Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6580
Revised: December 20, 2013
Accepted: January 19, 2014
Published online: June 7, 2014
Processing time: 225 Days and 6.4 Hours
AIM: To identify the subset of patients with stage IB gastric cancer with an unfavorable prognosis.
METHODS: Overall survival (OS) rates were examined in 103 patients with stage IB (T1N1M0 and T2N0M0) gastric cancer between January 2000 and December 2011. Univariate and multivariate analyses were performed to identify risk factors using a Cox proportional hazards model.
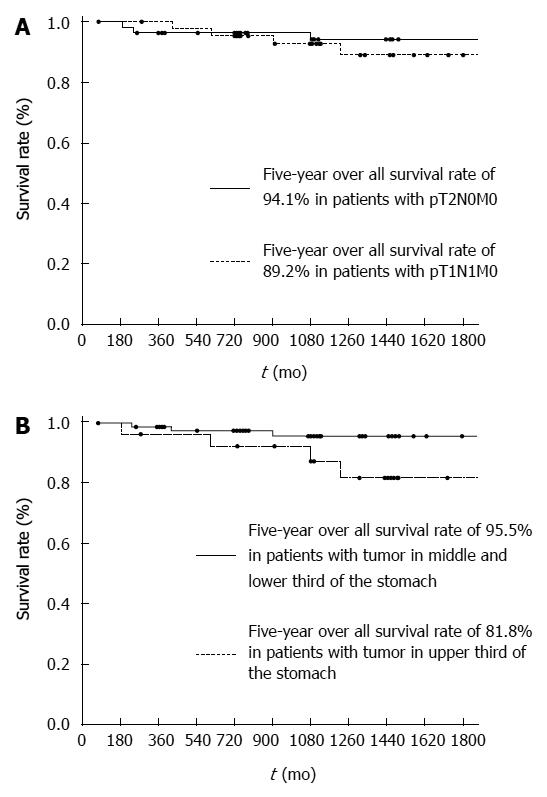
RESULTS: The OS rates of patients with T1N1 and T2N0 cancer were 89.2% and 94.1% at 5-years, respectively. Both univariate and multivariate analyses demonstrated that tumor location was the only significant prognostic factor. The OS rate was 81.8% at 5-years when the tumor was located in the upper third of the stomach and was 95.5% at 5-years when the tumor was located in the middle or lower third of the stomach (P = 0.0093).
CONCLUSION: These data may suggest that tumor location is associated with survival in patients with stage IB gastric cancer.
Core tip: This study identified the subset of patients with stage IB gastric cancer with an unfavorable prognosis. Overall survival (OS) rates were examined in 103 patients with stage IB gastric cancer. Both univariate and multivariate analyses demonstrated that tumor location was the only significant prognostic factor. The OS rate was 81.8% at 5-years when the tumor was located in the upper third of the stomach and was 95.5% at 5-years when the tumor was located in the middle or lower third of the stomach (P = 0.0093). Our data may suggest that tumor location is associated with survival in patients with stage IB gastric cancer.
- Citation: Aoyama T, Yoshikawa T, Fujikawa H, Hayashi T, Ogata T, Cho H, Yamada T, Hasegawa S, Tsuchida K, Yukawa N, Oshima T, Oba MS, Morita S, Rino Y, Masuda M. Prognostic factors in stage IB gastric cancer. World J Gastroenterol 2014; 20(21): 6580-6585
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6580
Gastric cancer is the fourth most common malignant disease and the second most frequent cause of cancer-related death worldwide[1]. East Asia has the highest occurrence of gastric cancer. It was shown from mass-screening programs and examinations that stage I gastric cancer has increased by up to 50% over the past two decades in Japan and South Korea[2].
Stage I gastric cancer is divided into IA and IB according to the third English edition of the Japanese Classification of Gastric Carcinoma. Five-year survival rate was reportedly 95.1% in stage IA and 88.9% in stage IB following surgery alone (90.2% in T2N0 and 87.6% in T1N1)[3]. Standard treatment for stage I is defined as surgery alone according to Japanese gastric cancer treatment guidelines[4]. Although the prognosis of stage IB is excellent, some patients recur even after curative surgery. Once recurrence has developed, the prognosis is limited, and is up to 1 year[5,6].
As a result of the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) phase III trial, S-1 became the first anti-cancer drug to have efficacy as adjuvant chemotherapy for stages 2 and 3 after curative gastrectomy for gastric cancer[7]. In updated results of the ACTS-GC phase III trial, the 5-year overall survival of stage 2 was 71.3% in the surgery alone group, but reached 84.2% in the S-1 group[8]. In the subset analyses, hazard ratio was lower in stage 2 than stage 3 and in node-negative disease than node-positive disease. Thus, S-1 was more effective, especially for relatively early disease. If we know which patients with stage IB recur after surgery, S-1 could prevent recurrence. However, prognostic factors of stage IB have not yet been fully clarified. The aim of the present study was to identify the subset of patients with stage IB gastric cancer with an unfavorable prognosis.
The patients were selected from the prospective database of the Kanagawa Cancer Center, Department of Gastrointestinal Surgery, Yokohama, Japan, according to the following criteria: (1) the patients had histologically-proven gastric adenocarcinoma; (2) the patients underwent curative resection for gastric cancer as a primary treatment between January 2000 and December 2011; (3) the patients with stage IB (T1N1M0 or T2N0M0) disease were diagnosed pathologically according to the third English edition of the Japanese Classification of Gastric Carcinoma[9]; and (4) the patients did not receive any adjuvant chemotherapy after surgery.
All patients underwent total or distal gastrectomy with D1+ or D2 lymph node dissection in accordance with the Japanese gastric cancer treatment guidelines published in 2010 (ver. 3)[4]. In distal gastrectomy, resected nodes included # 1, 3, 4sb, 4d, 5, 6, 7, 8a, and 9 in D1+ plus # 11p and 12a in D2. In total gastrectomy, resected nodes included # 1, 2, 3, 4sa, 4sb, 4d, 5, 6, 7, 8a, 9, and 11p in D1+ plus #10, 11d, and 12a in D2. In principal, D1+ lymphadenectomy was indicated for patients with cT1N0 tumors other than those for whom endoscopic mucosal resection or endoscopic submucosal dissection were recommended. D2 lymphadenectomy was indicated for patients with potentially curable T2-T4 tumors, as well as for those with cT1N+ tumors.
The patients received follow-up visits at outpatient clinics. Hematological tests and physical examinations were performed at least every three months for five years after surgery. CEA and CA19-9 tumor marker levels were determined at least every three months for five years. The patients underwent a computer tomography examination every six months during the first three years after surgery, and then every year for a further two years. The patients who received distal gastrectomy underwent an endoscopic examination every year for five years after the surgery.
The staging and clinicopathological characteristics of the tumors were based on the third English edition of the Japanese Classification of Gastric Carcinoma[9]. Overall survival (OS) was defined as the period between surgery and the occurrence of death. The data for patients who did not experience an event were treated as censored cases on the date of the final observation.
The OS curves were calculated using the Kaplan-Meier method and compared by the log-rank test. Cox’s proportional hazard model was used to perform univariate and multivariate analyses. The survival data were obtained from hospital records or from the city registry system. A P value of < 0.05 was defined as statistically significant. The SPSS software package (v11.0J Win, SPSS, Chicago, IL, United States) was used for all statistical analyses.
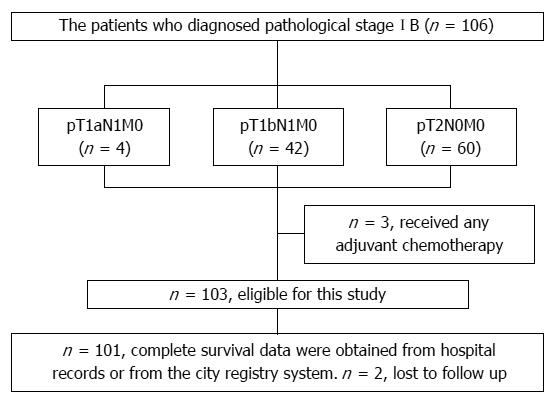
Between January 2000 and December 2011, a total of 106 patients underwent surgical resection and were diagnosed with pathological stage IB gastric cancer. The details of the stage IB patients are shown in Figure 1. Among these patients, 103 were eligible for the present study. Three patients were excluded from the study because they received postoperative adjuvant chemotherapy. The patients’ median age was 64 years (range 34 to 86 years). Sixty-five patients were male and 38 were female. The pathological stage was classified as T1N1 in 45 patients and T2N0 in 58 patients. Table 1 shows patient characteristics in the T1N1 group and T2N0 group. Fifty-four patients received D1+ lymph node resection and 52 patients received D2 lymph node resection. The median follow-up period was 49.1 mo (range 2.7 to 103.6 mo). None of the patients died within 30 d after surgery. Two patients were lost from follow up. Five patients died due to recurrence during the study period. Of 5 patients with metastases, lymph node metastasis was observed in 3 patients and liver metastasis in 2 patients.
| Characteristics | pT1N1M0 group(n = 45) | pT2N0M0 group(n = 58) | P value |
| Age (yr) | 0.433 | ||
| < 70 | 32 | 37 | |
| ≥ 70 | 13 | 21 | |
| Gender | 0.510 | ||
| Male | 30 | 35 | |
| Female | 15 | 23 | |
| Performance status (ECOG) | 0.208 | ||
| 0 | 45 | 56 | |
| 1 | 0 | 2 | |
| Site of tumor | 0.719 | ||
| Upper third | 11 | 16 | |
| Middle third | 24 | 22 | |
| Lower third | 10 | 20 | |
| Maximal tumor diameter (mm) | 0.281 | ||
| ≤ 20 | 7 | 4 | |
| > 20, ≤ 40 | 22 | 27 | |
| > 40 | 16 | 27 | |
| Histological type | 0.931 | ||
| Differentiated | 19 | 24 | |
| Undifferentiated | 26 | 34 | |
| Lymphatic invasion | 0.010 | ||
| Negative | 26 | 47 | |
| Positive | 19 | 11 | |
| Vascular invasion | 0.256 | ||
| Negative | 32 | 35 | |
| Positive | 13 | 23 | |
The OS rates of the patients with T1N1 and T2N0 were 92.7% and 96.5% at 3-years, 89.2% and 94.1% at 5-years, respectively (Figure 2A). There was no significant difference between the two groups (P = 0.6738). Therefore, we grouped the patients with T1N1 and T2N0 disease together. Further prognostic analyses were then focused on the patients with stage T1N1 and T2N0 cancer. When the OS rates were stratified according to each clinical factor, a marginal difference was observed for tumor location (Table 2). Each clinicopathological factor was categorized as shown in Table 3 and analyzed for prognostic significance. Both univariate and multivariate analyses of the OS rates demonstrated that tumor location was the only significant prognostic factor (Table 3). The OS rates were 87.3% at 3-years and 81.8% at 5-years when the tumors were located at the upper third of the stomach and were 95.5% at 3-years and 95.5% at 5-years when the tumors were located at the middle or lower third of the stomach (P = 0.0093) (Figure 2B). Recurrence was observed in 3 patients who had tumors located at the upper third of the stomach and in 2 patients with tumors located at the middle or lower third of the stomach.
| Characteristics | No. of patients | 3-yr rate | 5-yr rate | P value |
| Age (yr) | 0.0637 | |||
| < 70 | 69 | 97.0% | 94.7% | |
| ≥ 70 | 34 | 89.7% | 85.2% | |
| Gender | 0.1512 | |||
| Female | 38 | 100% | 95.2% | |
| Male | 65 | 91.8% | 89.8% | |
| Tumor location | 0.0565 | |||
| L | 29 | 91.7% | 91.7% | |
| M | 47 | 97.7% | 97.7% | |
| U | 27 | 88.8% | 84.1% | |
| Pathological tumor diameter (mm) | 0.6106 | |||
| ≤ 20 | 11 | 90.9% | 90.9% | |
| > 20, ≤ 40 | 49 | 95.1% | 91.8% | |
| > 40 | 43 | 95.1% | 91.8% | |
| Histological type | 0.4419 | |||
| Differentiated | 43 | 92.5% | 92.5% | |
| Undifferentiated | 60 | 94.6% | 91.0% | |
| Number of lymph node metastases | 0.9013 | |||
| 0 | 58 | 94.1% | 94.1% | |
| 1 | 33 | 93.4% | 89.1% | |
| 2 | 12 | 90.9% | 90.9% | |
| Lymphatic invasion | 0.1645 | |||
| Negative | 73 | 95.4% | 93.2% | |
| Positive | 30 | 89.3% | 89.3% | |
| Vascular invasion | 0.7861 | |||
| Negative | 67 | 93.3% | 93.3% | |
| Positive | 36 | 93.5% | 89.0% | |
| Characteristics | Number | Univariate | Multivariate | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| Age (yr) | 0.078 | ||||||
| < 70 | 69 | 1.000 | |||||
| ≥ 70 | 34 | 3.147 | 0.879-11.271 | ||||
| Gender | 0.186 | ||||||
| Female | 38 | 1.000 | |||||
| Male | 65 | 4.118 | 0.506-33.527 | ||||
| Tumor location | 0.018 | 0.018 | |||||
| M or L | 76 | 1.000 | 1.000 | ||||
| U | 27 | 5.273 | 1.326-20.969 | 5.273 | 1.326-20.969 | ||
| Pathological tumor diameter (mm) | 0.874 | ||||||
| ≤ 40 | 60 | 1.000 | |||||
| > 40 | 43 | 1.123 | 0.268-4.704 | ||||
| Histological type | 0.482 | ||||||
| Differentiated | 43 | 1.000 | |||||
| Undifferentiated | 60 | 1.608 | 0.428-6.041 | ||||
| Number of lymph node metastases | 0.902 | ||||||
| 0 | 58 | 1.000 | |||||
| 1 | 33 | 1.293 | 0.287-5.830 | ||||
| 2 | 12 | 1.563 | 0.169-14.495 | ||||
| Lymphatic invasion | 0.177 | ||||||
| Negative | 73 | 1.000 | |||||
| Positive | 30 | 2.369 | 0.674-8.512 | ||||
| Vascular invasion | 0.786 | ||||||
| Negative | 67 | 1.000 | |||||
| Positive | 36 | 1.192 | 0.334-4.253 | ||||
The present study identified a subset of patients with stage IB gastric cancer who had unfavorable outcomes. We found that patients with stage T1N1 and T2N0 cancer had similar outcomes. Therefore, we grouped the patients with T1N1 and T2N0 disease together. Further prognostic analyses were then focused on patients with stage T1N1 and T2N0 cancer. In this study, the survival rates of patients with stage IB cancer were clearly associated with tumor location. When the tumors were located in the upper third of the stomach, the 5-year OS rate was only 81.8%, which was poorer than 84.2% in stage 2 patients who received S-1 adjuvant chemotherapy[8]. Thus, surgery alone may not be justified for stage IB when the tumors are located at the upper third of the stomach. According to the subset analysis of the ACTS-GC, S-1 was much more effective against stage II than stage IIIA or stage IIIB cancers[7]. Considering that S-1 was more effective, especially for relatively early disease, adjuvant S-1 could be an option for these patients. Some authors have reported the significance of tumor location in terms of the prognosis of gastric cancer. For example, Piso et al[10] evaluated 532 patients with gastric cancer, and reported that long-term survival was worse in patients with proximal disease than in those with distal tumors. The proximal stomach is a predominant site for undifferentiated-type tumors, which tend to have a poorer prognosis than differentiated-type tumors. Anatomically, the lymphatic drainage is complex, and tumors located in this region can metastasize to almost all lymph nodes except #5. Curative surgery for proximal tumors is D2 total gastrectomy with splenectomy, which is more invasive than that for distal cancer. Although the precise mechanism is unclear, multiple factors, including those described above, could explain why patients with proximal tumors had a poorer survival.
Yokoyama et al[11] previously demonstrated that undifferentiated-type adenocarcinoma was the only risk factor for the recurrence of stage IB gastric cancer. However, there were some differences between the present study and Yokoyama[11]’s study. First, the evaluation of staging was different. We classified stage using the third English edition of the Japanese Classification of Gastric Carcinoma, while Yokoyama et al[11] used the second English edition of the Japanese Classification of Gastric Carcinoma. In addition, Yokoyama et al[11] included T3N0 and T1N2 cancers, which are now classified as stage IIA. Ahn et al[3] analyzed stage-specific survival using the third English edition of the Japanese Classification of Gastric Carcinoma, and reported that the five-year survival was 88.9% for stage IB (90.2% in T1N1 and 87.6% in T2N0, respectively) and 83.1% for stage IIA (84.0% in T1N2 and 82.1% in T3N0, respectively). Thus, survival was worse in the latter cases than in the former. Second, Yokoyama et al[11] included patients who received adjuvant chemotherapy in the analysis. Adjuvant chemotherapy could have affected survival[12,13].
There are some limitations in the present study. First, this was a retrospective single center study with a small sample size. Our findings could have been observed by chance in this series. Moreover, age or lymphatic invasion, which was marginally significant in the present study, might become more significant by increasing the number of patients or by extending the follow-up period. The only way to draw a definite conclusion is to collect recent data from many hospitals. However, our data help to clarify which parameters should be included in such a study. Therefore, we believe that our study will have an important clinical impact. Second, there is bias regarding time in this study. The data were collected between 2000 and 2010 and surgical procedures and perioperative care have changed. For example, the patients received two types of perioperative care, including conventional care before May 2009 and enhanced recovery after surgery after June 2009[14]. These factors might have affected our results.
In conclusion, our data may suggest that tumor location is associated with survival in patients with stage IB gastric cancer. As our study was a retrospective single-center study with a small sample size, a prospective multi-center study is necessary to confirm whether the patients with stage IB gastric tumors located in the upper third of the stomach have a poorer survival than those with tumors in other locations.
Stage I gastric cancer is divided into IA and IB according to the third English edition of the Japanese Classification of Gastric Carcinoma. Standard treatment for stage I is defined as surgery alone according to the Japanese gastric cancer treatment guidelines.
Five-year survival rate is reportedly 95.1% in stage IA and 88.9% in stage IB following surgery alone (90.2% in T2N0 and 87.6% in T1N1). Although the prognosis of stage IB is excellent, some patients recur even after curative surgery. Once recurrence has developed, the prognosis is limited, and is up to 1 year. If the authors know which patients with stage IB recur after surgery, S-1 could prevent recurrence. However, prognostic factors of stage IB have not yet been fully clarified. The aim of the present study was to identify the subset of patients with stage IB gastric cancer with an unfavorable prognosis.
In this study, the survival rates of patients with stage IB cancer were clearly associated with tumor location. When the tumors were located in the upper third of the stomach, the 5-year overall survival rate was only 81.8% which was poorer than 84.2% in stage 2 patients who received S-1 adjuvant chemotherapy.
This study suggested that surgery alone may not be justified for stage IB when tumors are located at the upper third of the stomach. According to the subset analysis of the ACTS-GC, S-1 was much more effective against stage II than stage IIIA or stage IIIB cancers. Considering that S-1 was more effective, especially for relatively early disease, adjuvant S-1 could be an option for these patients.
S-1 is an oral fluoropyrimidine, consisting of tegafur (a prodrug of fluorouracil), 5-chloro-2, 4-dihydropyrimidine (CDHP), and potassium oxonate.
There is still an open question what are the risk factors for the early recurrence of gastric cancer (even when it presents as a limited disease) as these patients might require additional treatment modalities besides the surgery, including but not limited to the adjuvant chemotherapy, intraperitoneal chemotherapy (EPIC, HIPEC), biological therapy, etc. There is a need for a systematic review of the available in the recent literature data on the prognostic factors associated with the early recurrences of early gastric cancer, and the research team has successfully contributed to this field by adding just another important paper.
P- Reviewers: Chen YJ, Dambrauskas Z, Gwang Ha Kim GH, Kayaalp C, Lee WJ S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Liu XM
| 1. | Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006;24:2188-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Saka M, Katai H, Fukagawa T, Nijjar R, Sano T. Recurrence in early gastric cancer with lymph node metastasis. Gastric Cancer. 2008;11:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116:5592-5598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 5. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1422] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 6. | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 7. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 8. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 10. | Piso P, Werner U, Lang H, Mirena P, Klempnauer J. Proximal versus distal gastric carcinoma--what are the differences? Ann Surg Oncol. 2000;7:520-525. [PubMed] |
| 11. | Yokoyama T, Kamada K, Tsurui Y, Kashizuka H, Okano E, Ogawa S, Obara S, Tatsumi M. Clinicopathological analysis for recurrence of stage Ib gastric cancer (according to the second English edition of the Japanese classification of gastric carcinoma). Gastric Cancer. 2011;14:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H, Tsuburaya A. Macroscopic tumor size as an independent prognostic factor for stage II/III gastric cancer patients who underwent D2 gastrectomy followed by adjuvant chemotherapy with S-1. Gastric Cancer. 2011;14:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, Cho H, Tsuburaya A. Risk factors for peritoneal recurrence in stage II/III gastric cancer patients who received S-1 adjuvant chemotherapy after D2 gastrectomy. Ann Surg Oncol. 2012;19:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, Tsuburaya A. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |