Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6666
Revised: January 24, 2014
Accepted: March 4, 2014
Published online: June 7, 2014
Processing time: 213 Days and 19 Hours
AIM: To investigate the correlation of toll-like receptor 4 (TLR4) gene Asp299Gly and Thr399Ile polymorphisms and acute pancreatitis (AP) risk and severity.
METHODS: To get a more precise estimation of the relationship, a comprehensive search was performed to examine all the eligible studies of TLR4 Asp299Gly and Thr399Ile polymorphisms and AP risk. The odds ratios with 95% confidence intervals were used to assess the strength of the association. Publication bias was analyzed by Begg’s funnel plots.
RESULTS: In total, six studies with 1255 cases and 998 controls were included in this meta-analysis. Totally, no significant associations were found between TLR4 Asp299Gly or Thr399Ile polymorphisms and AP risk using five models with high homogeneity (P > 0.05). Furthermore, stratification analysis by ethnicity or assay also found no significant association in these two polymorphisms (P > 0.05), and TLR4 Asp299Gly was not associated with AP severity (P > 0.05). In addition, no publication bias was found in these studies (P > 0.05).
CONCLUSION: Our current meta-analysis suggests that TLR4 Asp299Gly and Thr399Ile polymorphisms may not be risk factors to AP susceptibility.
Core tip: Toll-like receptor 4 (TLR4) is one of the central proinflammatory factors in the pathology of acute pancreatitis (AP). Nevertheless, the relationship between TLR4 polymorphisms and AP susceptibility has been controversial. Here, we performed a systematic meta-analysis of TLR4 polymorphisms and AP risk, and our data showed that TLR4 Asp299Gly and Thr399Ile polymorphisms may not be associated with AP susceptibility.
- Citation: Zhou XJ, Cui Y, Cai LY, Xiang JY, Zhang Y. Toll-like receptor 4 polymorphisms to determine acute pancreatitis susceptibility and severity: A meta-analysis. World J Gastroenterol 2014; 20(21): 6666-6670
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6666
Acute pancreatitis (AP) is a potentially lethal disease with a mortality rate ranging from 10% to 25% depending on the infectious status of the disease[1]. Therefore, AP is one of the major problems encountered by many clinical specialists. Clinically, AP is divided into two groups by the disease severity: mild AP (MAP) and severe AP (SAP). MAP is a self-limited disease while SAP has a fast malignant progression, even resulting in multiple organ failure and death[2]. However, the molecular mechanisms that explain why some people suffer from SAP and others have MAP, remains largely unknown up to now.
Recently more and more solid evidence has demonstrated that the involvement of the immune system and, largely, release of multiple proinflammatory factors have played a fundamental role in the pathogenesis of AP. Toll-like receptor 4 (TLR4) is one of these key factors in the inflammatory process of AP disease. It has been reported that there are two common single nucleotide polymorphisms (SNPs) exist in the coding region of TLR4: Asp299Gly and Thr399Ile. Many studies have investigated the relationship between these two TLR4 polymorphisms and AP risk. Nevertheless, the conclusions are still controversial. Therefore, we conducted a systematic meta-analysis of current data to clarify the association of TLR4 Asp299Gly and Thr399Ile polymorphisms and AP susceptibility.
A literature search was conducted to looking for eligible studies that explored the association between TLR4 polymorphisms and AP risk using Pubmed, Embase, Web of Science, CBM (China Biological Medicine Database) on September 27, 2013 with combinations of the following key words including (“toll like receptor” or “TLR”) and (“polymorphism” or “genotype” or “variant” or “mutation”) and “pancreatitis”. There was no language restriction in the literature search. All reference lists from relevant studies and reviews were hand searched for additional eligible studies. The studies with the latest sample size were included when there were republished studies.
Eligible studies had to meet all of the following criteria: (1) evaluating the association of TLR4 Asp299Gly or Thr399Ile polymorphisms and AP risk; (2) a case-control study; (3) it is of Hardy Weinberg equilibrium (HWE) in the control group; and (4) sufficient genotyping information to evaluating an odds ratio (OR) and 95% confidence interval (CI).
The following information of each study was extracted independently by two reviewers: the name of first author, year of publication, country, ethnicity, genotypes distribution in both AP and controls, P values for HWE evaluation, source of controls, sample size (case/control), and genotyping methods.
The pooled OR with its 95%CI was calculated to evaluate the strength of association between TLR4 polymorphisms and AP susceptibility in five different genetic models, and the Z test was used to determine the significance of the pooled OR. Cochran’s χ2-based Q statistic test was performed to assess possible heterogeneity between the individual studies[3]. The fixed-effects model was applied to calculate the pooled OR with its 95%CI when there was no obvious between-study heterogeneity, otherwise, the random-effects model was used[4,5]. In the case of zero cells, an appropriate continuity correction (addition of 0.5) was implemented[6]. Publication bias analysis was performed by the funnel plot and Egger’s test[4]. All P values are two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were done with Stata software (version 12.0).
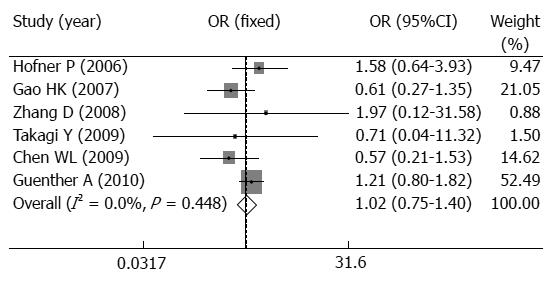
We collected 24 studies after database searches. After evaluation of title and abstract for the association of TLR4 polymorphisms and AP susceptibility, nine relevant studies were identified and retrieved for further investigation. Finally, six studies were identified according to the selection criteria. A total of 6 studies[7-12] with 1255 cases and 998 controls were included in this meta-analysis. In these studies, six studies[7-12] with 1255 cases and 998 controls were about the association of TLR4 Asp299Gly polymorphism and AP risk; three studies[7,8,11] with 815 cases and 744 controls were about the association of TLR4 Thr399Ile polymorphism and AP risk. Among these studies, five studies were published in English[7-11], and one study was in Chinese[12]. There were two studies of subjects of Caucasian descent[7,11], and four studies of subjects of Asian descent[8-10,12]. A classic polymerase chain reaction restriction fragment length polymorphism assay (PCR-RFLP) was used in four studies[8-10,12], a Taqman assay was conducted in two studies[7,11]. Table 1 listed the main characteristics of these six studies for two SNPs of TLR4 and AP. All five studies were consistent with HWE in the controls except for one study[11] (Table 1). Moreover, TLR4 Asp299Gly polymorphism and the severity of AP susceptibility from 4 studies[7,9,11,12] are also summarized in Table 2.
| SNP | Ref. | Year | Country | Ethnicity | AP | Control | P value for HWE | Source ofcontrols | AP | Control | Assay | ||||
| AA | AB | BB | AA | AB | BB | ||||||||||
| Asp299Gly | Hofner et al[11] | 2006 | Hungary | Caucasian | 84 | 7 | 1 | 64 | 7 | 2 | 0.01 | PB | 92 | 73 | Taqman |
| Gao et al[10] | 2007 | China | Asian | 101 | 22 | 0 | 71 | 9 | 0 | 0.59 | PB | 123 | 80 | PCR-RFLP | |
| Zhang et al[9] | 2008 | China | Asian | 238 | 0 | 0 | 121 | 0 | 0 | 1.00 | PB | 238 | 121 | PCR-RFLP | |
| Takagi et al[8] | 2009 | Japan | Asian | 202 | 0 | 0 | 286 | 0 | 0 | 1.00 | PB | 202 | 286 | PCR-RFLP | |
| Chen et al[12] | 2009 | China | Asian | 64 | 15 | 0 | 47 | 6 | 0 | 0.76 | PB | 79 | 53 | PCR-RFLP | |
| Guenther et al[7] | 2010 | Germany, United States | Caucasian | A 991 G 51 | A 725 G 45 | PB | 521 | 385 | Taqman | ||||||
| Thr399Ile | Hofner et al[11] | 2006 | Hungary | Caucasian | 85 | 6 | 1 | 64 | 7 | 2 | 0.01 | PB | 92 | 73 | Taqman |
| Takagi et al[8] | 2009 | Japan | Asian | 202 | 0 | 0 | 286 | 0 | 0 | 1.00 | PB | 202 | 286 | PCR-RFLP | |
| Guenther et al[7] | 2010 | Germany, United States | Caucasian | C 977 T49 | C 728 T 36 | PB | 521 | 385 | Taqman | ||||||
When those six studies were included in the meta-analysis, there was no obvious heterogeneity between the individual studies using five genetic models (P > 0.05). In overall analysis, TLR4 Asp299Gly polymorphism was not associated with AP risk when all studies were pooled into the meta-analysis using five genetic models (for A vs G: OR = 1.022, 95%CI: 0.748-1.397, P = 0.891; for AA vs GG: OR = 1.537, 95%CI: 0.466-5.075, P = 0.480; for AG vs GG: OR = 1.828, 95%CI: 0.454-7.368, P = 0.396; for AA + AG vs GG:OR = 1.576, 95%CI: 0.477-5.205, P = 0.456; for AA vs AG + GG:OR = 0.764, 95%CI: 0.458-1.277, P = 0.305, Table 3, Figure 1). Moreover, the five studies possessed highly homogeneity in all four genetic models (Pheterogeneity > 0.05, Table 3, Figure 1). Additionally, the further subgroup analysis by ethnicity or test assay showed that TLR4 Asp299Gly polymorphism was not a risk for in Asian or Caucasian populations or using PCR-RFLP assay or Taqman assay (P > 0.05, Table 3) with highly homogeneity.
| Test of association | Model | Test ofheterogeneity | ||||
| OR | 95%CI | P value | P value | I2(%) | ||
| Total | ||||||
| A vs G | 1.022 | 0.748-1.397 | 0.891 | F | 0.446 | 0.000 |
| AA vs GG | 1.537 | 0.466-5.075 | 0.480 | F | 0.971 | 0.000 |
| AG vs GG | 1.828 | 0.454-7.368 | 0.396 | F | 0.992 | 0.000 |
| AA vs AG/GG | 1.576 | 0.477-5.205 | 0.456 | F | 0.973 | 0.000 |
| AA/AG vs GG | 0.764 | 0.458-1.277 | 0.305 | F | 0.570 | 0.000 |
| Asian | ||||||
| A vs G | 0.628 | 0.349-1.133 | 0.122 | F | 0.874 | 0.000 |
| AA vs GG | 1.273 | 0.316-5.126 | 0.734 | F | 0.965 | 0.000 |
| AG vs GG | 1.760 | 0.338-9.153 | 0.502 | F | 0.966 | 0.000 |
| AA vs AG/GG | 1.326 | 0.330-5.339 | 0.691 | F | 0.961 | 0.000 |
| AA/AG vs GG | 0.606 | 0.330-1.115 | 0.107 | F | 0.861 | 0.000 |
| Caucasian | ||||||
| A vs G | 1.264 | 0.869-1.839 | 0.221 | F | 0.592 | 0.000 |
| AA vs GG | 2.625 | 0.233-29.591 | 0.435 | F | - | - |
| AG vs GG | 2.000 | 0.146-27.447 | 0.604 | F | - | - |
| AA vs AG/GG | 2.563 | 0.228-28.840 | 0.446 | F | - | - |
| AA/AG vs GG | 1.477 | 0.540-4.039 | 0.448 | F | - | - |
| PCR-RFLP | ||||||
| A vs G | 0.628 | 0.349-1.133 | 0.122 | F | 0.874 | 0.000 |
| AA vs GG | 1.273 | 0.316-5.126 | 0.734 | F | 0.965 | 0.000 |
| AG vs GG | 1.760 | 0.338-9.153 | 0.502 | F | 0.966 | 0.000 |
| AA vs AG/GG | 1.326 | 0.330-5.339 | 0.691 | F | 0.961 | 0.000 |
| AA/AG vs GG | 0.606 | 0.330-1.115 | 0.107 | F | 0.861 | 0.000 |
| Taqman | ||||||
| A vs G | 1.264 | 0.869-1.839 | 0.221 | F | 0.592 | 0.000 |
| AA vs GG | 2.625 | 0.233-29.591 | 0.435 | F | - | - |
| AG vs GG | 2.000 | 0.146-27.447 | 0.604 | F | - | - |
| AA vs AG/GG | 2.563 | 0.228-28.840 | 0.446 | F | - | - |
| AA/AG vs GG | 1.477 | 0.540-4.039 | 0.448 | F | - | - |
Considering the two different types of AP (MAP and SAP), we then assessed the association of TLR4 Asp299Gly polymorphism and two different types of AP risk using an allele genetic model. Our meta-analysis showed that the TLR4 Asp299Gly polymorphism has no association with MAP risk or SAP risk or the severity of AP using a fixed-effects model (P > 0.05, Table 4).
| Comparison | Test of association | Model | Test ofheterogeneity | Test ofpublication bias | |||
| OR | 95%CI | P value | P value | I2(%) | P value | ||
| MAP vs SAP | 1.040 | 0.658-1.643 | 0.866 | F | 0.219 | 32.300 | 0.124 |
| MAP vs Control | 1.112 | 0.747-1.655 | 0.601 | F | 0.159 | 42.000 | 0.955 |
| SAP vs Control | 1.054 | 0.691-1.609 | 0.807 | F | 0.334 | 11.800 | 0.020 |
For TLR4 Thr399Ile polymorphism, overall no association was found between TLR4 Thr399Ile polymorphism and AP risk using four genetic models (for C vs T: OR = 1.090, 95%CI: 0.736-1.614, P = 0.667; for CC vs TT: OR = 1.523, 95%CI: 0.258-9.012, P = 0.643; for CT vs TT: OR = 1.455, 95%CI: 0.166-12.756, P = 0.735; for CC + CT vs TT: OR = 1.494, 95%CI: 0.253-8.844, P = 0.658; for CC vs CT + TT: OR = 1.530, 95%CI: 0.580-4.041, P = 0.390, Table 5) with highly homogeneity.
| Geneticmodels | Test of association | Model | Test of heterogeneity | |||
| OR | 95%CI | P value | P value | I2(%) | ||
| C vs T | 1.090 | 0.736-1.614 | 0.667 | F | 0.503 | 0.000 |
| CC vs TT | 1.523 | 0.258-9.012 | 0.643 | F | 0.481 | 0.000 |
| CT vs TT | 1.455 | 0.166-12.756 | 0.735 | F | 0.823 | 0.000 |
| CC vs CT/TT | 1.494 | 0.253-8.844 | 0.658 | F | 0.493 | 0.000 |
| CC/CT vs TT | 1.530 | 0.580-4.041 | 0.390 | F | 0.560 | 0.000 |
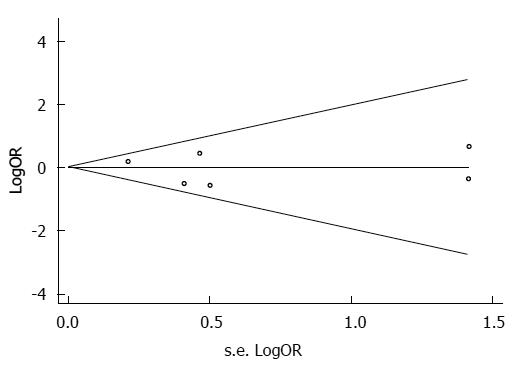
A funnel plot of these six included studies was symmetrical and didn’t suggest a possibility of publication bias (Figure 2). The statistical results from Egger’s test still did not show publication bias for TLR4 Asp299Gly polymorphism (for A vs G Pegger = 0.659; for AA vs GG Pegger = 0.204; for AG vs GG Pegger = 0.051; for AA + AG vs GG Pegger = 0.250; for AA vs AG + GG Pegger = 0.594) in all five genetic models (Figure 2).
The early phase of severe AP progression is commonly accompanied by activation of monocytes, polymorphonuclear granulocytes and macrophages, and the activated monocytes are the index of AP severity. Many factors and multiple pathways participated in the regulation of innate immune response of AP. Toll-like receptors (TLRs) can recognize pathogen-associated molecular patterns and protect bodies by initiating inflammatory reactions to destroy the invaders, thus playing pivotal roles in immune regulation. TLR4 has been extensively explored in inflammatory reactions and immune responses among the TLR family. TLR4 is commonly secreted by immune cells and can bind to its receptor Gram-negative bacterial lipopolysaccharide (LPS) as well as to a series of diverse ligands, such as heat-shock proteins, in both exogenous and endogenous situations[13,14]. It has been reported that about 29 SNPs have been found in the TLR4 gene till now[15]. Among these SNPs, Asp299Gly and Thr399Ile are the most common mutations in TLR4 gene. Asp299Gly is an A to G conversion which results in the replacement of Asp by Gly, while Thr399Ile is a C to T conversion which results in the replacement of Thr by Ile. Mutant Gly299 and Ile399 change the fourth exon structure of TLR4 protein, thus affecting the binding sites of ligands and bringing about interruption of TLR4 to LPS pathway[16].
In the past decade, accumulated studies demonstrated that bacterial infection in the necrotic tissues of pancreases is the major reason for death from SAP, and the chief pathogenic bacteria of infection in necrotic tissues are Gram-negative bacteria[2,17]. Many studies showed that Asp299Gly mutation of the TLR4 gene has changed the susceptibility of hosts to Gram-negative bacteria and turnover of individual bacterial infection[18-20]. The recent findings have been inconclusive for the association of TLR4 Asp299Gly and Thr399Ile polymorphisms and AP susceptibility[6-12,15,21], therefore, we performed this meta-analysis to clarify this association.
In this meta-analysis, we finally collected six studies with 1255 cases and 998 controls, and our meta-analysis indicated that no significant associations were found between TLR4 Asp299Gly or Thr399Ile polymorphisms and AP risk using five models with high homogeneity. Furthermore, subgroup analysis showed no significant association in these two polymorphisms by ethnicity or assay, and TLR4 Asp299Gly was not associated with AP severity. Our current meta-analysis indicates that both Asp299Gly and Thr399Ile polymorphisms of TLR4 gene may not be risk factors to AP susceptibility, implying that the polymorphisms of TLR4 have little effect in the pathogenesis of AP although TLR4 is one of the key genes in AP progression.
In summary, our meta-analysis implies that TLR4 gene polymorphisms were not significantly associated with AP susceptibility. However, the connection between TLR4 gene polymorphisms to AP susceptibility remains to be addressed in future investigations with a larger number of subjects.
Acute pancreatitis (AP) is a potentially lethal disease and many proinflammatory factors play important roles in the pathogenesis of AP.
Toll-like receptor 4 (TLR4) Asp299Gly and Thr399Ile polymorphisms have been found to interrupt the binding of TLR4 to lipopolysaccharide pathway, however, the results remains unclear.
In this paper, the authors for the first time conducted a systematic meta-analysis to evaluate the association between TLR4 polymorphisms and AP risk, and the results suggest that TLR4 Asp299Gly and Thr399Ile polymorphisms play little role in the pathogenesis of AP.
This study helped people to further understand the relationship between TLR4 Asp299Gly and Thr399Ile polymorphisms and AP susceptibility.
This paper deals with a hot topic of association between gene single nucleotide polymorphisms and AP risk.
P- Reviewer: Abdin AA S- Editor: Zhai HH L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Yousaf M, McCallion K, Diamond T. Management of severe acute pancreatitis. Br J Surg. 2003;90:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Isenmann R, Beger HG. Natural history of acute pancreatitis and the role of infection. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Bagos PG. A unification of multivariate methods for meta-analysis of genetic association studies. Stat Appl Genet Mol Biol. 2008;7:Article31. [PubMed] |
| 4. | Kavvoura FK, Ioannidis JP. Methods for meta-analysis in genetic association studies: a review of their potential and pitfalls. Hum Genet. 2008;123:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Bagos PG. Genetic model selection in genome-wide association studies: robust methods and the use of meta-analysis. Stat Appl Genet Mol Biol. 2013;12:285-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 7. | Guenther A, Aghdassi A, Muddana V, Rau B, Schulz HU, Mayerle J, Kraft M, Whitcomb DC, Lerch MM, Weiss FU. Toll-like receptor 4 polymorphisms in German and US patients are not associated with occurrence or severity of acute pancreatitis. Gut. 2010;59:1154-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Takagi Y, Masamune A, Kume K, Satoh A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene is associated with susceptibility to acute pancreatitis in Japan. Hum Immunol. 2009;70:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Zhang D, Zheng H, Zhou Y, Yu B, Li J. TLR and MBL gene polymorphisms in severe acute pancreatitis. Mol Diagn Ther. 2008;12:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Gao HK, Zhou ZG, Li Y, Chen YQ. Toll-like receptor 4 Asp299Gly polymorphism is associated with an increased risk of pancreatic necrotic infection in acute pancreatitis: a study in the Chinese population. Pancreas. 2007;34:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Hofner P, Balog A, Gyulai Z, Farkas G, Rakonczay Z, Takács T, Mándi Y. Polymorphism in the IL-8 gene, but not in the TLR4 gene, increases the severity of acute pancreatitis. Pancreatology. 2006;6:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Chen WL, Li WL, Su JR, Han SG, Yi XY. Analysis of Toll-like receptor 4 polymorphisms in patients with acute pancreatitis. Chin Remed Clinic. 2009;9:685-688. |
| 13. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3900] [Cited by in RCA: 3804] [Article Influence: 135.9] [Reference Citation Analysis (0)] |
| 14. | Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 469] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Umemura T, Katsuyama Y, Hamano H, Kitahara K, Takayama M, Arakura N, Kawa S, Tanaka E, Ota M. Association analysis of Toll-like receptor 4 polymorphisms with autoimmune pancreatitis. Hum Immunol. 2009;70:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1473] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 17. | Baron TH, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 515] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 19. | Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Corbett SA, Calvano SE, Lowry SF. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186:1522-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 413] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Raby BA, Klimecki WT, Laprise C, Renaud Y, Faith J, Lemire M, Greenwood C, Weiland KM, Lange C, Palmer LJ. Polymorphisms in toll-like receptor 4 are not associated with asthma or atopy-related phenotypes. Am J Respir Crit Care Med. 2002;166:1449-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Gao HK, Zhang XG, Zhou ZG, Li Y, Chen YD. Association between toll like receptor 4 (896A& gt; G) mutations and pancreatic necrotic infection in severe acute pancreatitis. Sichuan Daxue Xuebao Yieueban. 2007;38:617-619. [PubMed] |