Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6632
Revised: February 11, 2014
Accepted: March 8, 2014
Published online: June 7, 2014
Processing time: 234 Days and 9.8 Hours
AIM: To demonstrate the presence and biological activity of human papilloma virus (HPV) in gastric cancer (GAC) tissues.
METHODS: The study involved 84 surgically treated patients with gastric adenocarcinoma, regardless of the clinical stage of the disease. The presence of HPV DNA of high oncogenic risk types in formalin-fixed, paraffin-embedded tumor samples was determined using quantitative polymerase chain reaction analysis. A stringent protocol of prevention of cross- and environmental contamination was applied during DNA isolation, and amplification, as well as confirmation of the biological activity of the virus in tumor cells, was implemented. The study utilized the Real-time High Risk HPV test, which detects the DNA of 14 HPV subtypes that are considered to have high oncogenic potential. The overexpression of the p16INK4a protein assessed immunohistochemically was considered confirmation of the HPV infection.
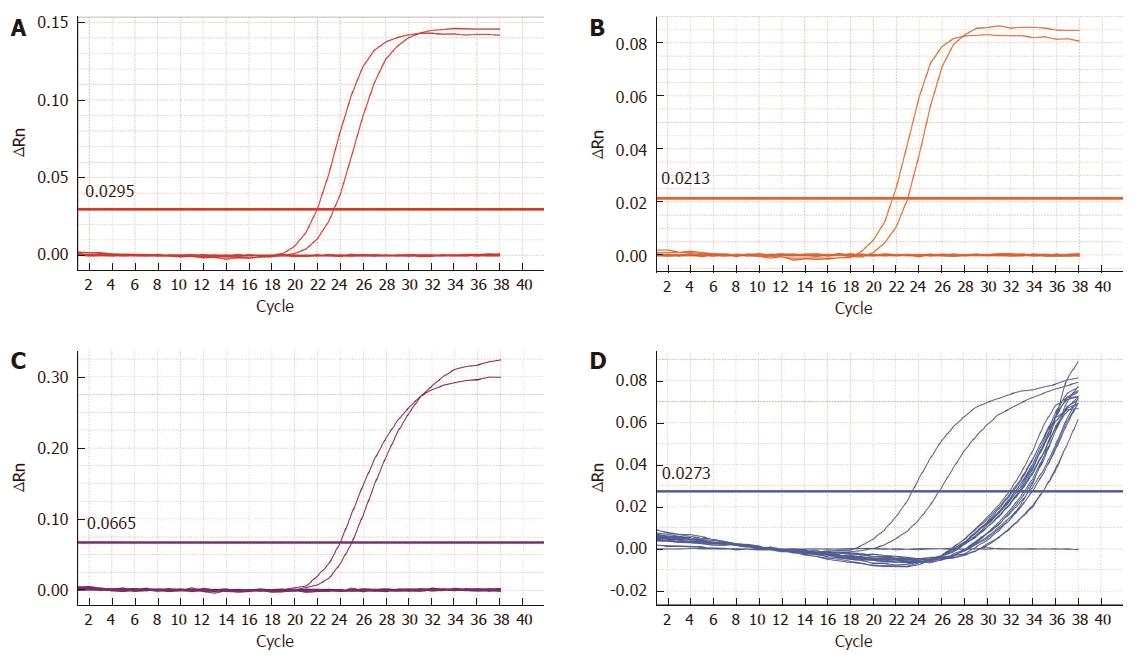
RESULTS: Among the 89 patients initially included in the study group, diagnostic results were obtained for 84 individuals. In five cases, either the histopathological material was too scant to isolate the necessary amount of DNA, or the isolated DNA was significantly degraded, resulting in the failure of internal control amplification within the predefined number of 35 cycles. Those patients were excluded from further analysis. The amplification of HPV DNA was demonstrated in none of the 84 tissue samples; thus, all cases were considered to have a negative DNA status of highly oncogenic HPV subtypes. Immunohistochemical staining provided diagnostic results for all of the examined tissue samples, and excluded the accumulation of the p16INK4a protein in tumor cells, thus confirming the lack of active HPV infection in all of the individuals.
CONCLUSION: The study does not confirm the presence or biological activity of HPV in tumor tissues. Thus, the relationship between GAC and HPV infection, in the Central European population seems doubtful.
Core tip: The study aimed to demonstrate the presence and biological activity of human papilloma virus (HPV) in gastric cancer tissues. The genomes of 14 HPV subtypes of high oncogenic potential were assessed using quantitative polymerase chain reaction in 84 tumor samples. A stringent protocol for preventing sample contamination, and confirming the biological activity of the virus in the tumor cells, was applied. The study did not confirm either the presence of the HPV genome or viral activity in the examined tumor tissues.
- Citation: Snietura M, Waniczek D, Piglowski W, Kopec A, Nowakowska-Zajdel E, Lorenc Z, Muc-Wierzgon M. Potential role of human papilloma virus in the pathogenesis of gastric cancer. World J Gastroenterol 2014; 20(21): 6632-6637
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6632.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6632
Alimentary tract carcinoma is a group of cancers characterized by a high risk of occurrence and a relatively high mortality rate. Recently, significant progress has been made in understanding the epidemiology, pathology and pathogenesis of alimentary tract carcinomas. Gastric cancer is the third leading cause of cancer death in both sexes worldwide (723000 deaths, 8.8% of the total)[1]. The greater proportion of the disease occurs within the male population in developing countries - mostly East Asia, South America and Eastern Europe. A downward trend has also been observed in the incidence of this cancer[1,2]. Among the etiological factors, the most significant is improper eating habits, particularly a diet rich in cured and smoked meat products, with low antioxidant content. Additionally tobacco smoking and alcohol consumption have a significant effect on the gastric cancer development. An important role is also played by chronic Helicobacter pylori (H. pylori) or Epstein-Barr virus (EBV) infection[2-5]. Neoplastic transformation is usually a long, highly complex and multi-stage process. that is caused by various genetic and epigenetic changes. human papilloma virus (HPV) is a suspected risk factor of neoplastic transformation. Abundant evidence has demonstrated the oncogenic properties of HPV in studies on anal[6,7], oral[8,9] and pharyngeal cancers[8,10], suggesting a role for the virus in the pathogenesis of cancer of other sections of the alimentary tract. Only a few studies have addressed the role of HPV in the epidemiology and development of gastric cancer (GAC), and they are limited mainly to South and East Asia. However, the results obtained seem divergent, and the conclusions drawn are controversial.
The present study was aimed to evaluate the presence of HPV DNA in GAC tissues using quantitative polymerase chain reaction (PCR) method and to indirectly confirm active infection through a demonstration of the p16INK4a protein overexpression using the immunohistochemical method.
The study involved 89 consecutive patients treated surgically for GAC from 2007 to 2013, regardless of the clinical stage of the disease. The inclusion criteria were as follows: age > 18 years and confirmed gastric adenocarcinoma. The exclusion criteria included the following: previous diagnosis of malignant carcinoma and previous anti-cancer therapy (radiotherapy, chemotherapy) for GAC. Paraffin blocks of the tumor tissue, histopathological and clinical documentation allowing for the determination of the primary location of the lesion, histological type of the tumor, histopathological grading and clinical staging using the pTNM scale were studied. Clinical and histopathological characteristics of the patients who were included in the study are presented in Table 1.
| Feature | Value | |
| Gender | Female | 38 |
| Male | 46 | |
| Total | 84 | |
| Age | Median | 64 yr |
| Range | 18-85 yr | |
| Ethnicity | Caucasian | 84 |
| Other | 0 | |
| Histopathological type | Adenocarcinoma | 84 |
| Intestinal type | 13 | |
| Mucous type | 17 | |
| Signet ring type | 5 | |
| Histopathological grading | G1 | 3 |
| G2 | 35 | |
| G3 | 46 | |
| Clinical staging according to the pTNM scale | T | T1-7, T2-16, T3-51, T4-10 |
| N | N0-23, N1-28, N2-19, N3-11, Nx-3 | |
| M | M0-62, M1-20, Mx-2 | |
Isolation of the genomic DNA aseptically from the paraffin tissue blocks was confirmed using a Maxwell AS2000 instrument and the Maxwell 16 FFPE Plus LEV DNA Purification Kit (Promega Corporation, Medison, WI, United States). The purity of the obtained isolates and DNA concentration was measured using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc. Waltham, MA, United States). The presence of HPV DNA of high oncogenic risk types was confirmed using a quantitative PCR (Q-PCR) analysis. The study utilized the RealTime High Risk HPV test (Abbott Laboratories, Abbott Park, IL, United States) to detect the DNA of 14 HPV subtypes that are considered to have high oncogenic risk. The usefulness of the test in the assessment of HPV status in formalin-fixed, paraffin-embedded tissue samples has been demonstrated elsewhere[8,10].
The reaction was a multiplex PCR using 5 (3 forward and 2 reverse) consensual starters for conservative sequences in the L1 gene of the virus, allowing simultaneous DNA amplification of several subtypes of the virus[11]. The detection of amplification products was completed using a set of fluorescence marked hybridization probes, specific for individual subtypes of the virus. That method allows for the separate detection of HPV for types 16 and 18 and for one or more of the other less common types: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. This configuration of the test covers over 93% of the high oncogenic risk subtypes reported for the European population[12]. The entire amplification and reaction product detection process was realized using the RT7500 Fast platform (Life Technologies Inc., Carlsbad, CA, United States). Although the test uses quantitative PCR, its result is of qualitative character - confirming or excluding the presence of an individual type of the virus. The results with a threshold cycle less than or equal to 32 (CT≤ 32) were considered positive. An internal control in the form of human β-globin was included in each sample. Amplification of the β-globin gene confirmed the isolation of a sufficient amount of DNA of satisfactory quality and excluded the possibility of false-negative results.
Commercially available negative and positive controls (RealTime High Risk HPV Control Kit, Abbott Laboratories, Abbott Park, IL, United States) were also included in each run to verify that the sample processing, amplification and detection steps were performed correctly. The negative control was formulated with DNA containing the β-globin sequence and poly-dA:dT as carrier DNA. The positive control contained HPV16, HPV18, HPV58 and β-globin sequences and carrier DNA.
A possible biological activity of the virus was confirmed immunohistochemically based on the accumulation of the p16INK4a protein caused by inhibition of the retinoblastoma gene (pRB) by the viral oncoprotein E7.
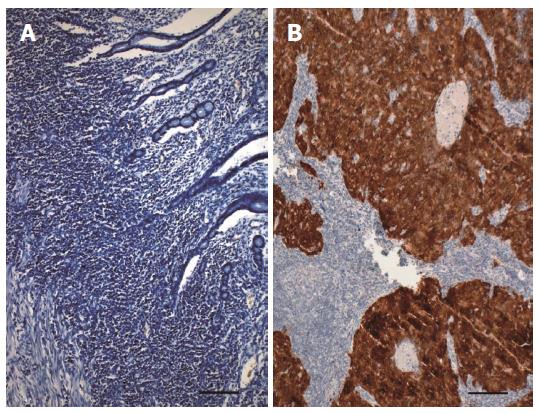
Determination of the p16INK4a protein overexpression was completed on 3-μm-thick paraffin sections, following their dewaxing and rehydratation. The antigen retrieval procedure was carried out in Antigen Retrieval Solution (Mtm Laboratories Inc., Heidelberg, Germany) (100 mmol/L Tris, 10 mmol/L EDTA (pH 9.0), 15 mmol/L sodium azide). The presence of the p16INK4a protein in the examined tissues was detected using mouse anti-human p16INK4a monoclonal antibody, clone E6H4 (Mtm Laboratories Inc., Heidelberg, Germany) at a ready-to-use concentration and was visualized using the HRP/DAB+ system (Dako Denmark A/S, Glostrup, Denmark). Contrast staining was completed using hematoxylin according to Meyer. Each batch was supplemented with a positive control in the form of a section of squamous cell carcinoma of a tonsil with a known, positive HPV status (demonstrating a strong and uniform color reaction) and negative controls in which the primary antibody was replaced by TBS buffer.
The intensity of the cytoplasmic immunohistochemical reaction was evaluated under a light microscope BX41 (Olympus Corporation, Tokyo, Japan) at magnifications of 100 x and 200 x using a semiquantitative three-grade scale: total lack of p16INK4a expression in the tumor tissue; focal staining in separated cells; and moderate or strong color reaction involving most of the cancer cells. Only a moderate or strong color reaction was considered a positive result.
The present study was conducted in accordance with the guidelines of the Declaration of Helsinki and its subsequent amendments, and informed consent was obtained from all of the patients.
Among the 89 patients initially included in the study, diagnostic results were obtained for 84 samples. In five cases, either the histopathological material was insufficient to isolate the necessary DNA amount or the isolated DNA was significantly degraded, resulting in the failure of internal control amplification within the predefined number of 35 cycles. Those patients were excluded from further analysis. The amplification of high risk HPV DNA was demonstrated in none of the 84 tissue samples; thus, all of the cases were considered negative (Figure 1).
Immunohistochemical staining provided diagnostic results for all of the examined samples, and excluded the accumulation of the p16INK4a protein, confirming the lack of active HPV infection in all individuals. In 80 cases the complete absence of staining with the specific anti-p16INK4a antibody was observed. In the other four cases, a focal, weakly expressed reaction within the neighboring epithelium, in a single tumor or in stromal cells was observed; (all of these expression patterns were classified as negative results) (Figure 2).
In GAC, dietary and lifestyle factors primarily contribute to the risk of developing cancer, in addition to infections with H. pylori or EBV[1-4]. It was demonstrated that approximately 9% of GAC display EBV in the tumor cells, although its effect on carcinogenesis and the development of GAC remains unclear[5,13,14]. Some authors believe that there may be a correlation between HPV infection and the development of GAC similar to that found for EBV. However, the role of HPV in GAC has not been yet extensively studied. Therefore, some recent papers have aimed at demonstrating of a correlation between HPV and GAC and other alimentary tract carcinomas. Unfortunately, those papers provided contradictory data. In studies from various authors, the incidence of HPV in patients with alimentary tract carcinomas is highly variable, depending on the selected detection technique. Currently, PCR is most often used due to its high sensitivity, but its disadvantage is its low specificity[15-18]. The data variability may be a result of using not only various HPV detection methods but also various study material collection procedures and sites, various methods of specimen protection against viral contamination, geographical differences and various selected subtypes of high oncogenic risk HPV (mostly HPV16 and HPV18). Ma et al[19] using liquid and in situ PCR found HPV16E6 genes in 37.5% of GAC (15/40) and stated that the gastric adenoid epithelium may be a target of HPV-dependent carcinogenesis. Xu et al[20] using the in situ hybridization technique, found HPV in 68% of the examined GAC samples and even in 20% (10/50) of the normal gastric mucosa tissue samples. Among 23 cases of concurrent esophageal squamous cell carcinoma (ESCC) and GCA (gastric cardia cancer), Ding et al[21] using PCR, found HPV DNA in 29% of GCA and 47% of ESCC cases. Although HPV occurred less commonly in GCA than it did in ESCC, higher p16INK4a expression was observed in GCA than in ESCC (75% vs 25%, respectively; P < 0.05). In some older studies on EBV, some authors demonstrated the presence of EBV or HPV (less commonly) in GAC; in some cases, the simultaneous presence of both viruses was revealed[22,23]. In contrast, Koshiol et al[24] found no HPV16/18 in any of the tissues collected from 144 cardia cancers. They used a standardized protocol to minimize the potential for environmental HPV to contaminate the tissues. Control β-globin, and therefore the DNA quality, was adequate in 75% of the cases (108/144). Among the 108 cases, all were negative for HPV DNA based on Linear Array and E6/E7-based PCR. Yuan et al[25] investigated the relationship between GAC and HPV. They performed PCR analyses of tissue samples from 98 patients with gastroduodenal diseases, including GAC, in a region presenting a high incidence rate of GAC. HPV genotypes were detected using the HPV GenoArray test kit (Hybribio Ltd, Hong Kong). HPV DNA was not detected in any of the patients’ tissues, including: GAC cells, adjacent dysplastic epithelium, surrounding lymphocytes, and paired normal gastric mucosa. The results of Kamangar et al[26]’s prospective, seroepidemiological study in a high-risk region of GAC in China did not support a major role for HPV 16, HPV 18 and HPV 73 in GAC etiology. Lagergren et al[27] performed a population-based, case-control study and found no association between HPV16 and an inverse association between HPV 18, and adenocarcinomas of the esophagus or gastroesophageal junction (OR = 0.2; 95%CI: 0.1-0.7). Other studies have also ruled out the presence of HPV in GAC or have found that it has low significance[28,29].
In the present study, we used the unique combination of viral DNA detection and confirmation of transcriptional activity of the virus, by demonstrating of p16INK4a accumulation in target cells. This algorithm based on p16 immunostaining followed by GP5+/6+ PCR in the p16-positive cases proposed by Smeets et al[30] is claimed to be 100% sensitive and specific. Moreover, it should be noted that the expression of p16INK4a is a sensitive surrogate marker of HPV infection but is not limited only to HPV-positive tumors, and the use of this marker alone as an indicator of biologically relevant HPV infections inevitably entails the risk of including some HPV-negative p16INK4a-positive results[31].
Another distinguishing attribute of the present study is the application of quantitative PCR, which covers more than 93% of the subtypes of high-oncogenic-risk viruses, including HPV16, HPV18 and twelve less abundant subtypes. The analytical parameters of this assay were precisely described[12] and the usefulness of HPV detection in the formalin-fixed, paraffin-embedded tissue samples was demonstrated in our previous studies[8,10] and by independent investigators[32]. The results obtained in the study failed to detect the presence of the HPV genome in GAC, suggesting that the incidence of high-oncogenic-risk HPV in GAC tissue is very low; therefore, the potential participation of the virus in GAC development is highly doubtful.
In conclusion, infectious agents such as HPV are suspected to play causal roles in various human malignancies. However, the present study failed to confirm the presence of the HPV genome as well as any viral biological activity in GAC tissues. Therefore, any role of the virus in the pathogenesis of GAC, at least in the Caucasian population of Middle end Eastern Europe, is doubtful.
Despite the significant progress made in the understanding of the epidemiology, pathology and pathogenesis of alimentary tract carcinomas, gastric cancer remains the third leading cause of cancer death in both sexes worldwide. The highest mortality rates are observed in the developing countries of Eastern Asia and of Central and Eastern Europe. Among the factors that may lead to the development of gastric cancer, the most significant are as follows: a diet rich in cured and smoked meat products with low antioxidant content, tobacco smoking and alcohol consumption and long-lasting infection by the bacterium called Helicobacter pylori.
Some types of human papilloma virus (HPV) are proven risk factors of neoplastic transformation in cervical, anal, oral or pharyngeal cancers, suggesting a role for the virus in the pathogenesis of cancer of other sections of the alimentary tract, including gastric cancer.
Previous publications using different techniques of HPV detection showed contradictory results and were restricted mainly to the Asian population. In the present study, we used the unique combination of viral DNA detection using quantitative polymerase chain reaction (PCR) that can detect 14 oncogenic subtypes of the virus, and confirmation of tumor cell infection by demonstrating the changes in cellular metabolism caused by HPV. Using this combined approach, we could eliminate the false-positive results emerging from HPV presence in the alimentary tract without infecting its tissues. This study is the first from Middle and Eastern Europe characterized by one of the highest gastric cancer incidence and mortality rates. Obtained results confirmed neither the presence of virus nor its biological activity in the tested tissues of gastric cancer.
The potential participation of HPV in the development of gastric cancer, at least in the population of the Middle and Eastern Europe, is highly doubtful.
HPVs represent a large group of relatively small viruses that contain DNA as genetic material and can infect epithelial tissues of mammalians, including humans. Most HPVs are benign and cause skin or genital warts, but a subgroup, known as high oncogenic subtypes, are responsible for the development of several cancers, including cervical, anal and pharyngeal cancer.
In this study, the authors explored the presence of high-risk HPVs and the expression of p16INK4a in a cohort of 84 gastric cancer tissues using RT-PCR and IHC analysis. The author failed to detect HPVs and p16INK4a expression in all samples. Therefore, they claimed that the presence and role of high-risk HPVs in the pathogenesis of gastric adenocarcinoma in a European population are not evident. This study is interesting and within the scope of the journal.
P- Reviewers: Al Moustafa AE, Duerksen-Hughes P S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 - Stomach Cancer, 2014-01, cited 2014-01-20. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx? cancer=stomach. |
| 2. | Fenoglio-Preiser C, Muñoz N, Carneiro F, Powell SM, Correa P, Rugge M, Guilford P, Sasako M, Lambert R, Stolte M. Tumours of the Stomach. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press 2000; 35-66. |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25542] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 4. | Sekine K, Nagata N. Synchronous gastric cancer presenting different pathological features depending on the involvement of Epstein-Barr virus infection. Clin Res Hepatol Gastroenterol. 2013;37:111-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R, Meneses-Gonzalez F. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 6. | Steenbergen RD, de Wilde J, Wilting SM, Brink AA, Snijders PJ, Meijer CJ. HPV-mediated transformation of the anogenital tract. J Clin Virol. 2005;32 Suppl 1:S25-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Gervaz P, Hirschel B, Morel P. Molecular biology of squamous cell carcinoma of the anus. Br J Surg. 2006;93:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Śnietura M, Jaworska M, Pigłowski W, Goraj-Zając A, Woźniak G, Lange D. High-risk HPV DNA status and p16 (INK4a) expression as prognostic markers in patients with squamous cell cancer of oral cavity and oropharynx. Pol J Pathol. 2010;61:133-139. [PubMed] |
| 9. | Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 831] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 10. | Snietura M, Piglowski W, Jaworska M, Mucha-Malecka A, Wozniak G, Lange D, Suwinski R. Impact of HPV infection on the clinical outcome of p-CAIR trial in head and neck cancer. Eur Arch Otorhinolaryngol. 2011;268:721-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2225] [Cited by in RCA: 2190] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 12. | Huang S, Tang N, Mak WB, Erickson B, Salituro J, Li Y, Krumpe E, Schneider G, Yu H, Robinson J. Principles and analytical performance of Abbott RealTime High Risk HPV test. J Clin Virol. 2009;45 Suppl 1:S13-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500-1510. [PubMed] |
| 14. | Zhu S, Sun P, Zhang Y, Yan L, Luo B. Expression of c-myc and PCNA in Epstein-Barr virus-associated gastric carcinoma. Exp Ther Med. 2013;5:1030-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Shroyer KR, Kim JG, Manos MM, Greer CE, Pearlman NW, Franklin WA. Papillomavirus found in anorectal squamous carcinoma, not in colon adenocarcinoma. Arch Surg. 1992;127:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Snietura M, Waniczek D, Nowakowska-Zajdel E, Piglowski W, Kopec A, Muc-Wierzgon M. Does human papilloma virus participate in colorectal carcinogenesis? J Biol Regul Homeost Agents. 2012;26:757-762. [PubMed] |
| 17. | Burnett-Hartman AN, Newcomb PA, Mandelson MT, Galloway DA, Madeleine MM, Wurscher MA, Carter JJ, Makar KW, Potter JD, Schwartz SM. No evidence for human papillomavirus in the etiology of colorectal polyps. Cancer Epidemiol Biomarkers Prev. 2011;20:2288-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Deschoolmeester V, Van Marck V, Baay M, Weyn C, Vermeulen P, Van Marck E, Lardon F, Fontaine V, Vermorken JB. Detection of HPV and the role of p16INK4A overexpression as a surrogate marker for the presence of functional HPV oncoprotein E7 in colorectal cancer. BMC Cancer. 2010;10:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Ma TY, Liu WK, Chu YL, Jiang XY, An Y, Zhang MP, Zheng JW. Detection of human papillomavirus type 16 DNA in formalin-fixed, paraffin-embedded tissue specimens of gastric carcinoma. Eur J Gastroenterol Hepatol. 2007;19:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Xu WG, Zhang LJ, Lu ZM, Li JY, Ke Y, Xu GW. Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract. Zhonghua Yixue Zazhi. 2003;83:1910-1914. [PubMed] |
| 21. | Ding GC, Ren JL, Chang FB, Li JL, Yuan L, Song X, Zhou SL, Guo T, Fan ZM, Zeng Y. Human papillomavirus DNA and P16(INK4A) expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol. 2010;16:5901-5906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Khurshid A, Kazuya N, Hanae I, Manabu I. Infection of human papillomavirus (HPV) and Epstein-Barr virus (EBV) and p53 expression in human esophageal carcinoma. J Pak Med Assoc. 1998;48:138-142. [PubMed] |
| 23. | Anwar K, Nakakuki K, Imai H, Inuzuka M. Infection of human papillomavirus (hpv) and epstein-barr-virus (ebv) and p53 overexpression in human gastric-carcinoma. Int J Oncol. 1995;7:391-397. [PubMed] |
| 24. | Koshiol J, Wei WQ, Kreimer AR, Ren JS, Gravitt P, Chen W, Kim E, Abnet CC, Zhang Y, Kamangar F. The gastric cardia is not a target for human papillomavirus-induced carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2010;19:1137-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Yuan XY, Wang MY, Wang XY, Chang AY, Li J. Non-detection of Epstein-Barr virus and Human Papillomavirus in a region of high gastric cancer risk indicates a lack of a role for these viruses in gastric carcinomas. Genet Mol Biol. 2013;36:183-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Kamangar F, Qiao YL, Schiller JT, Dawsey SM, Fears T, Sun XD, Abnet CC, Zhao P, Taylor PR, Mark SD. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. Int J Cancer. 2006;119:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Lagergren J, Wang Z, Bergström R, Dillner J, Nyrén O. Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case-control study in Sweden. J Natl Cancer Inst. 1999;91:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Strickler HD, Schiffman MH, Shah KV, Rabkin CS, Schiller JT, Wacholder S, Clayman B, Viscidi RP. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur J Cancer Prev. 1998;7:305-313. [PubMed] |
| 29. | Saegusa M, Hashimura M, Takano Y, Ohbu M, Okayasu I. Absence of human papillomavirus genomic sequences detected by the polymerase chain reaction in oesophageal and gastric carcinomas in Japan. Mol Pathol. 1997;50:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 591] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 31. | Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 32. | Kocjan BJ, Maver PJ, Hosnjak L, Zidar N, Odar K, Gale N, Poljak M. Comparative evaluation of the Abbott RealTime High Risk HPV test and INNO-LiPA HPV Genotyping Extra test for detecting and identifying human papillomaviruses in archival tissue specimens of head and neck cancers. Acta Dermatovenerol Alp Panonica Adriat. 2012;21:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |