherapy in stage II tumors with H-MSI is not recommended.
With regard to rectum cancer, the use of adjuvant chemotherapy is recommended in both stages II and III disease, although its effectiveness in many cases is extrapolated from colon cancer studies. A meta-analysis published in 2012 and based on 21 randomized clinical trials in rectal cancer patients reported an increase in OS and PFS in patients receiving postoperative chemotherapy with 5-FU-based schemes[214].
No survival benefit has been reported for CRC adjuvant treatment using irinotecan[215,216] or new drugs such as bevacizumab [humanized monoclonal vascular endothelial growth factor (VEGF) antibody][217] or cetuximab (murine monoclonal antibody directed against epidermal growth factor receptor, EGFR), regardless of tumor KRAS status[218].
There is no evidence for the benefit of adjuvant chemotherapy in stage I CRC[205].
Approximately half of the patients diagnosed with CRC eventually develop metastases, mainly those of metachronic presentation. The most common site for metastases occurrence is the liver. Approximately 20% of patients are diagnosed with synchronous liver metastases. However, resection of hepatic metastatic disease may achieve healing in a group of patients, with 5-year DFS of 20% in those patients where liver metastases resection was achieved. For this reason, it is essential to select those patients with resectable or potentially resectable disease. In addition, some patients with extrahepatic metastases may also benefit from their resection[219]. This has made the metastatic CRC therapeutic approach more complex, with multiple treatment options that increasingly require a multidisciplinary medical team, which can combine locoregional treatment of metastases with systemic treatment to obtain disease resectability[220].
Surgery: Good prognostic factors in liver metastases resection have been postulated, including the presence of a low number of metastases, lesions smaller than 5 cm, no major vasculature affectation, absence or a low volume of extrahepatic disease, sufficient liver reserves and the presence of negative margins in liver resection[221,222]. Furthermore, although the presence of extrahepatic disease is a poor prognostic factor, the possibility of extrahepatic disease resection in selected patients may increase the DFS median[219]. Resection of lung metastases in punctual cases could have an impact on survival[223].
The surgical approach to peritoneal carcinomatosis along with hyperthermic intraperitoneal chemotherapy and perioperative systemic chemotherapy are considered palliative, although with encouraging results in terms of survival[224].
Local liver treatments: In daily clinical practice, other techniques (radiofrequency ablation[225], hepatic arterial chemotherapy[226] administered directly to the liver, radioembolization with yttrium-90 microspheres[227] and stereotactic radiotherapy[228]) have been incorporated in the treatment of selected patients with few liver metastases or as a complement to surgery to achieve resectability. The role of such treatments is not fully defined in the absence, in most cases, of randomized trials. Stereotactic radiotherapy and radiofrequency are also being evaluated for the treatment of pulmonary metastases in selected patients[229].
Chemotherapy: In metastatic CRC treatment, chemotherapy can be used as a complement to metastases potentially curative by surgery as neoadjuvant treatment to achieve resectability of initially unresectable disease or as palliative therapy.
As adjuvant treatment, the administration of chemotherapy with 5-FU for 6 mo (Mayo scheme, which to date is considered suboptimal) has resulted in improvements in 5-year DFS[230]. Another study has compared the use of perioperative FOLFOX for 6 mo with surgical treatment alone in patients with up to 4 resectable liver metastases, demonstrating a better 3-year PFS. This scheme is currently considered first choice as adjuvant to surgery for liver metastases[231].
Regarding neoadjuvant treatment for potentially resectable liver disease, FOLFOX and FOLFIRI have exhibited similar response rates of approximately 30%[232], whereas the combination of oxaliplatin, irinotecan and 5-FU (FOLFOXIRI) exhibit higher reported response rates (66%), achieving a higher rate of resectability than FOLFIRI[233].
Palliative therapy with chemotherapy increases survival, can reduce the symptomatology and can improve quality of life. The treatment length may be 6 mo or until disease progression[234], depending mainly on toxicity. The choice of both first-line and subsequent treatments depends on previous treatments, the administration and toxicity profiles as well as the objectives to be achieved with the treatment. The use of 5-FU results in approximately 12-mo survival[235], being equivalent to Capecitabine treatment[236]. FOLFOX and FOLFIRI exhibit similar survival outcomes in first-line metastatic disease[232], with 15 mo OS, being also equivalent in oxaliplatin schemes the substitution of 5-FU by capecitabine[237].
New drugs: The addition of new drugs to the traditional chemotherapy schemes has not demonstrated efficacy as a complementary treatment in the resection of liver metastases.
Bevacizumab is a partially humanized monoclonal antibody against VEGF. Several studies have demonstrated the benefit of adding bevacizumab to oxaliplatin and irinotecan chemotherapy schemes. In first line therapy, the addition of bevacizumab to IFL scheme with irinotecan resulted in a statistically significant increase of both, PFS and OS[238]. Its combination with oxaliplatin schemes has resulted in an increased PFS but not OS[239,240].
The effectiveness of adding bevacizumab to chemotherapy is independent of the KRAS status[241]. Although the response rate with bevacizumab is approximately 50%[239], its role as neoadjuvant treatment with respectability intention is not clearly defined.
Cetuximab is a partially humanized monoclonal antibody against EGFR (epidermal growth factor receptor). Mutations in KRAS or BRAF in the signaling pathway downstream EGFR prevent tumors with mutated KRAS or BRAF from being sensitive to cetuximab treatment, being effective only in tumors with unmutated KRAS. Cetuximab and FOLFIRI combination increases PFS and OS[242]. In contrast, in first-line therapy, there is no benefit in statistically and clinically significant survival after the addition of cetuximab to FOLFOX[243]. Cetuximab has been shown to reverse resistance to irinotecan after progression to schemes containing the drug[244].
Panitumumab is a completely humanized monoclonal antibody against EGFR that is effective as monotherapy in patients in chemotherapy progression[245] or in combination. Its mechanism of action is similar to that of cetuximab; thus, the presence of KRAS mutations should also be considered. The addition of panitumumab to chemotherapy has demonstrated efficacy in PFS in combination with FOLFOX[246] in first-line therapy and in combination with FOLFIRI[247] in second-line therapy.
Aflibercept is a new molecule targeting against VEGF. The addition of aflibercept to FOLFIRI in second-line therapy increases PFS and OS[248] compared with chemotherapy alone.
The combination of chemotherapy with more than one biological agent (anti-EGFR and anti-VEGF) does not provide benefits but increases toxicity; therefore, it is contraindicated[249,250].
In Table 7, a summary of the different systematic treatments available for CRC is presented.
| Adjuvant therapy (stage III and stage II with high-risk features for systemic recurrence) |
| FOLFOX |
| CapeOx |
| If oxaliplatin is contraindicated or elderly patients |
| Capecitabine |
| 5-FU/Leucovorin |
| Metastatic disease (stage IV) |
| Resectable disease (lung, hepatic or peritoneal metastasis) |
| Consider surgery and/or locoregional treatment (radiofrequency, stereotactic radiotherapy) and 6 mo of perioperative chemotherapy (FOLFOX, CapeOx preferred) |
| Potentially resectable |
| FOLFOX |
| FOLFIRI |
| FOLFOXIRI |
| Cetuximab + FOLFIRI (only KRAS wild type) |
| Panitumumab + FOLFOX (only KRAS wild type) |
| Bevacizumab + FOLFOX |
| Unresectable (palliative) |
| FOLFOX |
| CapeOx |
| FOLFIRI |
| FOLFOX + Bevacizumab |
| CapeOx + Bevacizumab |
| FOLFOX + Panitumumab (only KRAS wild type) |
| FOLFIRI + Panitumumab (only KRAS wild type) |
| FOLFIRI + Cetuximab (only KRAS wild type) |
| FOLFIRI + Aflibercept |
| Capecitabine |
| 5-FU/Leucovorin |
| Cetuximab + Irinotecan (only KRAS wild type) |
| Cetuximab monotherapy (only KRAS wild type) |
| Panitumumab monotherapy (only KRAS wild type) |
| Regorafenib |
CRC is one of the most common cancers worldwide. Today, various techniques are available to detect CRC in its early stages or as precursor lesions, thereby preventing aggressive treatment.
Screening programs have helped make these techniques more accessible to the population, with FOBT, sigmoidoscopy and colonoscopy representing the most used. FIT is emerging as the best test for screening due to its better acceptance among populations. However, its sensitivity, yet distant from the gold standard (colonoscopy), requires further research.
The enormous work of basic and translational research in recent times has identified a large number of potential biomarkers. Although there is not sufficient evidence yet to recommend the analysis of biomarkers such as DNA, RNA or proteins in the blood or stool, it is likely that given the quick progression of technological tools in molecular biology, increasingly sensitive and less expensive, they will gradually be implemented in clinical practice and will most likely be developed in mass.
Chemotherapy remains the cornerstone of systemic treatment today, but several new targeted drugs have emerged in this filed in the last decade, improving the management of metastatic disease. The recent advances in molecular biology and the genetic classification of CRC are essential to individualize these therapies and will be basic for improving the treatment in the next years.
The authors greatly thank Olga Lopez for her unconditional technical and emotional support.
P- Reviewers: Konishi T, Kaumaya PTP, Vizoso FJ S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Farrington SM, Tenesa A, Barnetson R, Wiltshire A, Prendergast J, Porteous M, Campbell H, Dunlop MG. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet. 2005;77:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Piñol V, Castells A, Andreu M, Castellví-Bel S, Alenda C, Llor X, Xicola RM, Rodríguez-Moranta F, Payá A, Jover R. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 396] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 4. | Rustgi AK. Hereditary gastrointestinal polyposis and nonpolyposis syndromes. N Engl J Med. 1994;331:1694-1702. [PubMed] |
| 5. | Boland CR, Sinicrope FA, Brenner DE, Carethers JM. Colorectal cancer prevention and treatment. Gastroenterology. 2000;118:S115-S128. [PubMed] |
| 6. | Iacopetta B, Grieu F, Amanuel B. Microsatellite instability in colorectal cancer. Asia Pac J Clin Oncol. 2010;6:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Fam Cancer. 2004;3:93-100. [PubMed] |
| 8. | Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-793. [PubMed] |
| 9. | Nosho K, Irahara N, Shima K, Kure S, Kirkner GJ, Schernhammer ES, Hazra A, Hunter DJ, Quackenbush J, Spiegelman D. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3:e3698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 262] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. 2014; Available from: http: //globocan.iarc.fr/. |
| 11. | Saito H. Colorectal cancer screening using immunochemical faecal occult blood testing in Japan. J Med Screen. 2006;13 Suppl 1:S6-S7. [PubMed] |
| 12. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3659] [Article Influence: 304.9] [Reference Citation Analysis (2)] |
| 13. | Karsa LV, Lignini TA, Patnick J, Lambert R, Sauvaget C. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | López-Abente G, Ardanaz E, Torrella-Ramos A, Mateos A, Delgado-Sanz C, Chirlaque MD. Changes in colorectal cancer incidence and mortality trends in Spain. Ann Oncol. 2010;21 Suppl 3:iii76-iii82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Ribes J, Navarro M, Clèries R, Esteban L, Pareja L, Binefa G, Peris M, Fernández E, Borràs JM. Colorectal cancer mortality in Spain: trends and projections for 1985-2019. Eur J Gastroenterol Hepatol. 2009;21:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Borràs JM, Pareja L, Peris M, Espinàs JA. [Analysis of cancer incidence, survival and mortality according to the main tumoral localizations, 1985-2019: colorectal cancer]. Med Clin (Barc). 2008;131 Suppl 1:58-62. [PubMed] |
| 17. | Boyle P, Levin B; World Cancer Report. Cancer syte by syte-colorectal cancer. World cancer report 2008. Lyon: International Agency for Research on Cancer 2008; 374-379. |
| 18. | Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 19. | Fernandez E, La Vecchia C, Balducci A, Chatenoud L, Franceschi S, Negri E. Oral contraceptives and colorectal cancer risk: a meta-analysis. Br J Cancer. 2001;84:722-727. [PubMed] |
| 20. | Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925-943. [PubMed] |
| 21. | Gonzalez CA, Riboli E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46:2555-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 22. | Strong K, Wald N, Miller A, Alwan A. Current concepts in screening for noncommunicable disease: World Health Organization Consultation Group Report on methodology of noncommunicable disease screening. J Med Screen. 2005;12:12-19. [PubMed] |
| 23. | Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784-796. [PubMed] |
| 24. | Ciccolallo L, Capocaccia R, Coleman MP, Berrino F, Coebergh JW, Damhuis RA, Faivre J, Martinez-Garcia C, Møller H, Ponz de Leon M. Survival differences between European and US patients with colorectal cancer: role of stage at diagnosis and surgery. Gut. 2005;54:268-273. [PubMed] |
| 25. | Recommendations on cancer screening in the European union. Advisory Committee on Cancer Prevention. Eur J Cancer. 2000;36:1473-1478. [PubMed] |
| 26. | Zavoral M, Suchanek S, Zavada F, Dusek L, Muzik J, Seifert B, Fric P. Colorectal cancer screening in Europe. World J Gastroenterol. 2009;15:5907-5915. [PubMed] |
| 27. | Swan H, Siddiqui AA, Myers RE. International Colorectal Cancer Screening Programs: Population Contact Strategies, Testing Methods and Screening Rates. Pract Gastroenterol. 2012;36:8. |
| 28. | Segnan N, Patnick J, von Karsa L, editors . European guidelines for quality assurance in colorectal cancer screening and diagnosis. European guidelines for quality assurance in colorectal cancer screening and diagnosis - First edition. Luxembourg: European Commission, Publications Office of the European Union 2010; . |
| 29. | Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770-75; quiz 711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 30. | Rabeneck L, Paszat LF, Saskin R, Stukel TA. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol. 2010;105:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146:709-717. [PubMed] |
| 32. | Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 33. | The Norther-European Initiative on Colorectal Cancer (NordICC). Clinical Trials.gov [online]. Accessed on Sep 15, 2013. Available from: http://clinicaltrials.gov/show/NCT00883792. |
| 34. | Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 35. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [PubMed] |
| 36. | Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866-2877. [PubMed] |
| 37. | Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856-861. [PubMed] |
| 38. | Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Allison JE. The best screening test for colorectal cancer is the one that gets done well. Gastrointest Endosc. 2010;71:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Imperiali G, Minoli G, Meucci GM, Spinzi G, Strocchi E, Terruzzi V, Radaelli F. Effectiveness of a continuous quality improvement program on colonoscopy practice. Endoscopy. 2007;39:314-318. [PubMed] |
| 41. | Farraye FA, Wong M, Hurwitz S, Puleo E, Emmons K, Wallace MB, Fletcher RH. Barriers to endoscopic colorectal cancer screening: are women different from men? Am J Gastroenterol. 2004;99:341-349. [PubMed] |
| 42. | Medina GG, McQueen A, Greisinger AJ, Bartholomew LK, Vernon SW. What would make getting colorectal cancer screening easier? Perspectives from screeners and nonscreeners. Gastroenterol Res Pract. 2012;2012:895807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Ritvo P, Myers RE, Paszat L, Serenity M, Perez DF, Rabeneck L. Gender differences in attitudes impeding colorectal cancer screening. BMC Public Health. 2013;13:500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A, Castiglione G, Crosta C, Ederle A, Fantin A. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132:2304-2312. [PubMed] |
| 45. | Graser A, Stieber P, Nagel D, Schäfer C, Horst D, Becker CR, Nikolaou K, Lottes A, Geisbüsch S, Kramer H. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 46. | Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, Yee J, Henderson J, Hatten P, Burdick S. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet. 2005;365:305-311. [PubMed] |
| 47. | Sung JJ, Chan FK, Leung WK, Wu JC, Lau JY, Ching J, To KF, Lee YT, Luk YW, Kung NN. Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology. 2003;124:608-614. [PubMed] |
| 48. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1457] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 49. | [Colorectal cancer prevention working group. Clinical practice guide for colorectal cancer. AEG, SemFyC y Centro Cochrane Iberoamericano. Barcelona: Elsevier; . |
| 50. | Levin TR, Palitz A, Grossman S, Conell C, Finkler L, Ackerson L, Rumore G, Selby JV. Predicting advanced proximal colonic neoplasia with screening sigmoidoscopy. JAMA. 1999;281:1611-1617. [PubMed] |
| 51. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. [PubMed] |
| 52. | Farraye FA, Wallace M. Clinical significance of small polyps found during screening with flexible sigmoidoscopy. Gastrointest Endosc Clin N Am. 2002;12:41-51. [PubMed] |
| 53. | Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, Bresalier R, Andriole GL, Buys SS, Crawford ED. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 758] [Article Influence: 58.3] [Reference Citation Analysis (1)] |
| 54. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1139] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 55. | Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 453] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 56. | Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 57. | Scheitel SM, Ahlquist DA, Wollan PC, Hagen PT, Silverstein MD. Colorectal cancer screening: a community case-control study of proctosigmoidoscopy, barium enema radiography, and fecal occult blood test efficacy. Mayo Clin Proc. 1999;74:1207-1213. [PubMed] |
| 58. | Winawer SJ, Stewart ET, Zauber AG, Bond JH, Ansel H, Waye JD, Hall D, Hamlin JA, Schapiro M, O’Brien MJ. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med. 2000;342:1766-1772. [PubMed] |
| 59. | Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17-23. [PubMed] |
| 60. | von Wagner C, Ghanouni A, Halligan S, Smith S, Dadswell E, Lilford RJ, Morton D, Atkin W, Wardle J. Patient acceptability and psychologic consequences of CT colonography compared with those of colonoscopy: results from a multicenter randomized controlled trial of symptomatic patients. Radiology. 2012;263:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Rosman AS, Korsten MA. Meta-analysis comparing CT colonography, air contrast barium enema, and colonoscopy. Am J Med. 2007;120:203-210.e4. [PubMed] |
| 62. | Sosna J, Morrin MM, Kruskal JB, Lavin PT, Rosen MP, Raptopoulos V. CT colonography of colorectal polyps: a metaanalysis. AJR Am J Roentgenol. 2003;181:1593-1598. [PubMed] |
| 63. | Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Savarino JE, van Ballegooijen M, Kuntz KM, Zauber AG. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population. J Natl Cancer Inst. 2010;102:1238-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Sweet A, Lee D, Gairy K, Phiri D, Reason T, Lock K. The impact of CT colonography for colorectal cancer screening on the UK NHS: costs, healthcare resources and health outcomes. Appl Health Econ Health Policy. 2011;9:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Obstein KL, Valdastri P. Advanced endoscopic technologies for colorectal cancer screening. World J Gastroenterol. 2013;19:431-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Quintero E, Hassan C, Senore C, Saito Y. Progress and challenges in colorectal cancer screening. Gastroenterol Res Pract. 2012;2012:846985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541-1549. [PubMed] |
| 68. | Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 646] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 69. | Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002;50:840-844. [PubMed] |
| 70. | Kewenter J, Brevinge H, Engarås B, Haglind E, Ahrén C. Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing. Results for 68,308 subjects. Scand J Gastroenterol. 1994;29:468-473. [PubMed] |
| 71. | Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126:1674-1680. [PubMed] |
| 72. | Pignone M, Campbell MK, Carr C, Phillips C. Meta-analysis of dietary restriction during fecal occult blood testing. Eff Clin Pract. 2001;4:150-156. [PubMed] |
| 73. | Burch JA, Soares-Weiser K, St John DJ, Duffy S, Smith S, Kleijnen J, Westwood M. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. J Med Screen. 2007;14:132-137. [PubMed] |
| 74. | Young GP, St John DJ, Winawer SJ, Rozen P. Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol. 2002;97:2499-2507. [PubMed] |
| 75. | Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99:1462-1470. [PubMed] |
| 76. | van Dam L, Kuipers EJ, van Leerdam ME. Performance improvements of stool-based screening tests. Best Pract Res Clin Gastroenterol. 2010;24:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Grazzini G, Ventura L, Zappa M, Ciatto S, Confortini M, Rapi S, Rubeca T, Visioli CB, Halloran SP. Influence of seasonal variations in ambient temperatures on performance of immunochemical faecal occult blood test for colorectal cancer screening: observational study from the Florence district. Gut. 2010;59:1511-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Vilkin A, Rozen P, Levi Z, Waked A, Maoz E, Birkenfeld S, Niv Y. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol. 2005;100:2519-2525. [PubMed] |
| 79. | Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422-428. [PubMed] |
| 80. | Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, Birkenfeld S, Leshno M, Niv Y. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146:244-255. [PubMed] |
| 81. | Fraser CG, Matthew CM, Mowat NA, Wilson JA, Carey FA, Steele RJ. Immunochemical testing of individuals positive for guaiac faecal occult blood test in a screening programme for colorectal cancer: an observational study. Lancet Oncol. 2006;7:127-131. [PubMed] |
| 82. | Guittet L, Bouvier V, Mariotte N, Vallee JP, Arsène D, Boutreux S, Tichet J, Launoy G. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut. 2007;56:210-214. [PubMed] |
| 83. | Milà N, García M, Binefa G, Borràs JM, Espinàs JA, Moreno V. [Adherence to a population-based colorectal cancer screening program in Catalonia (Spain), 2000-2008]. Gac Sanit. 2012;26:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1468] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 85. | Garcia M, Milà N, Binefa G, Borràs JM, Espinàs JA, Moreno V. False-positive results from colorectal cancer screening in Catalonia (Spain), 2000-2010. J Med Screen. 2012;19:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Macken E, Moreels T, Vannoote J, Siersema PD, Van Cutsem E. Quality assurance in colonoscopy for colorectal cancer diagnosis. Eur J Surg Oncol. 2011;37:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | National Institute for Health Research. Horizon Scanning Centre. New and emerging biochemical tests for the screening of colorectal cancer. Birmingham: University of Birmingham 2012; . |
| 88. | Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153-158. [PubMed] |
| 89. | Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108-1113. [PubMed] |
| 90. | Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268-274. [PubMed] |
| 91. | Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1816] [Cited by in RCA: 1657] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 92. | Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124-3130. [PubMed] |
| 93. | Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA, Bujnicki JM, Menigatti M. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 94. | Friedman DB, Hill S, Keller JW, Merchant NB, Levy SE, Coffey RJ, Caprioli RM. Proteome analysis of human colon cancer by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics. 2004;4:793-811. [PubMed] |
| 95. | Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y, Hernandez NS, Chen X, Ahmed S, Konishi K. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci USA. 2007;104:18654-18659. [PubMed] |
| 96. | Ostwald C, Linnebacher M, Weirich V, Prall F. Chromosomally and microsatellite stable colorectal carcinomas without the CpG island methylator phenotype in a molecular classification. Int J Oncol. 2009;35:321-327. [PubMed] |
| 97. | Ahlquist DA, Harrington JJ, Burgart LJ, Roche PC. Morphometric analysis of the “mucocellular layer” overlying colorectal cancer and normal mucosa: relevance to exfoliation and stool screening. Hum Pathol. 2000;31:51-57. [PubMed] |
| 98. | Klaassen CH, Jeunink MA, Prinsen CF, Ruers TJ, Tan AC, Strobbe LJ, Thunnissen FB. Quantification of human DNA in feces as a diagnostic test for the presence of colorectal cancer. Clin Chem. 2003;49:1185-1187. [PubMed] |
| 99. | Diehl F, Schmidt K, Durkee KH, Moore KJ, Goodman SN, Shuber AP, Kinzler KW, Vogelstein B. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 2008;135:489-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Zou H, Taylor WR, Harrington JJ, Hussain FT, Cao X, Loprinzi CL, Levine TR, Rex DK, Ahnen D, Knigge KL. High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology. 2009;136:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Sidransky D, Tokino T, Hamilton SR, Kinzler KW, Levin B, Frost P, Vogelstein B. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992;256:102-105. [PubMed] |
| 102. | Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704-2714. [PubMed] |
| 103. | Ahlquist DA, Sargent DJ, Loprinzi CL, Levin TR, Rex DK, Ahnen DJ, Knigge K, Lance MP, Burgart LJ, Hamilton SR. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441-50, W81. [PubMed] |
| 104. | Olson J, Whitney DH, Durkee K, Shuber AP. DNA stabilization is critical for maximizing performance of fecal DNA-based colorectal cancer tests. Diagn Mol Pathol. 2005;14:183-191. [PubMed] |
| 105. | Lidgard GP, Domanico MJ, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, Allawi H, Yab TC, Taylor WR. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013;11:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 106. | Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 419] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 107. | Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, Butz ML, Thibodeau SN, Rabeneck L, Paszat LF. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142:248-56; quiz e25-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 108. | Calistri D, Rengucci C, Casadei Gardini A, Frassineti GL, Scarpi E, Zoli W, Falcini F, Silvestrini R, Amadori D. Fecal DNA for noninvasive diagnosis of colorectal cancer in immunochemical fecal occult blood test-positive individuals. Cancer Epidemiol Biomarkers Prev. 2010;19:2647-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Song K, Fendrick AM, Ladabaum U. Fecal DNA testing compared with conventional colorectal cancer screening methods: a decision analysis. Gastroenterology. 2004;126:1270-1279. [PubMed] |
| 110. | Itzkowitz S, Brand R, Jandorf L, Durkee K, Millholland J, Rabeneck L, Schroy PC, Sontag S, Johnson D, Markowitz S. A simplified, noninvasive stool DNA test for colorectal cancer detection. Am J Gastroenterol. 2008;103:2862-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 111. | Takai T, Kanaoka S, Yoshida K, Hamaya Y, Ikuma M, Miura N, Sugimura H, Kajimura M, Hishida A. Fecal cyclooxygenase 2 plus matrix metalloproteinase 7 mRNA assays as a marker for colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2009;18:1888-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 112. | Altomare DF, Di Lena M, Giuratrabocchetta S. MicroRNA: future perspectives in colorectal cancer. Colorectal Dis. 2012;14:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 113. | Link A, Balaguer F, Shen Y, Nagasaka T, Lozano JJ, Boland CR, Goel A. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1766-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 114. | Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, Akasu T, Fujita S, Yamamoto S, Baba H, Matsumura Y. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res (Phila). 2010;3:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 115. | Kanaoka S, Yoshida K, Miura N, Sugimura H, Kajimura M. Potential usefulness of detecting cyclooxygenase 2 messenger RNA in feces for colorectal cancer screening. Gastroenterology. 2004;127:422-427. [PubMed] |
| 116. | Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128:192-206. [PubMed] |
| 117. | Bezabeh T, Somorjai R, Dolenko B, Bryskina N, Levin B, Bernstein CN, Jeyarajah E, Steinhart AH, Rubin DT, Smith IC. Detecting colorectal cancer by 1H magnetic resonance spectroscopy of fecal extracts. NMR Biomed. 2009;22:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 118. | Hardt PD, Ngoumou BK, Rupp J, Schnell-Kretschmer H, Kloer HU. Tumor M2-pyruvate kinase: a promising tumor marker in the diagnosis of gastro-intestinal cancer. Anticancer Res. 2000;20:4965-4968. [PubMed] |
| 119. | Schneider J, Schulze G. Comparison of tumor M2-pyruvate kinase (tumor M2-PK), carcinoembryonic antigen (CEA), carbohydrate antigens CA 19-9 and CA 72-4 in the diagnosis of gastrointestinal cancer. Anticancer Res. 2003;23:5089-5093. [PubMed] |
| 120. | Karl J, Wild N, Tacke M, Andres H, Garczarek U, Rollinger W, Zolg W. Improved diagnosis of colorectal cancer using a combination of fecal occult blood and novel fecal protein markers. Clin Gastroenterol Hepatol. 2008;6:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, Diaz LA, Goodman SN, David KA, Juhl H. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci USA. 2005;102:16368-16373. [PubMed] |
| 122. | Tamkovich SN, Cherepanova AV, Kolesnikova EV, Rykova EY, Pyshnyi DV, Vlassov VV, Laktionov PP. Circulating DNA and DNase activity in human blood. Ann N Y Acad Sci. 2006;1075:191-196. [PubMed] |
| 123. | Stracke ML, Soroush M, Liotta LA, Schiffmann E. Cytoskeletal agents inhibit motility and adherence of human tumor cells. Kidney Int. 1993;43:151-157. [PubMed] |
| 124. | Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541-573. [PubMed] |
| 125. | Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018-1023. [PubMed] |
| 126. | Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, García-Saenz JA, Vidaurreta M, Martín M, Arroyo M. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 127. | Tsouma A, Aggeli C, Pissimissis N, Lembessis P, Zografos GN, Koutsilieris M. Circulating tumor cells in colorectal cancer: detection methods and clinical significance. Anticancer Res. 2008;28:3945-3960. [PubMed] |
| 128. | Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer--a survey. Biochim Biophys Acta. 2007;1775:181-232. [PubMed] |
| 129. | Vlassov VV, Laktionov PP, Rykova EY. Circulating nucleic acids as a potential source for cancer biomarkers. Curr Mol Med. 2010;10:142-165. [PubMed] |
| 130. | Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 2147] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 131. | deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, Steiger KV, Grützmann R, Pilarsky C, Habermann JK. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 132. | Mullard A. Alnylam dealt blow. Nat Biotechnol. 2009;27:213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 133. | Grützmann R, Molnar B, Pilarsky C, Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F, Roblick UJ. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 134. | Lofton-Day C, Model F, Devos T, Tetzner R, Distler J, Schuster M, Song X, Lesche R, Liebenberg V, Ebert M. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414-423. [PubMed] |
| 135. | Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T, Osborn N. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317-325. [PubMed] |
| 136. | Tänzer M, Balluff B, Distler J, Hale K, Leodolter A, Röcken C, Molnar B, Schmid R, Lofton-Day C, Schuster T. Performance of epigenetic markers SEPT9 and ALX4 in plasma for detection of colorectal precancerous lesions. PLoS One. 2010;5:e9061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 137. | Liew CC, Ma J, Tang HC, Zheng R, Dempsey AA. The peripheral blood transcriptome dynamically reflects system wide biology: a potential diagnostic tool. J Lab Clin Med. 2006;147:126-132. [PubMed] |
| 138. | Han M, Liew CT, Zhang HW, Chao S, Zheng R, Yip KT, Song ZY, Li HM, Geng XP, Zhu LX. Novel blood-based, five-gene biomarker set for the detection of colorectal cancer. Clin Cancer Res. 2008;14:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 139. | Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3218] [Cited by in RCA: 3554] [Article Influence: 209.1] [Reference Citation Analysis (0)] |
| 140. | Aslam MI, Taylor K, Pringle JH, Jameson JS. MicroRNAs are novel biomarkers of colorectal cancer. Br J Surg. 2009;96:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 141. | Mostert B, Sieuwerts AM, Martens JW, Sleijfer S. Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn. 2011;11:259-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 901] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 143. | Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 750] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 144. | Kawahara M, Chia D, Terasaki PI, Roumanas A, Sugich L, Hermes M, Iguro T. Detection of sialylated LewisX antigen in cancer sera using a sandwich radioimmunoassay. Int J Cancer. 1985;36:421-425. [PubMed] |
| 145. | Cordero OJ, Ayude D, Nogueira M, Rodriguez-Berrocal FJ, de la Cadena MP. Preoperative serum CD26 levels: diagnostic efficiency and predictive value for colorectal cancer. Br J Cancer. 2000;83:1139-1146. [PubMed] |
| 146. | Melle C, Ernst G, Schimmel B, Bleul A, Thieme H, Kaufmann R, Mothes H, Settmacher U, Claussen U, Halbhuber KJ. Discovery and identification of alpha-defensins as low abundant, tumor-derived serum markers in colorectal cancer. Gastroenterology. 2005;129:66-73. [PubMed] |
| 147. | Leman ES, Schoen RE, Weissfeld JL, Cannon GW, Sokoll LJ, Chan DW, Getzenberg RH. Initial analyses of colon cancer-specific antigen (CCSA)-3 and CCSA-4 as colorectal cancer-associated serum markers. Cancer Res. 2007;67:5600-5605. [PubMed] |
| 148. | Walgenbach-Brunagel G, Burger B, Leman ES, Walgenbach KJ, Tolba R, Heukamp L, Hirner A, Getzenberg RH. The use of a colon cancer associated nuclear antigen CCSA-2 for the blood based detection of colon cancer. J Cell Biochem. 2008;104:286-294. [PubMed] |
| 149. | Waas ET, Hendriks T, Lomme RM, Wobbes T. Plasma levels of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 correlate with disease stage and survival in colorectal cancer patients. Dis Colon Rectum. 2005;48:700-710. [PubMed] |
| 150. | Hundt S, Haug U, Brenner H. Blood markers for early detection of colorectal cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2007;16:1935-1953. [PubMed] |
| 151. | Engwegen JY, Helgason HH, Cats A, Harris N, Bonfrer JM, Schellens JH, Beijnen JH. Identification of serum proteins discriminating colorectal cancer patients and healthy controls using surface-enhanced laser desorption ionisation-time of flight mass spectrometry. World J Gastroenterol. 2006;12:1536-1544. [PubMed] |
| 152. | Wang Q, Shen J, Li ZF, Jie JZ, Wang WY, Wang J, Zhang ZT, Li ZX, Yan L, Gu J. Limitations in SELDI-TOF MS whole serum proteomic profiling with IMAC surface to specifically detect colorectal cancer. BMC Cancer. 2009;9:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Japink D, Leers MP, Sosef MN, Nap M. CEA in activated macrophages. New diagnostic possibilities for tumor markers in early colorectal cancer. Anticancer Res. 2009;29:3245-3251. [PubMed] |
| 154. | Kraus S, Galazan L, Naumov I, Shmueli E, Shapira S, Kazanov D, Geva R, Figer A, Arber N. Identification of CD24, a Novel Biomarker for the Early Detection of Colorectal Cancer (CRC), Using Peripheral Blood Mononuclear Cells. Gastroenterology. 2008;134:A-184. |
| 155. | Kobold S, Luetkens T, Cao Y, Bokemeyer C, Atanackovic D. Prognostic and diagnostic value of spontaneous tumor-related antibodies. Clin Dev Immunol. 2010;2010:721531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 156. | Casal JI, Barderas R. Identification of cancer autoantigens in serum: toward diagnostic/prognostic testing? Mol Diagn Ther. 2010;14:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 157. | Lu H, Goodell V, Disis ML. Targeting serum antibody for cancer diagnosis: a focus on colorectal cancer. Expert Opin Ther Targets. 2007;11:235-244. [PubMed] |
| 158. | Babel I, Barderas R, Díaz-Uriarte R, Martínez-Torrecuadrada JL, Sánchez-Carbayo M, Casal JI. Identification of tumor-associated autoantigens for the diagnosis of colorectal cancer in serum using high density protein microarrays. Mol Cell Proteomics. 2009;8:2382-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 159. | Babel I, Barderas R, Diaz-Uriarte R, Moreno V, Suarez A, Fernandez-Aceñero MJ, Salazar R, Capellá G, Casal JI. Identification of MST1/STK4 and SULF1 proteins as autoantibody targets for the diagnosis of colorectal cancer by using phage microarrays. Mol Cell Proteomics. 2011;10:M110.001784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 160. | Feng B, Yue F, Zheng MH. Urinary markers in colorectal cancer. Adv Clin Chem. 2009;47:45-57. [PubMed] |
| 161. | Su YH, Wang M, Aiamkitsumrit B, Brenner DE, Block TM. Detection of a K-ras mutation in urine of patients with colorectal cancer. Cancer Biomark. 2005;1:177-182. [PubMed] |
| 162. | Hsu WY, Chen WT, Lin WD, Tsai FJ, Tsai Y, Lin CT, Lo WY, Jeng LB, Lai CC. Analysis of urinary nucleosides as potential tumor markers in human colorectal cancer by high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Clin Chim Acta. 2009;402:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 163. | Su YH, Wang M, Brenner DE, Ng A, Melkonyan H, Umansky S, Syngal S, Block TM. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 2004;6:101-107. [PubMed] |
| 164. | Song BP, Jain S, Lin SY, Chen Q, Block TM, Song W, Brenner DE, Su YH. Detection of hypermethylated vimentin in urine of patients with colorectal cancer. J Mol Diagn. 2012;14:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 165. | Ward DG, Nyangoma S, Joy H, Hamilton E, Wei W, Tselepis C, Steven N, Wakelam MJ, Johnson PJ, Ismail T. Proteomic profiling of urine for the detection of colon cancer. Proteome Sci. 2008;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 166. | Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, Platzer P, Lu S, Dawson D, Willis J. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124-1132. [PubMed] |
| 167. | Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC, Sontag S, Johnson D, Skoletsky J, Durkee K, Markowitz S. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111-117. [PubMed] |
| 168. | Li M, Chen WD, Papadopoulos N, Goodman SN, Bjerregaard NC, Laurberg S, Levin B, Juhl H, Arber N, Moinova H. Sensitive digital quantification of DNA methylation in clinical samples. Nat Biotechnol. 2009;27:858-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 169. | Huang Z, Li L, Wang J. Hypermethylation of SFRP2 as a potential marker for stool-based detection of colorectal cancer and precancerous lesions. Dig Dis Sci. 2007;52:2287-2291. [PubMed] |
| 170. | Wang DR, Tang D. Hypermethylated SFRP2 gene in fecal DNA is a high potential biomarker for colorectal cancer noninvasive screening. World J Gastroenterol. 2008;14:524-531. [PubMed] |
| 171. | Glöckner SC, Dhir M, Yi JM, McGarvey KE, Van Neste L, Louwagie J, Chan TA, Kleeberger W, de Bruïne AP, Smits KM. Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res. 2009;69:4691-4699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 172. | Ausch C, Kim YH, Tsuchiya KD, Dzieciatkowski S, Washington MK, Paraskeva C, Radich J, Grady WM. Comparative analysis of PCR-based biomarker assay methods for colorectal polyp detection from fecal DNA. Clin Chem. 2009;55:1559-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 173. | Zhang H, Song YC, Dang CX. Detection of hypermethylated spastic paraplegia-20 in stool samples of patients with colorectal cancer. Int J Med Sci. 2013;10:230-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 174. | Bosch LJ, Oort FA, Neerincx M, Khalid-de Bakker CA, Terhaar sive Droste JS, Melotte V, Jonkers DM, Masclee AA, Mongera S, Grooteclaes M. DNA methylation of phosphatase and actin regulator 3 detects colorectal cancer in stool and complements FIT. Cancer Prev Res (Phila). 2012;5:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 175. | Zhang J, Yang S, Xie Y, Chen X, Zhao Y, He D, Li J. Detection of methylated tissue factor pathway inhibitor 2 and human long DNA in fecal samples of patients with colorectal cancer in China. Cancer Epidemiol. 2012;36:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 176. | Leung WK, To KF, Man EP, Chan MW, Hui AJ, Ng SS, Lau JY, Sung JJ. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in fecal samples of patients with colorectal cancer or polyps. Am J Gastroenterol. 2007;102:1070-1076. [PubMed] |
| 177. | Nagasaka T, Tanaka N, Cullings HM, Sun DS, Sasamoto H, Uchida T, Koi M, Nishida N, Naomoto Y, Boland CR. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101:1244-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 178. | Baek YH, Chang E, Kim YJ, Kim BK, Sohn JH, Park DI. Stool methylation-specific polymerase chain reaction assay for the detection of colorectal neoplasia in Korean patients. Dis Colon Rectum. 2009;52:1452-1459; discussion 1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 179. | Azuara D, Rodriguez-Moranta F, de Oca J, Soriano-Izquierdo A, Mora J, Guardiola J, Biondo S, Blanco I, Peinado MA, Moreno V. Novel methylation panel for the early detection of colorectal tumors in stool DNA. Clin Colorectal Cancer. 2010;9:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 180. | Marshall KW, Mohr S, Khettabi FE, Nossova N, Chao S, Bao W, Ma J, Li XJ, Liew CC. A blood-based biomarker panel for stratifying current risk for colorectal cancer. Int J Cancer. 2010;126:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 181. | Yip KT, Das PK, Suria D, Lim CR, Ng GH, Liew CC. A case-controlled validation study of a blood-based seven-gene biomarker panel for colorectal cancer in Malaysia. J Exp Clin Cancer Res. 2010;29:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 182. | Schiedeck TH, Wellm C, Roblick UJ, Broll R, Bruch HP. Diagnosis and monitoring of colorectal cancer by L6 blood serum polymerase chain reaction is superior to carcinoembryonic antigen-enzyme-linked immunosorbent assay. Dis Colon Rectum. 2003;46:818-825. [PubMed] |
| 183. | Wu CW, Ng SS, Dong YJ, Ng SC, Leung WW, Lee CW, Wong YN, Chan FK, Yu J, Sung JJ. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 184. | Chao S, Ying J, Liew G, Marshall W, Liew CC, Burakoff R. Blood RNA biomarker panel detects both left- and right-sided colorectal neoplasms: a case-control study. J Exp Clin Cancer Res. 2013;32:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 185. | Wang JY, Hsieh JS, Chang MY, Huang TJ, Chen FM, Cheng TL, Alexandersen K, Huang YS, Tzou WS, Lin SR. Molecular detection of APC, K- ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J Surg. 2004;28:721-726. [PubMed] |
| 186. | Leung WK, To KF, Man EP, Chan MW, Bai AH, Hui AJ, Chan FK, Sung JJ. Quantitative detection of promoter hypermethylation in multiple genes in the serum of patients with colorectal cancer. Am J Gastroenterol. 2005;100:2274-2279. [PubMed] |
| 187. | Tóth K, Sipos F, Kalmár A, Patai AV, Wichmann B, Stoehr R, Golcher H, Schellerer V, Tulassay Z, Molnár B. Detection of methylated SEPT9 in plasma is a reliable screening method for both left- and right-sided colon cancers. PLoS One. 2012;7:e46000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 188. | Lee BB, Lee EJ, Jung EH, Chun HK, Chang DK, Song SY, Park J, Kim DH. Aberrant methylation of APC, MGMT, RASSF2A, and Wif-1 genes in plasma as a biomarker for early detection of colorectal cancer. Clin Cancer Res. 2009;15:6185-6191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 189. | MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-460. [PubMed] |
| 190. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 752] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 191. | Lindsetmo RO, Joh YG, Delaney CP. Surgical treatment for rectal cancer: an international perspective on what the medical gastroenterologist needs to know. World J Gastroenterol. 2008;14:3281-3289. [PubMed] |
| 192. | Tytherleigh MG, Warren BF, Mortensen NJ. Management of early rectal cancer. Br J Surg. 2008;95:409-423. [PubMed] |
| 193. | West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 536] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 194. | Lee JK, Delaney CP, Lipman JM. Current state of the art in laparoscopic colorectal surgery for cancer: Update on the multi-centric international trials. Ann Surg Innov Res. 2012;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 195. | Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912-2919. [PubMed] |
| 196. | Madoff RD. Defining quality in colon cancer surgery. J Clin Oncol. 2012;30:1738-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 197. | Kapiteijn E, Marijnen CA, Colenbrander AC, Klein Kranenbarg E, Steup WH, van Krieken JH, van Houwelingen JC, Leer JW, van de Velde CJ. Local recurrence in patients with rectal cancer diagnosed between 1988 and 1992: a population-based study in the west Netherlands. Eur J Surg Oncol. 1998;24:528-535. [PubMed] |
| 198. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors . Colon and rectum. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer 2010; 143-164. |
| 199. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] |
| 200. | Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1489] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 201. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [PubMed] |
| 202. | Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 203. | Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980-987. [PubMed] |
| 204. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1348] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 205. | National Comprehensive Cancer Network. Guidelines Version 3.2013 Colon Cancer. Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 206. | Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939-944. [PubMed] |
| 207. | Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. [PubMed] |
| 208. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [PubMed] |
| 209. | Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 210. | Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408-3419. [PubMed] |
| 211. | Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020-2029. [PubMed] |
| 212. | Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20:3999-4005. [PubMed] |
| 213. | Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219-3226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 214. | Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;3:CD004078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 215. | Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, Fields AL, Mayer RJ. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456-3461. [PubMed] |
| 216. | Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, Topham C, Tabernero J, André T, Sobrero AF. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 217. | de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 218. | Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 219. | Pulitanò C, Bodingbauer M, Aldrighetti L, de Jong MC, Castillo F, Schulick RD, Parks RW, Choti MA, Wigmore SJ, Gruenberger T. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18:1380-1388. [PubMed] |
| 220. | Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212-2221. [PubMed] |
| 221. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318-21. [PubMed] |
| 222. | Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165-92, xi. [PubMed] |
| 223. | Girard P, Ducreux M, Baldeyrou P, Rougier P, Le Chevalier T, Bougaran J, Lasser P, Gayet B, Ruffié P, Grunenwald D. Surgery for lung metastases from colorectal cancer: analysis of prognostic factors. J Clin Oncol. 1996;14:2047-2053. [PubMed] |
| 224. | Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012;13:e362-e369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 225. | Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 304] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 226. | Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387-1395. [PubMed] |
| 227. | Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, Kettner E, Hildebrandt B, Dudeck O, Pech M. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35:1066-1073. [PubMed] |
| 228. | Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793-798. [PubMed] |
| 229. | Okunieff P, Petersen AL, Philip A, Milano MT, Katz AW, Boros L, Schell MC. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol. 2006;45:808-817. [PubMed] |
| 230. | Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982. [PubMed] |
| 231. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1444] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 232. | Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866-4875. [PubMed] |
| 233. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [PubMed] |
| 234. | Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol. 2006;24:394-400. [PubMed] |
| 235. | Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-Analysis Group in Cancer. Lancet. 2000;356:373-378. [PubMed] |
| 236. | Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282-2292. [PubMed] |
| 237. | Díaz-Rubio E, Tabernero J, Gómez-España A, Massutí B, Sastre J, Chaves M, Abad A, Carrato A, Queralt B, Reina JJ. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224-4230. [PubMed] |
| 238. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [PubMed] |
| 239. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2271] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 240. | Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 635] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 241. | Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 242. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 243. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (2)] |
| 244. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [PubMed] |
| 245. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [PubMed] |
| 246. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1394] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 247. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 248. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [PubMed] |
| 249. | Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 250. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 999] [Article Influence: 62.4] [Reference Citation Analysis (0)] |