Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6594
Revised: February 17, 2014
Accepted: March 7, 2014
Published online: June 7, 2014
Processing time: 157 Days and 3.6 Hours
AIM: To investigate that inflammatory markers can predict accurately the prognosis of hepatocelluar carcinoma (HCC) patients in living-donor liver transplantation (LDLT).
METHODS: From October 2000 to November 2011, 224 patients who underwent living donor liver transplantation for HCC at our institution were enrolled in this study. We analyzed disease-free survival (DFS) and overall survival (OS) after LT in patients with HCC and designed a new score model using pretransplant neutrophil-lymphocyte ratio (NLR) and C-reactive protein (CRP).
RESULTS: The DFS and OS in patients with an NLR level ≥ 6.0 or CRP level ≥ 1.0 were significantly worse than those of patients with an NLR level < 6.0 or CRP level < 1.0 (P = 0.049, P = 0.003 for NLR and P = 0.010, P < 0.001 for CRP, respectively). Using a new score model using the pretransplant NLR and CRP, we can differentiate HCC patients beyond the Milan criteria with a good prognosis from those with a poor prognosis.
CONCLUSION: Combined with the Milan criteria, new score model using NLR and CRP represent new selection criteria for LDLT candidates with HCC, especially beyond the Milan criteria.
Core tip: Although the Milan criteria are accepted as the standard selection criteria for liver transplantation candidates with hepatocelluar carcinoma (HCC), they are so strict; New selection criteria are needed to predict more accurately the prognosis of patients with HCC; Using a new score model using pretransplant neutrophil-lymphocyte ratio (NLR) and C-reactive protein (CRP), we can differentiate HCC patients beyond the Milan criteria with a good prognosis from those with a poor prognosis; Combined with the Milan criteria, a new score model using pretransplant NLR and CRP may represent new selection criteria for living-donor liver transplantation candidates with HCC, especially beyond the Milan criteria.
- Citation: Na GH, Kim DG, Han JH, Kim EY, Lee SH, Hong TH, You YK. Inflammatory markers as selection criteria of hepatocellular carcinoma in living-donor liver transplantation. World J Gastroenterol 2014; 20(21): 6594-6601
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6594.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6594
Among the several treatment modalities for hepatocellular carcinoma (HCC), liver transplantation (LT) and surgical resection are curative methods. Chronic liver disease is one of the main etiologies of HCC; approximately 80% of patients with HCC have cirrhosis[1]. LT is an ideal treatment for selected patients with HCC because it targets not only the tumor but also the underlying liver disease[2]. Since the introduction of the Milan criteria by Mazzaferro et al[3] in 1996, disease-free survival and overall survival after LT for patients with HCC meeting the Milan criteria have been equivalent to those of non-HCC patients. Although survival rates after LT have improved dramatically, HCC recurrence remains a significant problem. It has been demonstrated that about 10% of HCC patients within the Milan criteria experience HCC recurrence[4]. In contrast, some patients with HCC beyond the Milan criteria may have favorable outcomes[5]. There are some limitations to the Milan criteria that should be addressed in any new standard selection criteria for HCC patients. Inflammation is a critical component of tumor progression[6]. Several inflammatory markers, such as the neutrophil-lymphocyte ratio (NLR) and C-reactive protein (CRP), have been suggested as surrogate markers of treatment response and survival in patients with HCC[7-9]. However, few studies have examined the relationship between these factors and HCC recurrence after living-donor liver transplantation (LDLT).
The aim of the present study was to assess whether the pretransplant NLR and CRP levels can accurately predict disease-free survival and overall survival after LDLT in patients with HCC. Furthermore, we established a pretransplant score model that may assist in the selection of patients with HCC that would benefit from liver transplantation.
From October 2000 to November 2011, a total of 243 patients underwent LDLT for HCC at our transplant center. Nineteen patients were excluded from the study: 7 for postoperative mortality within 30 d after transplantation, 6 for preoperative infection, 4 for undergoing pretransplant locoregional treatment within 1 mo before transplantation, and 2 for massive alimentary tract bleeding within 1 mo before transplantation. After excluding these nineteen cases, the medical records of 224 patients were reviewed retrospectively. This study was approved by the Institutional Review Board of our center.
All patients who were to undergo transplantation for HCC were evaluated preoperatively by dynamic liver computed tomography (CT) and enhanced magnetic resonance imaging (MRI). Chest CT, bone scan, and positron emission tomography CT (PET CT) were performed to exclude distant metastasis and other primary malignancies. Contraindications for LT in patients with HCC included a tumor thrombus in the main portal vein, regional lymph node metastasis, and distant metastasis. The alpha-fetoprotein (AFP) level and the proteins induced by vitamin K absence or angiotensin-II (PIVKA-II) level were evaluated as tumor markers. Hepatitis viral markers and liver function tests were also assessed. Neutrophil and lymphocyte counts were routinely measured on the day before transplantation, with the NLR calculated by dividing the neutrophil count by the lymphocyte count. The serum CRP level was measured with a turbidimetric immunoassay (Wako Chemicals GmbH, Neuss, Germany). The pretransplant CRP level was not measured routinely until 2006 and then was checked routinely on the day before transplantation. When HCC was diagnosed, the treatment was based on the tumor stage and the patient’s liver function. Patients who were eligible for transplantation underwent DDLT or LDLT according to the meeting the Milan criteria and the availability of liver donor. Pretransplant locoregional treatments include hepatic resection, transarterial chemoembolization (TACE), radiofrequency ablation, and percutaneous ethanol injection, which were selected according to the Barcelona Clinic Liver Cancer scoring system. TACE was the primary treatment modality among pretransplant locoregional treatment modalities.
LDLT was performed according to a standard technique using a modified right lobe with middle hepatic vein reconstruction. For patients with ascites, aspiration and cytology were performed before beginning the operation. When lymph node enlargement was present, or in cases with suspicious metastatic disease, an intraoperative biopsy was performed. The operation was performed only in cases with negative biopsy results.
Immunosuppression regimens consisted of a triple drug regimen that included tacrolimus or cyclosporin, mycophenolatemofetil (MMF), and prednisolone. The dose of tacrolimus was adjusted to maintain levels of 7-10 ng/mL for the first postoperative month and 5-7 ng/mL thereafter. The dose of cyclosporin was adjusted to maintain levels of 100-150 ng/mL for the first postoperative month and 50-100 ng/mL thereafter. Steroids were withdrawn 1 mo after surgery, and MMF was withdrawn 6 mo after surgery. An interleukin-2 receptor blocker was administered on both the day of surgery and the fourth postoperative day.
Patients were followed weekly after hospital discharge until they were stable and then monthly for the first year, every 2 mo for 5 years, and then every 3 mo. Tumor markers were measured monthly during the first year, and then every 2 mo thereafter. Abdomen CT, chest CT, and bone scintigraphy were routinely performed every 4 mo for the first year, every 6 mo for the next year, and then annually. When tumor recurrence was suspected, MRI and/or PET-CT were performed.
Continuous variables are reported as mean ± SD and were compared using the Student t test. Categorical variables were analyzed using the χ2 test. To evaluate the risk factors for HCC recurrence, univariate analysis of risk factors was performed using the Kaplan-Meier method and evaluated using the log-rank test. Candidate predictors associated with a P value less than 0.2 on univariate analysis were entered into a multivariate analysis using Cox regression analysis. The CRP level was excluded from the multivariate analysis because the number of patients with an available CRP level was small compared with other clinico-pathological variables. Subgroup analysis by the Milan criteria was also conducted. Overall survival and disease-free survival were calculated using the Kaplan-Meier method and evaluated with the log-rank test. Statistical analysis was performed using SPSS (Chicago, IL, United States) 18.0 for Windows. A P value < 0.05 was considered to indicate statistical significance.
Among the 224 patients, 184 (82.1%) were male, and the mean age was 51.9 ± 6.9 years. The most common cause for LT was hepatitis B (n = 197, 87.9%), followed by hepatitis C (n = 13, 5.8%) and other causes (n = 14, 6.3%). Pretransplant locoregional treatments for HCC were performed in 167 patients (74.6%). Of the 224 patients, 133 (59.4%) met the Milan criteria. The mean Child-Pugh score was 8.2 ± 2.4, and the mean Model for End-stage Liver Disease (MELD) score was 12.8 ± 7.6 (Table 1).
| Parameter | Value |
| Mean age (yr)1 | 51.9 ± 6.9 |
| Male | 184 (82.1) |
| Diagnosis | |
| Hepatitis B | 197 (87.9) |
| Hepatitis C | 13 (5.8) |
| Others | 14 (6.3) |
| GRWR1 | 1.21 ± 0.27 |
| Child pugh score1 | 8.2 ± 2.4 |
| MELD score1 | 12.8 ± 7.6 |
| AFP (ng/mL)1 | 183.4 ± 762.7 |
| PIVKA-II (mAU/mL)1 | 206.5 ± 1118.7 |
| C-reactive protein (mg/dL)1 | 1.36 ± 2.74 |
| Neutrophil lymphocyte ratio1 | 3.47 ± 4.68 |
| Pretransplant treatment for HCC | 167 (74.6) |
| Tumor number1 | 2.6 ± 2.4 |
| Maximum tumor size (cm)1 | 3.2 ± 3.1 |
| Microvascular invasion | 44 (21.3) |
| Edmondson-steiner grade (III-IV) | 81 (42.9) |
| Within the milan criteria | 133 (59.4) |
| Follow-up duration (mo)1 | 48.9 ± 37.3 |
| (median: 68, range: 6-139) |
The median follow-up period was 68 mo (range, 6-139 mo). The 1, 3, and 5 year overall survival rates were 88.5%, 78.0% and 76.6%, respectively. During the follow-up period, 50 patients (22.3%) died. The cause of death was HCC recurrence in 31 patients (62.0%), technical complications in nine patients (18.0%), sepsis in five patients (10.0%), graft failure in three patients (6.0%), and other causes in two patients (4.0%). The 1, 3, and 5 year disease-free survival rates were 88.3%, 83.3% and 81.6%, respectively. Most HCC recurrences (n = 30, 81.1%) occurred within 2 years, with 26 patients (70.3%) experiencing HCC recurrence within 1 year. Two patients (5.4%) experienced HCC recurrence 5 years after transplantation.
To determine whether an elevated NLR level was correlated with HCC recurrence after LDLT, we performed the Kaplan-Meier analysis with the log-rank test. Using NLR cut-offs of 1, 2, 3, 4, 5, 6 and 7 and comparing disease-free survival and overall survival rates, we showed that an NLR of 6 was the most significant, with a χ2 value of 3.497 and a P value of 0.049 for disease-free survival and a χ2 value of 8.799 and a p value of 0.003 for overall survival (Table 2). Of the 224 patents, 27 (12.1%) had an NLR ≥ 6.0. Total bilirubin level (P = 0.006), Child-Pugh score (P < 0.001), MELD score (P < 0.001), and CRP level (P = 0.035) were significantly different between the patients with an NLR level ≥ 6.0 and those with an NLR level < 6.0. Tumor number and maximum tumor size, tumor biologic factors such as microvascular invasion and tumor grade, and tumor markers were not significantly different between the two groups of patients. Also, the NLR level was significantly correlated with the total bilirubin level (r = 0.384, P < 0.001), Child-Pugh score (r = 0.268, P < 0.001), MELD score (r = 0.419, P < 0.001), and CRP level (r = 0.220, P = 0.008) (Table 3).
| Disease-free survival | Overall survival | |||
| χ2 | P value | χ2 | P value | |
| Neutrophil-lymphocyte ratio (n = 224) | ||||
| NLR ≥ 1 (n = 195) | 1.041 | 0.308 | 0.125 | 0.724 |
| NLR ≥ 2 (n = 115) | 2.938 | 0.087 | 2.777 | 0.096 |
| NLR ≥ 3 (n = 70) | 0.746 | 0.388 | 3.308 | 0.069 |
| NLR ≥ 4 (n = 44) | 1.132 | 0.287 | 5.301 | 0.021 |
| NLR ≥ 5 (n = 34) | 2.383 | 0.123 | 7.257 | 0.007 |
| NLR ≥ 6 (n = 27) | 3.497 | 0.049 | 8.799 | 0.003 |
| NLR ≥ 7 (n = 22) | 1.379 | 0.240 | 6.411 | 0.001 |
| C-reactive protein (n = 145) | ||||
| CRP ≥ 0.5 (n = 72) | 1.359 | 0.244 | 4.032 | 0.045 |
| CRP ≥ 1.0 (n = 42) | 6.653 | 0.010 | 12.604 | < 0.001 |
| CRP ≥ 2.0 (n = 25) | 6.974 | 0.008 | 6.728 | 0.009 |
| Parameters | NLR < 6 | NLR≥6 | P value | CRP < 1 | CRP≥1 | P value |
| (n = 197) | (n = 27) | (n = 103) | (n = 42) | |||
| Mean age (yr)1 | 51.8 ± 7.0 | 52.2 ± 6.3 | 0.748 | 52.4 ± 6.9 | 52.4 ± 6.8 | 0.992 |
| Male | 164 (83.2) | 20 (74.1) | 0.243 | 81 (78.6) | 35 (83.3) | 0.522 |
| Etiology (HBV) | 172 (87.3) | 25 (92.6) | 0.345 | 90 (87.4) | 37 (88.1) | 0.991 |
| GRWR1 | 1.20 ± 0.27 | 1.24 ± 0.30 | 0.495 | 1.21 ± 0.29 | 1.23 ± 0.26 | 0.777 |
| Total bilirubin (g/dL)1 | 3.8 ± 6.8 | 11.1 ± 12.5 | 0.006 | 3.2 ± 5.8 | 11.5 ± 12.2 | < 0.001 |
| PT INR1 | 2.6 ± 15.3 | 1.7 ± 0.5 | 0.771 | 1.4 ± 0.3 | 6.6 ± 33.2 | 0.293 |
| Child Pugh score1 | 7.9 ± 2.3 | 9.8 ± 2.2 | < 0.001 | 7.8 ± 2.4 | 9.9 ± 1.9 | < 0.001 |
| MELD score1 | 11.7 ± 6.8 | 20.6 ± 8.8 | < 0.001 | 10.9 ± 6.0 | 19.4 ± 8.9 | < 0.001 |
| AFP (ng/mL)1 | 142 ± 366 | 490 ± 1995 | 0.383 | 128 ± 381 | 346 ± 1593 | 0.389 |
| NLR1 | 2.12 ± 1.19 | 13.3 ± 7.79 | < 0.001 | 2.54 ± 2.71 | 6.15 ± 7.37 | 0.003 |
| CRP (mg/dL)1 | 1.03 ± 2.13 | 3.55 ± 4.76 | 0.035 | 0.33 ± 0.29 | 3.87 ± 4.13 | < 0.001 |
| Tumor number1 | 2.5 ± 2.3 | 3.3 ± 2.8 | 0.162 | 2.7 ± 2.4 | 2.4 ± 2.1 | 0.352 |
| Maximal tumor size1 | 3.10 ± 2.37 | 4.03 ± 6.13 | 0.441 | 2.9 ± 1.5 | 4.1 ± 5.8 | 0.475 |
| Microvascular invasion (+) | 38 (21.0) | 6 (23.1) | 0.800 | 20 (21.1) | 6 (15.0) | 0.225 |
| E-S grade (III-IV) | 94 (57.3) | 14 (56.0) | 0.901 | 38 (44.7) | 17 (45.9) | 0.415 |
| Beyond the Milan criteria | 69 (36.5) | 14 (51.9) | 0.125 | 39 (39.0) | 15 (36.6) | 0.899 |
| Pretransplant locoregional treatment | 146 (74.1) | 21 (77.8) | 0.682 | 81 (78.6) | 30 (71.4) | 0.352 |
To determine whether the elevated CRP level was correlated with HCC recurrence after LDLT, we performed the Kaplan-Meier analysis with the log-rank test. Using CRP cut-offs of 0.5, 1.0 and 2.0 and comparing disease-free survival and overall survival rates, we showed that a CRP of 1.0 was the most significant, with a χ2 value of 6.653 and a P value of 0.010 for disease-free survival and a χ2 value of 12.604 and a P value of less than 0.001 for overall survival (Table 2). Of the 145 patents, 42 (29.0%) had a CRP ≥ 1.0. Total bilirubin level (P < 0.001), Child-Pugh score (P < 0.001), MELD score (P < 0.001), and NLR level (P = 0.003) were significantly different between patients with a CRP level ≥ 1.0 and those with a CRP level < 1.0, as with the results for NLR. Tumor number and maximum tumor size, tumor biologic factors such as microvascular invasion and tumor grade, and tumor markers were not significantly different between the two groups of patients. Also, the CRP level was significantly correlated with the total bilirubin level (r = 0.207, P = 0.012), Child-Pugh score (r = 0.216, P = 0.009), MELD score (r = 0.272, P = 0.001), the tumor number (r = 0.415, P < 0.001), and the NLR level (r = 0.220, P = 0.008) (Table 3).
On univariate analysis, the NLR level ≥ 6.0 (P = 0.049), CRP level ≥ 1.0 (P = 0.010), AFP ≥ 100 (P = 0.015), pretransplant locoregional treatment (P = 0.017), tumor size ≥ 5 cm (P < 0.001), and microvascular invasion (P = 0.024) were significantly associated with HCC recurrence after LDLT. According to multivariate analysis, AFP ≥ 100 (HR= 2.588, 95%CI: 1.187-5.645, P = 0.017) and tumor size ≥ 5 cm (HR = 6.014; 95%CI: 2.432-14.869, P < 0.001) were independent risk factors for HCC recurrence after LDLT. An NLR level ≥ 6.0 was a significant risk factor for tumor recurrence with marginal significance (HR = 2.512; 95%CI: 0.987-6.391, P = 0.053). Also, an NLR level ≥ 6.0 (P = 0.003), CRP level ≥ 1.0 (P < 0.001), AFP ≥ 100 (P = 0.048), pretransplant locoregional treatment (P = 0.023), and tumor size ≥ 5 cm (P = 0.001) were significantly associated with overall survival after LDLT on univariate analysis. An NLR level ≥ 6.0 was an only independent prognostic factor for poor survival on multivariate analysis (HR = 2.896; 95%CI: 1.399-5.996, P = 0.004) (Table 4).
| Disease free survival | Overall survival | |||||
| Univariate | Multivariate analysis | Univariate | Multivariate analysis | |||
| P value | HR (95%CI) | P value | P value | HR (95%CI) | P value | |
| Age ≥ 60 | 0.404 | 0.723 | ||||
| Male gender | 0.196 | 2.086 (0.595-7.307) | 0.250 | 0.411 | ||
| Etiology (HBV) | 0.977 | 0.775 | ||||
| GRWR ≥ 1.0 | 0.551 | 0.639 | ||||
| Child C | 0.978 | 0.810 | ||||
| AFP ≥ 100 | 0.015 | 2.588 (1.187-5.645) | 0.017 | 0.048 | 1.567 (0.822-2.987) | 0.172 |
| NLR ≥ 6 | 0.049 | 2.512 (0.987-6.391) | 0.053 | 0.003 | 2.896 (1.399-5.996) | 0.004 |
| Pretransplant locoregional treatment | 0.017 | 4.604 (1.074-19.740) | 0.040 | 0.023 | 1.946 (0.804-4.713) | 0.140 |
| Tumor number ≥ 2 | 0.604 | 0.913 | ||||
| Tumor size ≥ 5 cm | < 0.001 | 6.014 (2.432-14.869) | < 0.001 | 0.001 | 2.206 (0.968-5.028) | 0.060 |
| Microvascular invasion (+) | 0.024 | 1.369 (0.599-3.132) | 0.456 | 0.074 | 1.554 (0.769-3.141) | 0.220 |
| E-S grade (III-IV) | 0.172 | 1.040 (0.470-2.302) | 0.923 | 0.082 | 1.371 (0.715-2.630) | 0.342 |
| CRP ≥ 1.0 (n = 145) | 0.010 | < 0.001 | ||||
We analyzed disease-free survival and overall survival for patients according to the Milan criteria. For 133 patients (59.4%) within the Milan criteria, an NLR level ≥ 6.0 was associated with decreased overall survival (P = 0.037) but was not associated with disease-free survival (P = 0.541). A CRP level ≥ 1.0 was not associated with disease-free survival (P = 0.797) but was associated with decreased overall survival, but with only marginal significance (P = 0.054). A tumor size ≥ 5 cm was an only independent prognostic factor for HCC recurrence on multivariate analysis (HR = 6.980; 95%CI: 1.497-32.535), P = 0.013) (Table 5).
| Disease free survival | Overall survival | |||||
| Univariate | Multivariate analysis | Univariate | Multivariate analysis | |||
| P value | HR (95%CI) | P value | P value | HR (95%CI) | P value | |
| Within the Milan criteria | ||||||
| AFP ≥ 100 | 0.475 | 0.471 | ||||
| NLR ≥ 6 | 0.541 | 0.037 | 2.509 (0.945-6.658) | 0.065 | ||
| Pretransplant locoregional treatment | 0.193 | 2.126 (0.587-7.710) | 0.251 | 0.257 | ||
| Tumor number ≥ 2 | 0.764 | 0.459 | ||||
| Tumor size ≥ 5 cm | < 0.001 | 6.980 (1.497-32.535) | 0.013 | 0.681 | ||
| Microvascular invasion (+) | 0.619 | 0.307 | ||||
| E-S grade (III-IV) | 0.835 | 0.592 | ||||
| CRP ≥ 1.0 (n = 145) | 0.797 | 0.054 | ||||
| Beyond the Milan criteria | ||||||
| AFP ≥ 100 | 0.058 | 3.619 (1.184-11.063) | 0.024 | 0.082 | 2.456 (0.888-6.793) | 0.083 |
| NLR ≥ 6 | 0.034 | 3.973 (1.288-12.249) | 0.016 | 0.045 | 4.685 (1.607-13.657) | 0.005 |
| Pretransplant locoregional treatment | 0.096 | 0.972 | 0.086 | 0.971 | ||
| Tumor number ≥ 2 | 0.001 | 0.429 (0.108-1.701) | 0.228 | 0.016 | 0.493 (0.121-2.001) | 0.322 |
| Tumor size ≥ 5 cm | < 0.001 | 3.753 (0.901-15.626) | 0.069 | 0.003 | 2.354 (0.587-9.449) | 0.227 |
| Microvascular invasion (+) | 0.226 | 0.318 | ||||
| E-S grade (III-IV) | 0.083 | 2.191 (0.697-6.884) | 0.179 | 0.067 | 2.618 (0.895-7.651) | 0.079 |
| CRP ≥ 1.0 (n = 145) | 0.004 | 0.001 | ||||
For 91 patients (40.6%) beyond the Milan criteria, NLR level ≥ 6.0 (HR = 3.973; 95%CI: 1.288-12.249, P = 0.016) and AFP ≥ 100 (HR = 3.619; 95%CI: 1.184-11.063, P = 0.024) were independent risk factors for HCC recurrence after LDLT. An NLR level ≥ 6.0 was an only independent prognostic factor for poor survival on multivariate analysis (HR = 4.685; 95%CI: 1.607-13.657, P = 0.005). A CRP level ≥ 1.0 was significantly associated with both disease-free survival (P = 0.004) and overall survival (P = 0.001) on univariate analysis (Table 5).
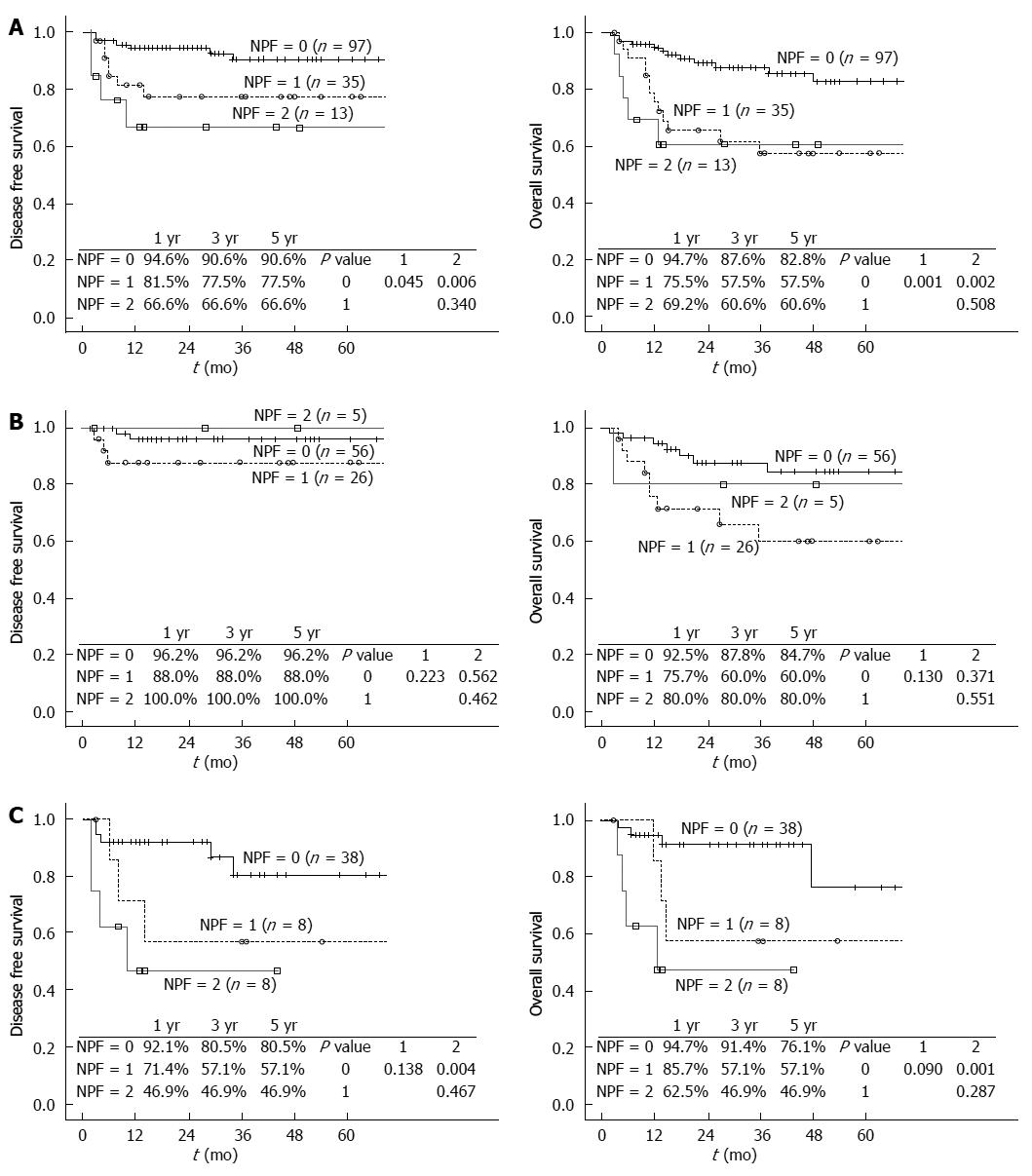
We established a pretransplant new prognostic factor score model based on the results for NLR and CRP. Each factor (NLR level ≥ 6.0 or CRP level ≥ 1.0) was given a score of 1, after which patients were divided into three groups according to prognostic scores: those with NLR level < 6.0 and CRP level < 1.0 [score 0, n = 97 (66.9%)], those with NLR level ≥ 6.0 or CRP level ≥ 1.0 [score 1, n = 35 (24.1%)], and those with NLR level ≥ 6.0 and CRP level ≥ 1.0 [score 2, n = 13 (9.0%)]. The disease-free survival for patients with a score of 1 or 2 was significantly lower than that for patients with a score of 0 (P = 0.045 and P = 0.006, respectively). The overall survival for patients with a score of 1 or 2 was also significantly lower than that for patients with a score of 0 (P = 0.001 and P = 0.002, respectively). For patients meeting the Milan criteria, the disease-free survival and the overall survival were not significantly different according to the prognostic score model using NLR and CRP. For patients beyond the Milan criteria, the disease-free survival and the overall survival were significantly superior in patients with a score of 0 compared to those in patients with a score of 2 (P = 0.004 and P = 0.001, respectively) (Figure 1).
LT is considered an ideal treatment for selected patients with HCC because it can treat not only the tumor but also the underlying liver disease. Since the introduction of the Milan criteria by Mazzaferro et al[3] in 1996, the disease-free survival and overall survival after LT for patients with HCC meeting the Milan criteria have been equivalent to those of non-HCC patients. LT has become the treatment of choice for cirrhotic patients with HCC. However, there are some limitations in the Milan criteria that must be adopted in the standard selection criteria for HCC patients. First, the Milan criteria depend exclusively on tumor size and number of pretransplant radiologic evaluations. Many reports have demonstrated that tumor biology such as microvascular invasion and tumor differentiation[10,11] and pretransplant tumor markers such as AFP and PIVKA-II[12,13] affect HCC recurrence after LT. Second, because the Milan criteria are so strict, many patients who may benefit from LT will be rejected. Many centers have reported good results despite expansion of the selection criteria[14,15]. Therefore, it is important to establish more appropriate selection criteria for LT candidates with HCC. The aim of the present study was to identify new factors related to DFS and OS after LT in order to expand the selection criteria in patients with HCC.
Inflammation is a critical component of tumor progression[6,16]. Many cancers generate from sites of infection, chronic irritation and inflammation. This process is thought to be related to upregulation of cytokines and inflammatory mediators. Inflammation promotes the proliferation and survival of tumor cells and angiogenesis and provides a good microenvironment for tumor growth[17]. It is reported that proinflammatory cytokines such as TNF, IL-1, and IL-6 as well as tumor associated macrophages and IL-17 are involved in this inflammatory process[18,19]. However, it is difficult to measure these cytokines and factors routinely before transplantation. NLR and CRP have been suggested as surrogate markers which can be easily measured preoperatively. An elevated NLR level is associated with poor outcomes in patients with several types of malignant tumors, including colorectal cancer[20], pancreatic cancer[21], intrahepatic cholangiocarcinoma[22], and HCC[7]. Furthermore, an elevated NLR is related to a poor prognosis in patients undergoing LT for HCC[23,24]. Also, a high CRP level has been shown to be significantly correlated with a poor outcome in patients undergoing hepatic resection for HCC[25,26].
In the present study, the pretransplant NLR and CRP level were predictive of disease-free survival and overall survival after LT in patients with HCC. These factors correlated with hepatic functional parameters such as the MELD score and Child-Pugh score rather than tumor size and number, and were more relevant to overall survival than disease-free survival. Also, the subgroup analysis using the Milan criteria showed that there were more significant differences in disease-free survival and overall survival of patients with HCC beyond the Milan criteria. Because the Milan criteria that adopted the standard selection criteria for HCC patients are so strict, many patients are excluded from LT who could actually benefit greatly from the transplantation. However, the new score model used in this study may help to identify patients with HCC who could benefit most from LT, beyond the limitations imposed by the Milan criteria. The present study is the first report to analyze NLR and CRP levels together and to compare both factors. A new score model using these factors may help to predict disease-free survival and overall survival after LDLT in patients with HCC. Our findings showed that the CRP level was more powerful than the NLR level in predicting disease-free survival and overall survival after LDLT in patients with HCC. However, in the present study, the sample size for the CRP level was smaller than that for the NLR level. If the sample size for the CRP level was increased, better results may be obtained.
There were some limitations to this study. First, it was of a retrospective design. The pretransplant CRP level was not measured routinely until 2006. Thus, the sample size available for the pretransplant CRP level was smaller than the sample sizes for the other data. Second, pretransplant NLR and CRP levels may have been affected by various clinical factors (including preoperative sepsis, recent pretransplant locoregional treatments, and massive alimentary bleeding). We attempted to reduce these confounding factors that can result in falsely elevated NLR and CRP through careful pretransplant examination.
In conclusion, pretransplant NLR and CRP levels are useful biomarkers to predict disease-free survival and overall survival after LDLT in patients with HCC. These factors are more useful in patients with HCC beyond the Milan criteria than in patients with HCC who meet the Milan criteria. Our score model may assist in the selection of patients with HCC who would benefit from LDLT, but would otherwise have been excluded by the Milan criteria.
Liver transplantation (LT) is an ideal treatment for selected patients with hepatocellular carcinoma (HCC) because it targets not only the tumor but also the underlying liver disease. Although survival rates after LT have improved dramatically, HCC recurrence remains a significant problem. Inflammation is a critical component of tumor progression. Several inflammatory markers, such as the neutrophil-lymphocyte ratio (NLR) and C-reactive protein (CRP), have been suggested as surrogate markers of treatment response and survival in patients with HCC.
Because the Milan criteria that adopted the standard selection criteria for HCC patients are so strict, many patients are excluded from LT who could actually benefit greatly from the transplantation. The aim of the present study was to identify new factors related to disease-free survival and overall survival after LT in order to expand the selection criteria in patients with HCC.
In the present study, the pretransplant NLR and CRP level were predictive of disease-free survival and overall survival after LT in patients with HCC. Also, the subgroup analysis using the Milan criteria showed that there were more significant differences in disease-free survival and overall survival of patients with HCC beyond the Milan criteria.
The score model of this paper may assist in the selection of patients with HCC who would benefit from living donor liver transplantation, but would otherwise have been excluded by the Milan criteria.
This is a retrospective study including 224 living related LTx for HCC with almost 60% meeting the Milan criteria for LTx. The main finding of the study is that by adding some markers of inflammation such as NLR and CPR there is evidence that the Milan criteria may improve. The authors have used statistical methods trying to find cutoffs for NLR and CRP with reliable results. The authors acknowledge the limitations of their study but the results are of particular interest if reproduced in a prospective way.
P- Reviewer: Fourtounas C S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Ishizaki Y, Kawasaki S. The evolution of liver transplantation for hepatocellular carcinoma (past, present, and future). J Gastroenterol. 2008;43:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Moreno P, Jaurrieta E, Figueras J, Benasco C, Rafecas A, Fabregat J, Torras J, Casanovas T, Casais L. Orthotopic liver transplantation: treatment of choice in cirrhotic patients with hepatocellular carcinoma? Transplant Proc. 1995;27:2296-2298. [PubMed] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5312] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 4. | Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 6. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11281] [Article Influence: 490.5] [Reference Citation Analysis (2)] |
| 7. | Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 8. | Zheng Z, Zhou L, Gao S, Yang Z, Yao J, Zheng S. Prognostic role of C-reactive protein in hepatocellular carcinoma: a systematic review and meta-analysis. Int J Med Sci. 2013;10:653-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Cescon M, Bertuzzo VR, Ercolani G, Ravaioli M, Odaldi F, Pinna AD. Liver transplantation for hepatocellular carcinoma: role of inflammatory and immunological state on recurrence and prognosis. World J Gastroenterol. 2013;19:9174-9182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 12. | Pérez-Saborido B, de los Galanes SJ, Menéu-Díaz JC, Romero CJ, Elola-Olaso AM, Suárez YF, Valencia VB, Moreno-González E. Tumor recurrence after liver transplantation for hepatocellular carcinoma: recurrence pathway and prognostic factors. Transplant Proc. 2007;39:2304-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Figueras J, Ibañez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, Mir J, Loinaz C, Herrera L, López-Cillero P. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl. 2001;7:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 313] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5765] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 17. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8326] [Article Influence: 489.8] [Reference Citation Analysis (0)] |
| 18. | Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 19. | Brodsky SV, Mendelev N, Melamed M, Ramaswamy G. Vascular density and VEGF expression in hepatic lesions. J Gastrointestin Liver Dis. 2007;16:373-377. [PubMed] |
| 20. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 844] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 21. | Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 24. | Xiao GQ, Liu C, Liu DL, Yang JY, Yan LN. Neutrophil-lymphocyte ratio predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. World J Gastroenterol. 2013;19:8398-8407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K, Takenaka K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Nagaoka S, Yoshida T, Akiyoshi J, Akiba J, Torimura T, Adachi H, Kurogi J, Tajiri N, Inoue K, Niizeki T. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int. 2007;27:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |