Published online Jun 14, 2014. doi: 10.3748/wjg.v20.i22.6774
Revised: February 14, 2014
Accepted: February 26, 2014
Published online: June 14, 2014
Processing time: 260 Days and 12.6 Hours
Ulcerative colitis (UC) is characterized by repeated flare-ups of inflammation that can lead to oncogenic insults to the colonic epithelial. UC-associated carcinogenesis presents a different sequence of tumorigenic events compared to those that contribute to the development of sporadic colorectal cancer. In fact, in UC, the early events are represented by oxidative DNA damage and DNA methylation that can produce an inhibition of oncosuppressor genes, mutation of p53, aneuploidy, and microsatellite instability. Hypermethylation of tumor suppressor and DNA mismatch repair gene promoter regions is an epigenetic mechanism of gene silencing that contribute to tumorigenesis and may represent the first step in inflammatory carcinogenesis. Moreover, p53 is frequently mutated in the early stages of UC-associated cancer. Aneuploidy is an independent risk factor for forthcoming carcinogenesis in UC. Epithelial cell-T-cell cross-talk mediated by CD80 is a key factor in controlling the progression from low to high grade dysplasia in UC-associated carcinogenesis.
Core tip: The ulcerative colitis (UC)-associated carcinogenesis presents a different sequence of tumorigenic events compared to those that contribute to the development of sporadic colorectal cancer. In fact, in UC, early events are represented by oxidative DNA damage and DNA methylation that can produce inhibition of oncosuppressor genes, mutation of p53, aneuploidy, and microsatellite instability. Epithelial cell-T-cell cross-talk mediated by CD80 is a key factor in controlling the progression from low to high grade dysplasia in UC-associated carcinogenesis.
- Citation: Scarpa M, Castagliuolo I, Castoro C, Pozza A, Scarpa M, Kotsafti A, Angriman I. Inflammatory colonic carcinogenesis: A review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World J Gastroenterol 2014; 20(22): 6774-6785
- URL: https://www.wjgnet.com/1007-9327/full/v20/i22/6774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i22.6774
Ulcerative colitis (UC) is historically known as a risk factor for developing intestinal cancers via mechanisms that remain incompletely understood. In a recent meta-analysis of population-based cohorts, UC increases the risk of colorectal cancer (CRC) 2.4-fold. Male sex, UC diagnosis at young age, and extensive colitis also increase this risk[1]. In European collaborative studies, northern countries were observed to have more inflammatory bowel disease (IBD)-related intestinal cancers than southern ones[2]. The cumulative risk of colon cancer is approximately 8% 20 years after the initial UC diagnosis, rising to 18% at 30 years[3,4]. Adenocarcinoma of the colon develops from a dysplastic precursor lesion. In UC patients, pre-malignant histological changes are broadly referred to as dysplasia rather than adenoma, since the dysplasia is very often not polypoid[5]. Even though recent studies reported that at least 25% of UC patients may be diagnosed with low grade dysplasia in a 10 year follow-up period, some studies, such as the one by Lim et al[6] and Lynch et al[7] in 1993, suggested that low grade dysplasia will develop in all UC patients if they are followed for an adequate length of time. Nevertheless, very recent epidemiological data seems to make uncertain these classical pillars in IBD-associated cancer knowledge. In fact, very recent Dutch data pointed out that a high proportion of IBD-associated CRCs develop before the recommended start of surveillance[8,9]. Moreover, an authoritative Danish study concluded that a diagnosis of UC or CD no longer seems to increase patients’ risk of CRC, although subgroups of patients with UC remain at an increased risk[10]. The decreasing risk for CRC from 1979 to 2008 might result from the improved therapies for IBD patients that have developed over that time[10].
In recent years, a causal link between chronic inflammation and gastrointestinal tract carcinogenesis has gained increasingly strong support[11,12]. In a recent Finnish study, the degree of inflammation and duration of disease were observed to cumulatively increase the risk for dysplasia and CRC in IBD patients[13]. A chronic inflammatory condition exposes IBD patients to a number of signals with potential tumorigenic effects. These signals include persistent activation of the nuclear factor-kappa B (NF-κB) and cyclooxygenase-2 (COX2) pathways, release of proinflammatory mediators such as tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6), and augmented levels of reactive oxygen and nitrogen species. An inflammatory microenvironment can contribute to colonic tumorigenesis via 3 major processes: (1) increasing oxidative stress, which causes direct DNA damage that contributes to tumor initiation; (2) activating prosurvival and anti-apoptotic pathways in epithelial cells that contribute to tumor promotion; and (3) creating a microenvironment that promotes sustained growth, neoangiogenesis, migration, and invasion of tumor cells, thus supporting tumor local progression and distant metastasis[14]. Precancerous lesions and invasive carcinoma in UC differ from sporadic ones in terms of a younger age at onset and flat mucosa within large fields of genetic abnormalities, rather than as isolated and visible exophytic lesions[15-17]. However, many of the genetic abnormalities observed in sporadic adenoma and carcinoma, including alterations in adenomatous polyposis coli (APC), p53, bcl-2, and K-ras genes, microsatellite instability, and aneuploidy, are also observed in UC-related neoplasms, albeit with a different frequency and timing in many cases[18-26].
This is a comprehensive overview of the available literature on pathogenesis and immunosurveillance mechanisms in inflammatory colonic carcinogenesis. A text word literature review was performed using PubMed and Medline databases. Although this was not a systematic review, the search terms used were as follows: colorectal AND cancer OR carcinoma AND UC OR IBD OR AND pathogenesis OR immune surveillance. The reference lists of identified articles were searched for further relevant publications. Two researchers (Scarpa M and Pozza A) independently selected the studies, which were limited to clinical studies published between January 1980 to July 2013 and in the English language. Unpublished data and data published in abstract form only were excluded, as these were unlikely to contain sufficient methodological information to allow valid conclusions to be made. Whenever discordance regarding study inclusion existed, the two researchers negotiated an agreement.
Genomic instability includes microsatellite instability (MSI) associated with mutant phenotypes and chromosome instability (CIN) characterized by gross chromosomal abnormalities[27]. Three fundamental intracellular mechanisms are involved in the repairing of DNA damage: nucleotide excision repair (NER), base excision repair, and mismatch repair (MMR). Their alteration/inactivation can lead to MSI. On the other hand, CIN is typically associated with the progressive accumulation of mutations in oncosuppressor genes and oncogenes[16]. Defects in DNA MMR genes and CIN pathways are responsible for a variety of hereditary cancer predisposition syndromes, including hereditary non-polyposis colorectal carcinoma, Bloom syndrome, ataxia-telangiectasia, and Fanconi anemia[27]. Furthermore, besides the many genetic contributors to CIN and MSI, there are also epigenetic factors that can be equally damaging to cell-cycle control. Hypermethylation of oncosuppressor and DNA MMR gene promoter regions is an epigenetic mechanism of gene silencing involved in colorectal carcinogenesis. Finally, telomere shortening has been demonstrated to increase genetic instability and tumor formation in mice models[27].
In UC, colonocytes are subject to high levels of genetic damage. In fact, chronic inflammation of the colon can contribute to carcinogenesis by increasing oxidative stress which promotes DNA damage, thus contributing to tumor initiation. Oxidative DNA damage is more evident in patients with UC and dysplasia[28,29]. Furthermore, the severity of colitis inflammation has been associated with high levels of reactive oxygen species (ROS) and reduced defenses to oxidative stress. Both of these mechanisms might contribute to oxidative DNA damage[25,30-36]. ROS induce genetic damage either as base alterations, “abasic” sites, or as strand breaks, and each of these damage types could cause genomic instability. Therefore, an interesting hypothesis is that in a subgroup of UC patients who could be defined as “progressors”, the mucosal epithelium is damaged by ROS, producing genomic instability and eventually carcinogenesis initiation and progression. This hypothesis is supported by the observation of chromosomal instability and MSI in the non-dysplastic mucosa of UC patients with dysplasia and cancer[25,37,38]. Genomic instability occurs with the same frequency (10%) throughout the whole neoplastic progression in UC. Therefore, genomic instability does not accumulate as the neoplasia progresses, but rather occurs very early and persists at a steady level. The constant presence of instability may be linked to the maximal tolerated degree of genetic damage and the dynamic rate of cell turnover in the inflamed colon.
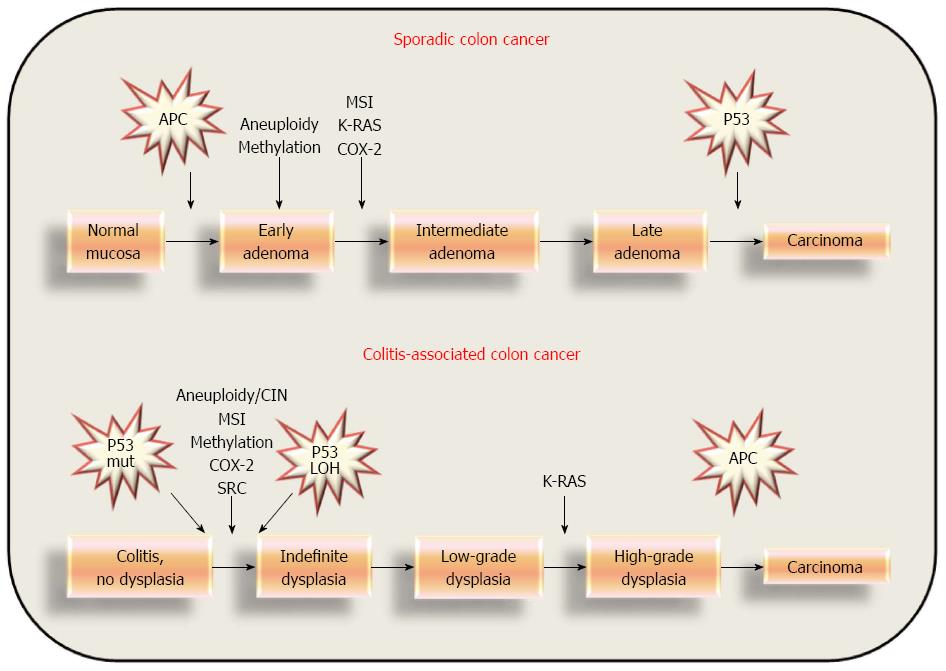
Genomic instability rate in the colonic mucosa without dysplasia from patients with UC and dysplasia is significantly higher than that of UC patients who are completely dysplasia/cancer-free. On the other hand, in normal mucosa from patients with sporadic colon cancers and adenomas, genomic instability is not observed[39]. On the contrary, in patients with widespread UC, genomic instability precedes neoplastic transformation, and may be related to the extension of chronic inflammation. These observations suggest once again the peculiarity of the pathogenesis of UC CRC, and can in part explain the difference from sporadic cancers: in fact, UC neoplasia is frequently multifocal, widespread, and may occur in flat mucosa. The differences in UC-related and sporadic colorectal carcinogenesis is shown in Figure 1.
On the other hand, non-progressor patients may be exposed to a lower oxidative stress in their colon than progressors, or may present a lower susceptibility for genomic instability after oxidative stress because of better protective mechanisms. For example, glutathione and glutathione S-transferase levels vary among UC patients and may influence ROS levels[40]. Nevertheless, a small amount of heterogeneity was also observed in the non-progressor group: 20% of patients demonstrated increased genomic instability in the colon without any trace of dysplasia or cancer at the optimized combination of sensitivity and specificity. Curiously enough, the genomic instability rate approximates the percentage of patients with no dysplasia or cancer that one might expect to develop a cancer in the following 20 to 30 years[41]. Nevertheless, the question as to why some UC patients have a mutator phenotype, while others do not, still remains open[42].
UC-related colonic carcinogenesis can also be associated with MSI. In fact, it was demonstrated that MSI can be caused by ROS[43]. Microsatellites are short repetitive sequences (1- to 5-nucleotide) of DNA that are randomly distributed throughout the whole genome. The stability of these sequences is a good measure of the general integrity of the genome. MSI reflects a gain or loss of repeat units in a germline microsatellite allele, suggesting the clonal expansion that is typical of a cancer. A high rate of MSI in severe long standing UC is probably related to the genomic instability produced by repeated inflammatory insults. Therefore, the influence of inflammation should be considered when estimating MSI in UC[44]. Indeed, although the great number of molecular mechanisms involved in the increased risk of CRC in UC is still unclear, it appears to be related to MSI[25,45]. The prevailing hypothesis is that overproduction of free radicals saturates the ability of the cell to repair oxidative DNA damage prior to replication[25,46]. Another hypothesis is that prolonged and repeated oxidative insults directly inactivate DNA MMR genes[47]. One study reported half of UC mucosal samples with high MSI as having MLH1 hypermethylation[48]. However, differently from what is observed in hereditary non-polyposis colon cancer, other studies found little evidence for MMR defects as a cause of MSI in UC[49,50]. These data suggest the possibility that mechanisms other than MMR defects exist. Recently, adaptive increased activity of 3-methyladenine DNA glycosylase (AAG) and apurinic endonuclease (APE1) in areas of UC colon undergoing active inflammation was observed[51]. Interestingly, this imbalanced increase appeared to be associated with the MSI observed in UC. These data were consistent with a possible novel mechanism by which patients with chronic colonic inflammation acquire MSI. UC patients were demonstrated to have increased AAG and APE1 enzyme activity in epithelial areas of their colon with active inflammation, and those with MSI have the largest increase and imbalance in the levels of AAG and APE1 in inflamed areas of their colons. These observations showed that the adaptive imbalanced increase of these enzymes may have DNA-damaging effects and contribute to carcinogenesis in chronic colonic inflammation[51].
In inflammatory conditions, ROS induce DNA damage[31], and since mitochondrial DNA (mtDNA) lacks histones and related protective systems, mutations accumulate there more than in nuclear DNA[52]. The human mitochondrial genome includes a 16.5-kb circular double-stranded DNA molecule encoding 13 polypeptides of the respiratory chain, 22 transfer RNAs, and 2 ribosomal RNAs necessary for protein synthesis. Since the correct expression of the complete mitochondrial genome is necessary for the maintenance of mitochondrial functions, including electron transport, even small changes in the mtDNA sequence can cause profound functional impairment that enhances generation of free radicals, which in turn increase the extent of DNA mutation. Free radicals can act as initiators and/or promoters that can cause DNA damage, and thus they can activate oncogenes and inactivate oncosuppressors[34,53]. Therefore, oxidative injury to mitochondria in a chronic inflammation situation may contribute to the early stages of carcinogenesis. Human cancers are characterized by mutations of mtDNA[54-56] and, curiously enough, accumulation of mtDNA mutations in cancerous tissue seems to be related to the grading of malignancy. An interesting hypothesis is that genetic instability in the process of carcinogenesis results in the high rate of mtDNA mutation in the colorectal mucosa of individuals with UC, and the increased instability of genes in mtDNA are consistent with the high incidence of CRC in individuals with UC.
Recently, Nishikawa et al[54,57] observed that the number of mtDNA mutations in the colonic mucosa in UC patients is significantly higher than that found in other types of malignancies. Moreover, the rate and the timing of genetic mutations underlying sporadic cancer (adenoma-carcinoma sequence) and UC-associated carcinogenesis seem to be different. Although the definite mechanism of these differences is still unknown, increased oxidative stress in the UC colon[58,59] appears to be a major cause of DNA damage[60]. Thus, the high mtDNA mutation rate in the colonic epithelial cells of UC patients is associated with mutation of nuclear DNA in long-lasting inflammation. The observation that the great majority of mtDNA mutations in UC patients were homoplasmic in nature suggests that these mutations had become dominant in their mucosa. Mitochondrial DNA with certain types of mutations are characterized by the generation of abnormal proteins and increased electron leakage from the electron transport chain, and therefore the amounts of endogenously produced free radicals may be increased in these cells. Finally, in tissues with chronic inflammation, the resulting increase in oxidative stress acts to enhance the mutation of either mtDNA or, probably, nuclear DNA, thereby promoting the early stage of tumorigenesis. Given its clonal nature and the large number of mtDNA copies, mutation of the mitochondrial genome in the colonic mucosa of UC patients is suggestive of genomic instability that enhances carcinogenesis. The high incidence of mtDNA mutation in the colonic mucosa of subjects with UC indicates that the DNA mutation rate is enhanced in their epithelial cells by the oxidative stress produced by chronic inflammation and, hence, malignant transformation can occur more easily than in normal subjects[57].
Finally, DNA hypermethylation may play a role in UC carcinogenesis. Altered genomic methylation is a well-recognized characteristic of tumor cells, and specific aberrant methylation events occur in the early steps of colorectal carcinogenesis, leading to profound modifications in gene expression[61]. In fact, the aberrant methylation of H-cadherin (CDH13) beginning at an early stage of colorectal tumorigenesis frequently silences the expression of this tumor suppressor gene in colorectal adenomas and cancers[62]. Moreover, besides germ-line mutations associated with hereditary familial adenomatous polyposis and somatic mutations in sporadic colorectal tumors, hypermethylation provides an important mechanism for impairing APC function[63]. Furthermore, hypermethylation of the CpG island in the cellular DNA-repair protein O-6-methylguanine-DNA-methyltransferase (MGMT) gene[64] and in the MLH1 gene is associated with the reduced gene expression observed in the majority of sporadic primary CRCs with MSI[65]. Finally, in gastrointestinal cancer RUNX3 hypermethylation decreases transforming growth factor-β (TGF-β)/BMP signaling[66]. In our series, the methylation of these genes occurred in more than half of the patients (data not yet published). Garrity-Park et al[67] evaluated the methylation status of 10 genes [p16, p14, runt-related transcript factor-3 (RUNX3), COX-2, E-cadherin, methylated-in-tumor-1 (MINT1), MINT31, HPP1, estrogen receptor, and SLC5A8] in mucosal samples from UC-CRC tumors and non-neoplastic colonic tissue from both UC-CRC cases and UC controls. Methylated promoters of RUNX3, MINT1, and COX-2 resulted in potential biomarkers of the presence of CRC in patients with UC, and so these genes might also be used as biomarkers for colorectal dysplasia.
Furthermore, in UC-associated carcinogenesis, hypermethylation of the promoter of Death-Associated Protein Kinase (DAPK) was observed in long-standing UC patients[68]. DAPK is a pro-apoptotic protein implied in various apoptotic cascades. Kuester et al[68] observed that DAPK is overexpressed in inflamed colonic epithelium, suggesting a protective role of this molecule. Therefore, its inactivation mediated by promoter hypermethylation might be critical for the accumulation of epithelial cells with genomic damage in inflamed epithelium of UC, and might contribute to the initiation of the neoplastic process and development of UC-associated carcinoma. Increased expression of DNA methyltransferase (DNMT)-1 in non-neoplastic mucosa may either precede or be a relatively early event in UC-related carcinogenesis, and may be useful to predict the risk of colorectal neoplasia in UC[69]. In fact, in our series of UC patients DNMT1, DNMT3a, and DNMT3b, mRNA expression resulted in being significantly higher than in patients without an inflammatory condition (data not yet published).
Aneuploidy is an independent risk factor for tumorigenesis in UC. A less favorable prognosis in patients with UC-related CRC compared with those with sporadic CRC has been reported. UC-related neoplasms presented a significantly higher rate of aneuploidy than sporadic CRC. UC-related CRC and aneuploid sporadic CRC have a similarly lower than that of diploid sporadic CRC. Aneuploidy resulted in being the strongest independent prognostic marker for R0-resected CRC patients[70].
Preneoplastic lesions and invasive cancers associated with UC usually develop as multiple and superficially extended lesions called DALMs (dysplasia-associated lesion or mass)[71-74]. DALMs are frequent in the most inflamed colonic areas. Thus, a chronic inflammation - dysplasia - carcinoma sequence has been suggested[75]. Comparisons of the molecular alteration profiles between sporadic and UC-associated CRCs have shown clear differences. The timing and frequency of the gene alterations in UC-related cancers appear to be unique. Mutations of APC and of K-ras genes are less frequent[76,77] in UC-related cancer than sporadic ones. LOH at the APC loci in UC was noted in dysplasia with associated carcinoma, but LOH of APC was not present either in cases of non-dysplastic epithelium or in high grade dysplasia alone. Conversely, LOH of APC is present in 20% of colonic adenomas[78,79]. In contrast, p53 is frequently mutated at the early stages of UC-related carcinogenesis; 33%-67% in dysplasia and 83%-95% in UC-related cancer[20,80]. Moreover, loss of heterozygosity (LOH) of the p53 gene and src activation occur in UC non-dysplastic epithelium, UC-associated dysplasia, and in UC-associated carcinoma, whereas there is an absence of LOH of p53 in regions with negative, indefinite, or low grade dysplastic histology[81]. Mutations in the ras proto-oncogene are present in 40%-60% of sporadic colon cancers and are probably an early event; in contrast, these mutations are less frequently seen in UC-related cancer, and are probably a late event[22,82,83]. Finally, network analysis discovered that Sp1 and c-myc proteins may play roles in UC in the early and late stages of carcinogenesis, respectively. Two over-expressed proteins in the non-dysplastic tissue of UC progressors, CPS1 and S100P, were further confirmed by IHC analysis[84]. Finally, telomerase and ILK activation occurs during the later stages of carcinoma progression, whereas upregulation of survivin, c-MYB, and Tcf-4 is a feature of the early stage development of neoplasia, and thus they might serve as early indicators for UC-associated colorectal carcinogenesis[85]. These distinctive molecular patterns seem to result from different aetiological factors and microenvironments that characterize the adenoma-carcinoma sequence or UC-associated carcinogenesis[75].
Recently, van der Woude et al[86] observed that Bcl-xl was not expressed in chronic UC, but was clearly present in UC-related cancer tumor cells. Furthermore, they found interesting differences in the expression of Fas and Bcl-xl between UC-related cancer and sporadic carcinoma. Fas expression was strong in most UC-related dysplasia and tumor cells, whereas it was weak in sporadic carcinoma tumor cells. Moreover, Bcl-xl expression was important in chronic UC tumor cells, but was only weak in sporadic colon cancer cells[86]. However, the different expression patterns of proapoptotic and anti-apoptotic proteins did not result in actual differences in apoptosis[85]. Activated caspase 3 staining, used as a marker of apoptosis, was weakly represented in both chronic UC-associated colon cancer and sporadic colon cancer, and may be the result of the decreased apoptosis rate in the presence of increased cell proliferation[86].
The inflammation process leads to the activation of the transcription factor NF-κB, which stimulates the expression of many genes promoting cell survival, including anti-apoptotic genes[12,85,87]. NF-κB regulation of inducible nitric oxide synthase (iNOS) and COX-2 in the gastritis-metaplasia-gastric cancer sequence and in the metaplasia-dysplasia-adenocarcinoma sequence in Barrett’s esophagus have been extensively assessed[88]. Nitric oxide, produced by iNOS, was demonstrated to inhibit apoptosis by downregulating caspase activity[89]. In a paper by Watson et al[90], increased expression of iNOS in UC-associated dysplasia was described, whereas iNOS expression was absent in UC-associated carcinoma.
COX-2 is an inducible cyclooxygenase whose production is stimulated by IL-1, TNF, and many other inflammatory mediators[90,91]. COX-2 was demonstrated to play a role in the reparative process after mucosal injury in the gastrointestinal tract[90,91]. Multiple studies reported COX-2 overexpression (either protein or mRNA levels) in colonic adenomas and carcinomas, suggesting that this enzyme is definitely involved in sporadic colorectal carcinogenesis[92-94]. In a study by Agoff et al[26], COX-2 expression was examined at protein and mRNA levels on several mucosal samples in total colectomy specimens from UC patients who had developed dysplasia or carcinoma, which they showed that COX-2 overexpression in UC-related neoplasms occurs at the early stages, beginning in mucosa that is only diploid and still negative for dysplasia, and in mucosa that is not yet inflamed. Moreover, they showed that COX-2 protein overexpression detected by immunohistochemistry in mucosal samples occurs early on in UC-related neoplastic progression.
Two potential mechanisms may be involved in the relationship between COX-2 overexpression and neoplastic progression in UC: one related to malondialdehyde levels, and one related to the up-regulation of bcl-2[84]. The first hypothesis suggests that increased COX-2 activity, in part related to the normal physiological response to injury and inflammation, may induce DNA damage through increased production of malondialdehyde, a mutagenic by-product of COX-mediated prostaglandin synthesis and lipid peroxidation[40,95]. This malondialdehyde production would be in addition to that produced by the constitutive activity of COX-1, which is thought to be important in sporadic colorectal neoplasia[90]. In support of this hypothesis, elevated levels of malondialdehyde have been observed both in sporadic colon cancer and in IBD[96-99]. After tumor initiation, COX-2 may promote its progression by increasing expression of bcl-2[100,101]. In fact, bcl-2 mediates the resistance to apoptosis, and bcl-2 up-regulation was also observed in UC-associated neoplasia. Moreover, overexpression of bcl-2 is reversible by both nonspecific COX inhibitors[101] and by highly selective COX-2 inhibitors[102]. Genes involved in UC-related carcinogenesis are shown in Table 1.
| Gene | Function | Ref. |
| Mutation/overexpression | ||
| Apc | Wnt signaling pathway inhibition | 23,80,81,88,89 |
| Bcl-xl | Apoptosis suppression | 90 |
| Ptgs2 | Inflammation promotion and apoptosis inhibition | 26 |
| iNos | Apoptosis inhibition through NO production | 90 |
| Kras | Cell survival promotion and apoptosis suppression | 22,80,81,84-86 |
| Tp53 | Cell-cycle regulation | 20,82,83,87 |
| Tnfrsf6 | Apoptosis promotion | 90 |
| Smad3 | Wnt signaling pathway component | 121 |
| Aberrant methylation | ||
| p16 | Cell-cycle regulation | 71 |
| Mlh1 | DNA mismatch repair | 69 |
| Runx3 | Transcription factor | 71 |
| Dapk | Induction of cell death | 72 |
The importance of IL6/p-STAT3 in patients with inflammation-induced CRC has recently been demonstrated[103]. In fact, IL-6 is a critical tumor promoter during early CAC tumorigenesis. In addition to enhancing proliferation of tumor-initiating cells, IL-6 produced by lamina propria myeloid cells protects normal and premalignant intestinal epithelial cells from apoptosis. By binding to its gp130-associated receptor, IL-6 activates three separate signaling pathways, namely Shp2-Ras-ERK, JAK1/2-Stat3, and PI3K-Akt-mTOR[104] and, according to their results, Grivennikov concluded that among these, Stat3 is a critical IL-6 effector in colitis-associated cancer induction[103]. In fact, Stat3 has the capacity to mediate IL-6- and IL-11-dependent IEC survival and to promote proliferation through G1 and G2/M cell-cycle progression as the common tumor cell-autonomous mechanism that bridges chronic inflammation to tumor promotion[105]. Moreover, IL-6 signaling also seems to affect tumor growth during later stages of CAC[106]. IL-6 signaling during that stage increases TNF-α production, and its interference with TNF-α signaling inhibits tumor growth and reduces IL-6 production. Such cross-regulation was also observed in the case of IL1 and IL-6; IL-1 can induce IL-6 production in colon cancer cell lines[107]. The role for suppressor cytokines is more controversial. In fact, TGF-β signaling in colonic myeloid cells is significantly involved in the development of colitis-associated cancer[108]. In fact, Suppressor of Cytokine Signaling 3 (SOCS3) seems to be involved in UC pathogenesis, and its absence seems critical for CRC progression[109]. Oncogenic Smad3 signaling, altered by chronic inflammatory conditions and eventual somatic mutations, promotes UC-associated neoplastic progression through the upregulation of growth-related proteins[110].
The inconsistencies between the high frequency of colonic dysplasia and the much lower incidence of invasive cancer suggests the presence of mechanisms of surveillance that may prevent malignant progression of neoplasms in the colon in most cases. Observations that proctocolectomy specimens with preoperative UC and dysplasia showed cancer or dysplasia only in 64% cases[111] and, that 64% of UC patients with low grade dysplasia (LGD) had indefinite or no dysplasia after 4-year follow-up[16], suggest the presence of an efficient immune surveillance mechanism based on T-lymphocytes activation, ensuring the elimination of developing tumor cells.
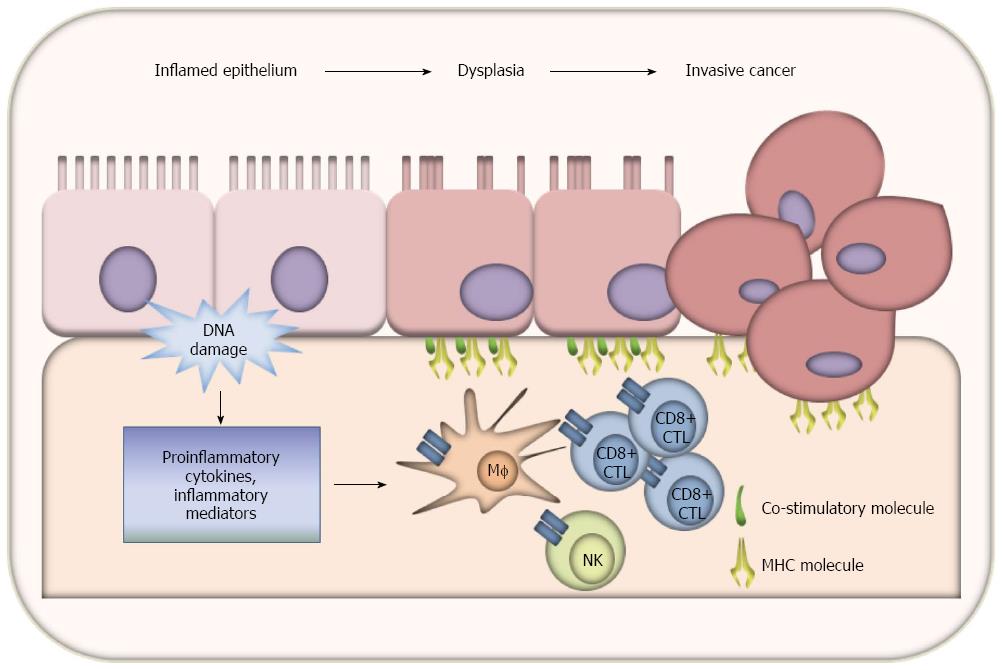
Tumor cell escape from immunosurveillance enables unrestrained neoplastic cell growth and metastatic diffusion. The immune escape is thought to be facilitated both by active defense of tumor cells and by defects in function of the immune system[112,113]. Both CD4 and CD8 T lymphocytes are responsible for anti-tumor immunity[114,115]. The effective activation of naive T lymphocytes implies the engagement of the T cell receptor (TCR) with the major histocompatibility complex (MHC)-antigen-complex in the presence of co-stimulation molecules that promote an effective interaction of APC and T cells[116-118]. The presentation of MHC-antigen-complex without co-stimulatory signals leads to T-cell energy[119]. These co-stimulatory signals are provided by the interaction of CD80 or CD86 on APC surface, with their receptors expressed by T-cells[120,121]. CD80 or CD86 binding to CD28 induces tyrosine phosphorylation of several substrates and enhances T cell activation promoted by the MHC-TCR interaction[122]. An increase in CD4/CD8 ratio was observed in sentinel lymph nodes draining dysplastic epithelium compared to normal mucosa. The increase in CD4(+) T cells in relation to CD8(+) T cells correlated with the degree of dysplasia reflected by a significant increase in the ratio against low-grade dysplasia compared to indefinite dysplastic lesions. The T-cell response was specific to antigens from dysplastic epithelial lining, as seen in proliferation assays. This observation suggests an important surveillance role for the immune system against premalignant intestinal lesions in patients with long-standing UC[123]. The products of oncogenes or oncosuppressor mutated proteins can act as potentially immunogenic proteins, and are expressed by CRC cells without any rejection by the immune system. Moreover, antigen presenting cells infiltrating colorectal carcinoma express MHC molecules, but do not express CD80 or CD86[124]. In vitro, the observation of CD80 and CD86 expression by human carcinoma cells lines up well with the regulation by IFN that was attributed to the early stage of carcinogenesis when they were selected[125]. In fact, the role of co-stimulatory molecules in the immune response to tumor initiation and progression has already been suggested by Antonia et al[126], who showed in 1995 that surface CD80 expression can be induced by an oncogenic insult, and its downregulation at a later stage in the carcinogenesis process may lead to their escape from immunosurveillance mechanisms. In previous work by our group, we showed that there is significant and specific CD80 overexpression in the colon mucosa of patients with UC and dysplasia that is downregulated at later stages in carcinogenesis. On the other hand, in the non-inflammatory carcinogenesis pathway, CD80 is significantly less expressed[127,128]. Our more recent data show that the proportion of epithelial cells acting as antigen presenting cells peaks in the dysplastic colonic mucosa of UC patients, and that the activation of CD8+ T cells can be mediated by epithelial cells through a CD80-dependent mechanism. Moreover, in a murine model of inflammatory colonic carcinogenesis, we demonstrated that CD80 inhibition significantly increased the high grade dysplasia rate and extension, whereas enhancing CD80 signaling with anti-CTLA4 antibody significantly decreased these lesions (data not yet published). These data suggest that, in UC-associated carcinogenesis, the progression from dysplasia to invasive cancer is controlled not by a mere immunoediting process, such as that observed in sporadic invasive cancer by Galon et al[129], but a truly effective immunosurveillance mechanism mediated by CD80 expression on epithelial cells (data not yet published). Immune surveillance mechanisms in UC-related carcinogenesis are shown in Figure 2.
Patients with UC undergo repeated episodes of colonic inflammation that are associated with various tumorigenic events, and the sequence of these events is different from that which contributes to the development of sporadic CRC. In fact, in UC, early events are represented by DNA methylation that produces inhibition of oncosuppressor genes, mutation of p53, aneuploidy, and MSI. Hypermethylation of tumor suppressor and DNA MMR gene promoter regions is an epigenetic mechanism of gene silencing that can be involved in tumorigenesis and may also represent the first step in inflammatory carcinogenesis. Moreover, p53 is frequently mutated in the early stages of UC-associated carcinogenesis. Aneuploidy is an independent risk factor for forthcoming carcinogenesis in UC. Epithelial cell-T-cell cross-talk mediated by CD80 is a key factor in controlling the progression from LGD to HGD in UC-associated carcinogenesis.
The authors are extremely grateful to Ms. Christina Drace for her kind help in the final editing of the manuscript.
P- Reviewers: Faria GR, Schicho R S- Editor: Wen LL L- Editor: Rutherford A E- Editor: Ma S
| 1. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 2. | Katsanos KH, Tatsioni A, Pedersen N, Shuhaibar M, Ramirez VH, Politi P, Rombrechts E, Pierik M, Clofent J, Beltrami M. Cancer in inflammatory bowel disease 15 years after diagnosis in a population-based European Collaborative follow-up study. J Crohns Colitis. 2011;5:430-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2079] [Article Influence: 86.6] [Reference Citation Analysis (1)] |
| 4. | Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 395] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Itzkowitz S. Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice. J Clin Gastroenterol. 2003;36:S70-S4; discussion S94-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Lynch DA, Lobo AJ, Sobala GM, Dixon MF, Axon AT. Failure of colonoscopic surveillance in ulcerative colitis. Gut. 1993;34:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 128] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Baars JE, Kuipers EJ, van Haastert M, Nicolaï JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol. 2012;47:1308-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Lutgens MW, Vleggaar FP, Schipper ME, Stokkers PC, van der Woude CJ, Hommes DW, de Jong DJ, Dijkstra G, van Bodegraven AA, Oldenburg B. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-81.e1; quiz e13-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 11. | van der Woude CJ, Kleibeuker JH, Jansen PL, Moshage H. Chronic inflammation, apoptosis and (pre-)malignant lesions in the gastro-intestinal tract. Apoptosis. 2004;9:123-130. [PubMed] |
| 12. | Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G626-G634. [PubMed] |
| 13. | Nieminen U, Jussila A, Nordling S, Mustonen H, Färkkilä MA. Inflammation and disease duration have a cumulative effect on the risk of dysplasia and carcinoma in IBD: a case-control observational study based on registry data. Int J Cancer. 2014;134:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | O’Connor PM, Lapointe TK, Beck PL, Buret AG. Mechanisms by which inflammation may increase intestinal cancer risk in inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1411-1420. [PubMed] |
| 15. | Brentnall TA. Risk factors for development of colorectal cancer in inflammatory bowel disease. Advances in Inflammatory Bowel Disease. London: Kluwer Academic Publishers 1998; pp 159-167. |
| 16. | Zisman TL, Bronner MP, Rulyak S, Kowdley KV, Saunders M, Lee SD, Ko C, Kimmey MB, Stevens A, Maurer J. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm Bowel Dis. 2012;18:2240-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Goldgraber MB, Humphreys EM, Kirsner JB, Palmer WL. Carcinoma and ulcerative colitis, a clinical-pathologic study. I. Cancer deaths. Gastroenterology. 1958;34:809-839. [PubMed] |
| 18. | Kern SE, Redston M, Seymour AB, Caldas C, Powell SM, Kornacki S, Kinzler KW. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107:420-428. [PubMed] |
| 19. | Park WS, Pham T, Wang C, Pack S, Mueller E, Mueller J, Vortmeyer A, Zhuang Z, Fogt F. Loss of heterozygosity and microsatellite instability in non-neoplastic mucosa from patients with chronic ulcerative colitis. Int J Mol Med. 1998;2:221-224. [PubMed] |
| 20. | Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369-378. [PubMed] |
| 21. | Bronner MP, Culin C, Reed JC, Furth EE. The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol. 1995;146:20-26. [PubMed] |
| 22. | Burmer GC, Levine DS, Kulander BG, Haggitt RC, Rubin CE, Rabinovitch PS. c-Ki-ras mutations in chronic ulcerative colitis and sporadic colon carcinoma. Gastroenterology. 1990;99:416-420. [PubMed] |
| 23. | Tarmin L, Yin J, Harpaz N, Kozam M, Noordzij J, Antonio LB, Jiang HY, Chan O, Cymes K, Meltzer SJ. Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res. 1995;55:2035-2038. [PubMed] |
| 24. | Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611-1620. [PubMed] |
| 25. | Brentnall TA, Crispin DA, Bronner MP, Cherian SP, Hueffed M, Rabinovitch PS, Rubin CE, Haggitt RC, Boland CR. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56:1237-1240. [PubMed] |
| 26. | Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol. 2000;157:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Charames GS, Bapat B. Genomic instability and cancer. Curr Mol Med. 2003;3:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | D’Incà R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Scarpa M, Cardin R, Bortolami M, Kotsafti A, Scarpa MC, Pozza A, Maran G, Picciocchi M, Ruffolo C, D’Incà R. Mucosal immune environment in colonic carcinogenesis: CD80 expression is associated to oxidative DNA damage and TLR4-NFκB signalling. Eur J Cancer. 2013;49:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915-7922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3967] [Cited by in RCA: 3633] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 31. | Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633-19636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 682] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 32. | Buffinton GD, Doe WF. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med. 1995;19:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1729] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 34. | Feig DI, Reid TM, Loeb LA. Reactive oxygen species in tumorigenesis. Cancer Res. 1994;54:1890s-1894s. [PubMed] |
| 35. | Holmes EW, Yong SL, Eiznhamer D, Keshavarzian A. Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig Dis Sci. 1998;43:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 281] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Rabinovitch PS, Dziadon S, Brentnall TA, Emond MJ, Crispin DA, Haggitt RC, Bronner MP. Pancolonic chromosomal instability precedes dysplasia and cancer in ulcerative colitis. Cancer Res. 1999;59:5148-5153. [PubMed] |
| 38. | Willenbucher RF, Aust DE, Chang CG, Zelman SJ, Ferrell LD, Moore DH, Waldman FM. Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol. 1999;154:1825-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Stoler DL, Chen N, Basik M, Kahlenberg MS, Rodriguez-Bigas MA, Petrelli NJ, Anderson GR. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc Natl Acad Sci USA. 1999;96:15121-15126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Mukai FH, Goldstein BD. Mutagenicity of malonaldehyde, a decomposition product of peroxidized polyunsaturated fatty acids. Science. 1976;191:868-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 245] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Dröge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Chen R, Rabinovitch PS, Crispin DA, Emond MJ, Koprowicz KM, Bronner MP, Brentnall TA. DNA fingerprinting abnormalities can distinguish ulcerative colitis patients with dysplasia and cancer from those who are dysplasia/cancer-free. Am J Pathol. 2003;162:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Jackson AL, Chen R, Loeb LA. Induction of microsatellite instability by oxidative DNA damage. Proc Natl Acad Sci USA. 1998;95:12468-12473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Ishitsuka T, Kashiwagi H, Konishi F. Microsatellite instability in inflamed and neoplastic epithelium in ulcerative colitis. J Clin Pathol. 2001;54:526-532. [PubMed] |
| 45. | O’Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH, Crispin DA. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 46. | Loeb KR, Loeb LA. Genetic instability and the mutator phenotype. Studies in ulcerative colitis. Am J Pathol. 1999;154:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Chang CL, Marra G, Chauhan DP, Ha HT, Chang DK, Ricciardiello L, Randolph A, Carethers JM, Boland CR. Oxidative stress inactivates the human DNA mismatch repair system. Am J Physiol Cell Physiol. 2002;283:C148-C154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 48. | Fleisher AS, Esteller M, Harpaz N, Leytin A, Rashid A, Xu Y, Liang J, Stine OC, Yin J, Zou TT. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 2000;60:4864-4868. [PubMed] |
| 49. | Cawkwell L, Sutherland F, Murgatroyd H, Jarvis P, Gray S, Cross D, Shepherd N, Day D, Quirke P. Defective hMSH2/hMLH1 protein expression is seen infrequently in ulcerative colitis associated colorectal cancers. Gut. 2000;46:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Noffsinger AE, Belli JM, Fogt F, Fischer J, Goldman H, Fenoglio-Preiser CM. A germline hMSH2 alteration is unrelated to colonic microsatellite instability in patients with ulcerative colitis. Hum Pathol. 1999;30:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Hofseth LJ, Khan MA, Ambrose M, Nikolayeva O, Xu-Welliver M, Kartalou M, Hussain SP, Roth RB, Zhou X, Mechanic LE. The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation. J Clin Invest. 2003;112:1887-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Croteau DL, Bohr VA. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem. 1997;272:25409-25412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 338] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 53. | Trush MA, Kensler TW. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic Biol Med. 1991;10:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 228] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Nishikawa M, Nishiguchi S, Shiomi S, Tamori A, Koh N, Takeda T, Kubo S, Hirohashi K, Kinoshita H, Sato E. Somatic mutation of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Res. 2001;61:1843-1845. [PubMed] |
| 55. | Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 618] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 56. | Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 609] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 57. | Nishikawa M, Oshitani N, Matsumoto T, Nishigami T, Arakawa T, Inoue M. Accumulation of mitochondrial DNA mutation with colorectal carcinogenesis in ulcerative colitis. Br J Cancer. 2005;93:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Oshitani N, Kitano A, Okabe H, Nakamura S, Matsumoto T, Kobayashi K. Location of superoxide anion generation in human colonic mucosa obtained by biopsy. Gut. 1993;34:936-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Tomobuchi M, Oshitani N, Matsumoto T, Kitano A, Seki S, Arakawa T. In situ generation of nitric oxide by myenteric neurons but not by mononuclear cells of the human colon. Clin Exp Pharmacol Physiol. 2001;28:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 353] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 61. | Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 522] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 62. | Toyooka S, Toyooka KO, Harada K, Miyajima K, Makarla P, Sathyanarayana UG, Yin J, Sato F, Shivapurkar N, Meltzer SJ. Aberrant methylation of the CDH13 (H-cadherin) promoter region in colorectal cancers and adenomas. Cancer Res. 2002;62:3382-3386. [PubMed] |
| 63. | Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60:4366-4371. [PubMed] |
| 64. | Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1643] [Cited by in RCA: 1654] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 65. | Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870-6875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1373] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 66. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 835] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 67. | Garrity-Park MM, Loftus EV, Sandborn WJ, Bryant SC, Smyrk TC. Methylation status of genes in non-neoplastic mucosa from patients with ulcerative colitis-associated colorectal cancer. Am J Gastroenterol. 2010;105:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Kuester D, Guenther T, Biesold S, Hartmann A, Bataille F, Ruemmele P, Peters B, Meyer F, Schubert D, Bohr UR. Aberrant methylation of DAPK in long-standing ulcerative colitis and ulcerative colitis-associated carcinoma. Pathol Res Pract. 2010;206:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Fujii S, Katake Y, Tanaka H. Increased expression of DNA methyltransferase-1 in non-neoplastic epithelium helps predict colorectal neoplasia risk in ulcerative colitis. Digestion. 2010;82:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Gerling M, Meyer KF, Fuchs K, Igl BW, Fritzsche B, Ziegler A, Bader F, Kujath P, Schimmelpenning H, Bruch HP. High Frequency of Aneuploidy Defines Ulcerative Colitis-Associated Carcinomas: A Comparative Prognostic Study to Sporadic Colorectal Carcinomas. Ann Surg. 2010;Jun 4; Epub ahead of print. [PubMed] |
| 71. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [PubMed] |
| 72. | Collins RH, Feldman M, Fordtran JS. Colon cancer, dysplasia, and surveillance in patients with ulcerative colitis. A critical review. N Engl J Med. 1987;316:1654-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 213] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 281] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Okayasu I, Fujiwara M, Takemura T, Toyoshima H, Nakamura K. Development of colorectal cancer in ulcerative colitis, clinicopathological study of 347 patients and new concepts of cancer. Development from analysis of mucosal cell proliferation activity. Stomach Intestine (Tokyo). 1993;28:171-179. |
| 75. | Yoshida T, Matsumoto N, Mikami T, Okayasu I. Upregulation of p16(INK4A) and Bax in p53 wild/p53-overexpressing crypts in ulcerative colitis-associated tumours. Br J Cancer. 2004;91:1081-1088. [PubMed] |
| 76. | Greenwald BD, Harpaz N, Yin J, Huang Y, Tong Y, Brown VL, McDaniel T, Newkirk C, Resau JH, Meltzer SJ. Loss of heterozygosity affecting the p53, Rb, and mcc/apc tumor suppressor gene loci in dysplastic and cancerous ulcerative colitis. Cancer Res. 1992;52:741-745. [PubMed] |
| 77. | Fujimori T, Satonaka K, Yamamura-Idei Y, Nagasako K, Maeda S. Non-involvement of ras mutations in flat colorectal adenomas and carcinomas. Int J Cancer. 1994;57:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Fogt F, Vortmeyer AO, Goldman H, Giordano TJ, Merino MJ, Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Fogt F, Urbanski SJ, Sanders ME, Furth EE, Zimmerman RL, Deren JJ, Noffsinger AE, Vortmeyer AO, Hartmann CJ, Odze RL. Distinction between dysplasia-associated lesion or mass (DALM) and adenoma in patients with ulcerative colitis. Hum Pathol. 2000;31:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Yin J, Harpaz N, Tong Y, Huang Y, Laurin J, Greenwald BD, Hontanosas M, Newkirk C, Meltzer SJ. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993;104:1633-1639. [PubMed] |
| 81. | Burmer GC, Rabinovitch PS, Haggitt RC, Crispin DA, Brentnall TA, Kolli VR, Stevens AC, Rubin CE. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602-1610. [PubMed] |
| 82. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 83. | Itzkowitz SH, Greenwald B, Meltzer SJ. Colon carcinogenesis in inflammatory bowel disease. Inflamm Bowel Dis. 1995;1:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Brentnall TA, Pan S, Bronner MP, Crispin DA, Mirzaei H, Cooke K, Tamura Y, Nikolskaya T, Jebailey L, Goodlett DR. Proteins That Underlie Neoplastic Progression of Ulcerative Colitis. Proteomics Clin Appl. 2009;3:1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Svec J, Musílková J, Bryndová J, Jirásek T, Mandys V, Kment M, Pácha J. Enhanced expression of proproliferative and antiapoptotic genes in ulcerative colitis-associated neoplasia. Inflamm Bowel Dis. 2010;16:1127-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | van der Woude CJ, Moshage H, Homan M, Kleibeuker JH, Jansen PL, van Dekken H. Expression of apoptosis related proteins during malignant progression in chronic ulcerative colitis. J Clin Pathol. 2005;58:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95:3452-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 88. | van der Woude CJ, Jansen PL, Tiebosch AT, Beuving A, Homan M, Kleibeuker JH, Moshage H. Expression of apoptosis-related proteins in Barrett’s metaplasia-dysplasia-carcinoma sequence: a switch to a more resistant phenotype. Hum Pathol. 2002;33:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 90. | Watson AJ. Chemopreventive effects of NSAIDs against colorectal cancer: regulation of apoptosis and mitosis by COX-1 and COX-2. Histol Histopathol. 1998;13:591-597. [PubMed] |
| 91. | Sakamoto C. Roles of COX-1 and COX-2 in gastrointestinal pathophysiology. J Gastroenterol. 1998;33:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 93. | Williams CS, Luongo C, Radhika A, Zhang T, Lamps LW, Nanney LB, Beauchamp RD, DuBois RN. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 94. | Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785-3789. [PubMed] |
| 95. | Basu AK, Marnett LJ. Unequivocal demonstration that malondialdehyde is a mutagen. Carcinogenesis. 1983;4:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Baur G, Wendel A. The activity of the peroxide-metabolizing system in human colon carcinoma. J Cancer Res Clin Oncol. 1980;97:267-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Hendrickse CW, Kelly RW, Radley S, Donovan IA, Keighley MR, Neoptolemos JP. Lipid peroxidation and prostaglandins in colorectal cancer. Br J Surg. 1994;81:1219-1223. [PubMed] |
| 98. | Oliva MR, Ripoll F, Muñiz P, Iradi A, Trullenque R, Valls V, Drehmer E, Sáez GT. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol Carcinog. 1997;18:232-243. [PubMed] |
| 99. | Chiarpotto E, Scavazza A, Leonarduzzi G, Camandola S, Biasi F, Teggia PM, Garavoglia M, Robecchi A, Roncari A, Poli G. Oxidative damage and transforming growth factor beta 1 expression in pretumoral and tumoral lesions of human intestine. Free Radic Biol Med. 1997;22:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 1565] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 101. | Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362-366. [PubMed] |
| 102. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] |
| 103. | Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1770] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 104. | Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 754] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 105. | Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 106. | Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 589] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 107. | Legrand-Poels S, Schoonbroodt S, Piette J. Regulation of interleukin-6 gene expression by pro-inflammatory cytokines in a colon cancer cell line. Biochem J. 2000;349 Pt 3:765-773. [PubMed] |
| 108. | Li J, Liu Y, Wang B, Xu Y, Ma A, Zhang F, Ge C, Yang Z, Li J, Liu Y. Myeloid TGF-β signaling contributes to colitis-associated tumorigenesis in mice. Carcinogenesis. 2013;34:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 109. | Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 110. | Kawamata S, Matsuzaki K, Murata M, Seki T, Matsuoka K, Iwao Y, Hibi T, Okazaki K. Oncogenic Smad3 signaling induced by chronic inflammation is an early event in ulcerative colitis-associated carcinogenesis. Inflamm Bowel Dis. 2011;17:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Kiran RP, Ali UA, Nisar PJ, Khoury W, Gu J, Shen B, Remzi FH, Hammel JP, Lavery IC, Fazio VW. Risk and location of cancer in patients with preoperative colitis-associated dysplasia undergoing proctocolectomy. Ann Surg. 2014;259:302-309. [PubMed] |
| 112. | Ugurel S, Uhlig D, Pföhler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother. 2004;53:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 266] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 114. | Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357-2368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 976] [Cited by in RCA: 985] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 115. | Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 363] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 116. | Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 830] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 117. | Chen L, Ashe S, Brady WA, Hellström I, Hellström KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 772] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 118. | Janeway CA, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 637] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 119. | Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1378] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 120. | Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 237] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 121. | June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 975] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 122. | Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1192] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 123. | Karlsson M, Lindberg K, Karlén P, Ost A, Thörn M, Winqvist O, Eberhardson M. Evidence for immunosurveillance in intestinal premalignant lesions. Scand J Immunol. 2010;71:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Chaux P, Moutet M, Faivre J, Martin F, Martin M. Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7-1 and B7-2 costimulatory molecules of the T-cell activation. Lab Invest. 1996;74:975-983. [PubMed] |
| 125. | Li J, Yang Y, Inoue H, Mori M, Akiyoshi T. The expression of costimulatory molecules CD80 and CD86 in human carcinoma cell lines: its regulation by interferon gamma and interleukin-10. Cancer Immunol Immunother. 1996;43:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Antonia SJ, Muñoz-Antonia T, Soldevila G, Miller J, Flavell RA. B7-1 expression by a non-antigen presenting cell-derived tumor. Cancer Res. 1995;55:2253-2256. [PubMed] |
| 127. | Scarpa M, Behboo R, Angriman I, Cecchetto A, D’Incà R, Termini B, Barollo M, Ruffolo C, Polese L, Sturniolo GC. Expression of costimulatory molecule CD80 in colonic dysplasia in ulcerative colitis: an immunosurveillance mechanism against colorectal cancer? Int J Colorectal Dis. 2006;21:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 128. | Scarpa M, Bortolami M, Cecchetto A, Faggian D, Kotsafti A, Ruffolo C, Navaglia F, Pozza A, D’Incà R, Plebani M. Mucosal immune environment in colonic carcinogenesis: CD80 up-regulation in colonic dysplasia in ulcerative colitis. Eur J Cancer. 2011;47:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4910] [Article Influence: 258.4] [Reference Citation Analysis (0)] |