Published online Jun 7, 2014. doi: 10.3748/wjg.v20.i21.6554
Revised: September 27, 2013
Accepted: November 3, 2013
Published online: June 7, 2014
Processing time: 357 Days and 18 Hours
AIM: To compare the binding of cholecystokinin (CCK)-8 to CCK receptors in sling and clasp fibers of the human lower esophageal sphincter.
METHODS: Esophageal sling and clasp fibers were isolated from eight esophagectomy specimens, resected for squamous cell carcinoma in the upper two thirds of the esophagus, which had been maintained in oxygenated Kreb’s solution. Western blot was used to measure CCK-A and CCK-B receptor subtypes in the two muscles. A radioligand binding assay was used to determine the binding parameters of 3H-CCK-8S to the CCK receptor subtypes. The specificity of binding was determined by the addition of proglumide, which blocks the binding of CCK to both receptor subtypes.
RESULTS: There was no significant difference between the sling and clasp fibers of the human lower esophageal sphincter in the amount of CCK-A [integrated optical density (IOD) value: 22.65 ± 0.642 vs 22.328 ± 1.042, P = 0.806] or CCK-B receptor protein (IOD value: 13.20 ± 0.423 vs 12.45 ± 0.294, P = 0.224) as measured by Western blot. The maximum binding of radio-labeled CCK-8S was higher in the sling fibers than in the clasp fibers (595.75 ± 3.231 cpm vs 500.000 ± 10.087 cpm, P < 0.001) and dissociation constant was lower (Kd: 1.437 ± 0.024 nmol/L vs 1.671 ± 0.024 nmol/L, P < 0.001). The IC50 of the receptor specific antagonists were lower for the CCK-A receptors than for the CCK-B (P < 0.01).
CONCLUSION: CCK binding modulates the contractile function of the lower esophageal sphincter through differential binding to the CCK-A receptor on the sling and clasp fibers.
Core tip: We isolated the sling and clasp muscles which help form the human lower esophageal sphincter. The expression of cholecystokinin (CCK)-A and CCK-B receptors was measured in the two muscles. The binding of 3H-CCK-8S to the CCK receptors was studied to determine the binding characteristics of the hormone to the receptor subtypes. It is concluded that the CCK-A receptor probably plays a more important role than the CCK-B receptor in mediating the contractile function of lower esophageal sphincter, through a combination of more receptors and a stronger binding affinity.
- Citation: Liu JF, Zhang J, Liu XB, Drew PA. Investigation of cholecystokinin receptors in the human lower esophageal sphincter. World J Gastroenterol 2014; 20(21): 6554-6559
- URL: https://www.wjgnet.com/1007-9327/full/v20/i21/6554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i21.6554
The lower esophageal sphincter (LES) is the incrassate muscle bundle located at the esophagogastric junction, and includes the sling fibers from the greater curvature and clasp fibers from the lesser curvature of the stomach[1]. The LES can open to allow liquids or solids to enter the stomach, or to permit vomiting or belching. At other times the basal tone of the LES is intended to prevent abnormal reflux of gastric contents into the esophagus[2]. The regulation of the LES is complex, involving interplay between the nervous and hormonal systems, as well as local myogenic influences[3,4]. In particular, gastrointestinal peptide hormones play important roles in its regulation[5,6].
The cholecystokinins (CCK) are a family of peptide hormones which have important roles in regulating gastrointestinal motility and the delivery of nutrients to the small intestine[7-10]. The individual members are identified by the number of amino acids in the hormone following post-translational modification of the CCK gene product, preprocholecystokinin (e.g., CCK-58, CCK-8). The receptors for CCK are divided into two subtypes, CCK-A and CCK-B, based on their affinities to CCK analogues, gastrin and specific antagonists. Gastrin and CCK are similar in structure, and both bind to CCK-B receptors. We have previously shown that human sling and clasp fibers contract in response to both gastrin and CCK-8, but the sling fibers have stronger contractions than the clasp fibers[6].
Structural and functional abnormalities of the LES may result in esophageal diseases[11,12]. An incompetent LES permits gastro-esophageal reflux, which damages the esophageal epithelial lining and may lead to complications such as esophagitis, Barrett’s esophagus or cancer[13,14]. Currently, antireflux surgery, typically Nissen fundoplication, is the mainstay treatment to prevent reflux, but it is invasive and has a number of potential side-effects. A better understanding of the physiology of the LES may lead to medical interventions to prevent or reduce reflux, avoiding the need for surgery. In this study we investigated if the differential response of sling and clasp fibers to CKK-8 correlates with differential binding characteristics of CCK-8 to the CCK receptors in these fibers.
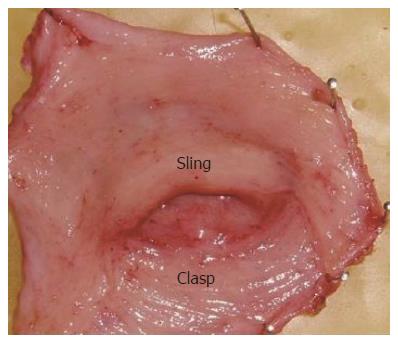
Specimens of the esophagogastric junction were obtained from 5 men and 3 women (mean age 53 years, range 45-75 years) who underwent esophagectomy for squamous cell carcinoma in the upper two-thirds of the esophagus. Patients with heartburn symptoms or motility disorders, such as achalasia of the esophagus or dermatosclerosis, were excluded from the study. Immediately after removal from the patient the esophagogastric junction tissue was placed in oxygenated Kreb’s solution and transported to the laboratory. Frozen section histology was performed to confirm absence of tumor in the specimens. The sling and clasp fibers (Figure 1) were separated as described previously[6]. The experimental protocol was approved by the Ethics Committee of the Fourth Hospital, Hebei Medical University.
Membrane proteins were isolated using the Eukaryotic Membrane Protein Extraction kit (Pierce, Rockford, IL, United States), following the manufacturer’s instructions. The CCK-A and CCK-B receptors in the membrane protein preparation were detected by Western blot as previously described[15]. A goat polyclonal antibody to human CCK-A receptor (1:400 dilution) and a goat polyclonal antibody to human CCK-B receptor (Santa Cruz, United States) were each used at a dilution of 1:400. The secondary antibody was a donkey anti-goat IgG conjugated to peroxidase, used at a 1:2000 dilution and developed with DAB. The integrated optical density (IOD) of each band was determined using the Gel-Pro analyzer software package (Media Cybernetics, United States).
The binding studies were carried out as described by Salvatore et al[16]. The membrane protein isolate was incubated, at a final concentration of 0.15 mg/mL with serial dilutions of 3H-CCK-8S (Sigma, United States), ranging from 6.4 nmol/L to 0.05 nmol/L in a total volume of 200 μL for 10 h at 4 °C with shaking every 30 min. The specificity of binding was determined by the addition of 5 μmol/L proglumide, which blocks the binding of CCK to both receptor subtypes. Bound ligand was isolated by filtration under vacuum on Whatman GF/B filters which were then washed three times with ice-cold HEPES buffer (130 mmol/L NaCl, 5 mmol/L MgCl2 and 10 mmol/L HEPES, pH 7.4). Bound radioactivity was measured by liquid scintillation counting (Model LS 6500, Beckman Instruments, Fullerton, CA, United States). Nonspecific binding, defined as the binding of radiolabelled ligand in the presence of 10 mmol/L CCK, was subtracted from the total binding measured in each assay. Competitive inhibition was determined using 7.5 nmol/L 3H-CCK-8S with CR1409 as an antagonist to the CCK-A receptor or CR2945 to the CCK-B receptor (Sigma, United States). Data were analyzed and competitive curves constructed from the mean of triplicate measurements. The dissociation constant of the radioligand receptor complex (Kd) and the maximum binding value (Bmax) were calculated using GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, CA, United States). The inhibitory binding constant Ki was calculated from the IC50 according to the Cheng-Prusoff equation, Ki = IC50/(l + L/Kd), where L is the concentration of the radioligand, IC50 is the concentration of drug causing 50% inhibition of the specific radioligand binding, and Kd is dissociation constant[17].
Data are expressed as mean ± SD, and groups were compared by the paired Student’s t test using the SPSS statistical program. Differences were considered statistically significant when P < 0.05.
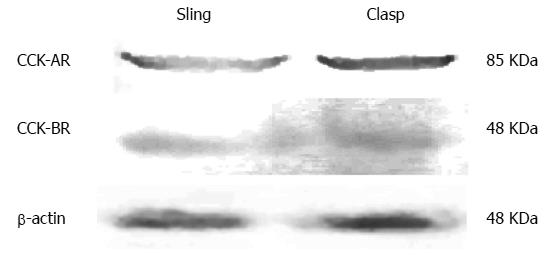
Both CCK-A and CCK-B receptors were measured in the membrane protein extract of the sling and the clasp muscle fibers by Western blot (Figure 2). The results in Table 1 show that the IOD for the beta-actin loading control did not differ between the fibers. There were no significant differences between the sling and clasp fibers in the IOD for the CCK-A (t = 0.263, P = 0.806) or the CCK-B (t = 1.439, P = 0.224) receptors.
| n | CCK-AR | CCK-BR | |
| Clasp fibers | 8 | 22.328 ± 1.042 | 12.45 ± 0.294 |
| Sling fibers | 8 | 22.65 ± 0.642 | 13.20 ± 0.423 |
| t | 0.263 | 1.439 | |
| P value | 0.806 | 0.224 |
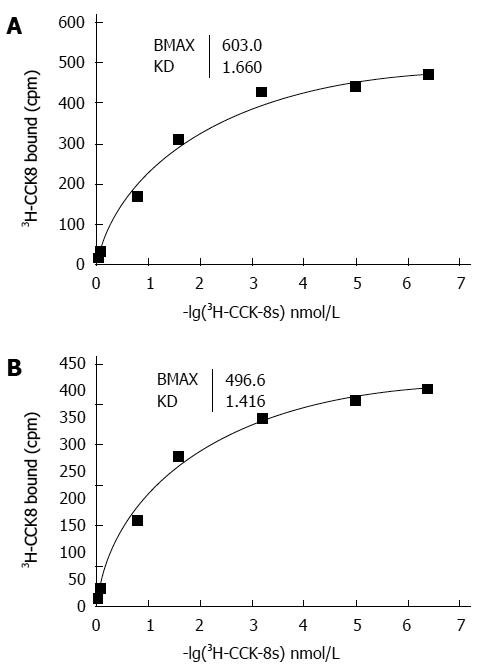
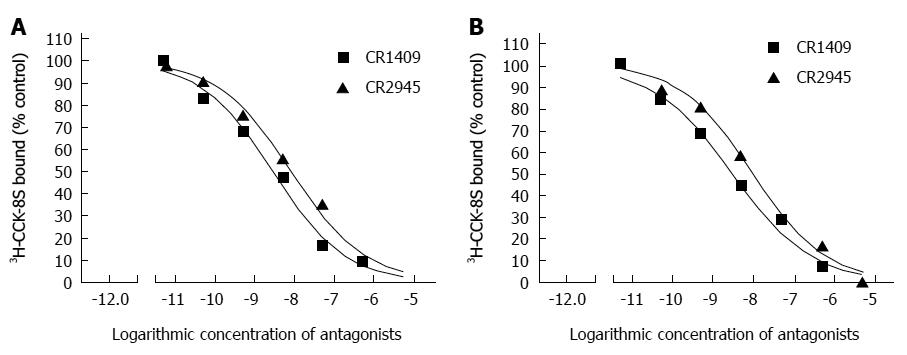
The binding of 3H-CCK-8S to the CCK receptor was specific in both the sling and clasp fibers. A typical saturation isotherm plot for the binding of 3H-CCK-8S to human cell membrane protein extract is shown in Figure 3. The mean Bmax and Kd values for all eight sling and clasp muscle preparations analysed (clasp fibers: 500.00 ± 10.09 vs 1.671 ± 0.024; sling fibers: 595.75 ± 3.23 vs 1.437 ± 0.024; t: 9.040 vs 6.898) differed significantly between the sling and the clasp fibers (each P < 0.001). The results in Figure 4 show the competitive inhibition curves of the specific CCK-A receptor antagonist, CR1409, and the specific CCK-B receptor antagonist, CR2945, for the binding of 3H-CCK-8S to the membrane protein extract from the sling and clasp fibers. The mean IC50 values for CR1409 and CR2945 for all eight sling and clasp muscle preparations analysed are shown in Table 2. There were no significant differences between the sling and clasp fibers in the IC50 for CR1409 (t = 1.72, P = 0.161) or CR2945 (t = 1.93, P = 0.126). In both the sling and clasp fibers the IC50 for CR1409 was significantly higher than that for CR2945 (P = 0.001 and P < 0.001 respectively). The pKi values were also greater for CR1409 than for CR2495 in both the sling and clasp fibers (Table 2) (each P < 0.001).
| n | CR1409 | CR2945 | t | P value | |
| IC50 values | |||||
| Clasp fibers | 8 | 3.165 ± 0.187 | 9.583 ± 0.501 | 11.99 | < 0.001 |
| Sling fibers | 8 | 2.798 ± 0.104 | 8.147 ± 0.551 | 9.53 | 0.001 |
| t | 1.72 | 1.93 | |||
| P value | 0.161 | 0.126 | |||
| pKi values | |||||
| Clasp fibers | 8 | 8.476 ± 0.065 | 8.018 ± 0.028 | 12.69 | 0.001 |
| Sling fibers | 8 | 8.556 ± 0.022 | 8.090 ± 0.042 | 11.73 | 0.001 |
| t | 1.653 | 2.754 | |||
| P value | 0.156 | 0.058 |
The gastrointestinal hormone CCK plays an important role in the regulation of gastrointestinal motility. We have previously shown that the sling and clasp fibers, which contribute to the tone of the LES, contract in response to CCK. The strength of the response differs between these fibers, with the sling fibers contracting more strongly than the clasp fibers[6]. We report here that the binding of CCK-8 to the CCK receptor subtypes differs between these two types of fibers, which may provide an explanation for the difference in their response to the hormone.
The expression and distribution of the two CCK receptor subtypes, CCK-A and CCK-B, vary between tissues and organs, and within different parts of the same organ, within a species[15]. CR1409 is a selective antagonist of the CCK-A receptor[18], with concentration dependent inhibitory effects on CCK-8 mediated responses in vivo, such as satiety or contraction of the smooth muscle of the ileum or gallbladder[19]. CR-2945 is a potent, selective and reversible non-peptide antagonist of CCK-B receptor. It is a candidate new generation, non-sedative anxiolytic, as well being considered a treatment for acid-related dyspeptic symptoms, where visceral motility and acid exposure play a role[20]. We found that the IC50 for CR1409 was less than that for CR2945 in both the sling and clasp muscles, with the pKi for CR1409 greater than that for CR2945. There were no significant differences in the IC50 of CR1409 and CR2945 between the clasp fibers and sling fibers, implying that there were no differences in the affinity of each antagonist to its receptor between the two fibers. The difference in the Bmax for the binding of the radiolabelled CCK-8S between the sling fibers and clasp fibers suggest that there are more CCK receptors in the sling fibers than in the clasp fibers. Our results are consistent with the ability of CCK to induce contraction of the sling and clasp fibers by binding to both receptor subtypes, with induction of stronger contractions in the sling fibers because of higher binding to the CCK-A receptor.
A number of studies show that CCK can reduce LES pressure, most commonly following a meal, when plasma levels of the hormone are significantly elevated. Boulant et al[21] found in dogs that CCK infusion increased transient LES relaxations (TLESRs), and that a CCK-A receptor antagonist (but not a CCK-B receptor antagonist) prevented this increase, without affecting the basic tone of the LES. Ledeboer et al[22] demonstrated in humans that the TLESRs following a fat meal, which are CCK mediated, are reduced by the CCK-A receptor antagonist loxiglumide. Masclee et al[23] reported that ingestion of cholestyramine in humans resulted in a reduction of LES pressure, again an effect which could be abolished by loxiglumide, and similar findings following infusion of a triglyceride meal were reported by Trudgill et al[11]. The postprandial reduction in LES pressure is complex, involving at least CCK and nitric oxide[12,22,24]. Salapatek et al[25] found in cats that CCK induced LES contraction through a preganglionic cholinergic mechanism involving a nicotinic synapse, but induction of relaxation occurred predominantly at a postganglionic site involving adrenergic modulation. Based on these experiments it was proposed that there is animal-to-animal variability in the balance of excitatory and inhibitory mechanisms to the LES, which determines the effect of a mediator which is capable of activating both mechanisms[25].
The most likely explanation for the difference in action of CCK described in these reports and our study is that we studied the effect of the hormone on contraction of the isolated sling and clasp fibers in vitro, whereas in the other studies the LES pressure was measured in vivo[25]. The location of a high pressure zone at the gastroesophageal junction and the asymmetric distribution of the pressure, as measured by esophageal manometry, are in concordance with the position and arrangement of the two bundles of fibers[26]. Thus, while it is clear that the sling and clasp fibers contribute to the generation of the LES pressure, they are not the only muscles involved. Additionally, in vitro studies permit the measurement of effects due to CCK alone, whereas with in vivo studies it is difficult to control for all the variables which may impact on a function as complex as that which regulates LES pressure.
In conclusion, we found no difference in the amount of CCK-A or CCK-B receptors between human sling and clasp fibers, as measured by Western blot, but the sling fibers bound more radiolabelled CCK-8S than the clasp fibers. Our binding and inhibition data are consistent with the CCK-A receptor playing an important role in mediating the contractile function of the LES, acting through differential effects on the sling and clasp fibers.
The sling fibers of the human lower esophageal sphincter contract more strongly in response to gastrin and cholecystokinin (CCK)-8 than the clasp fibers. Authors investigate a possible explanation that the binding of CCK-8 to CCK receptor subtypes differs between these fibers.
It is well known that the muscles comprising the lower esophageal sphincter have specific structural and physiological characteristics. The regulation of the lower esophageal sphincter is complex, involving an interplay between the nervous and hormonal systems, as well as local myogenic influences. In particular, gastrointestinal peptide hormones play an important role in the regulation of the sphincter. Authors have previously shown that the human sling fibers have stronger contractions than the clasp fibers in response to gastrin and CCK-8. In this study they show that this may result at least in part differences in binding of CCK-8 to the CCK receptor subtypes between the sling and clasp fibers of the human lower esophageal sphincter.
The majority of published studies on the lower esophageal sphincter have involved animals such as guinea pigs, cats, and dogs. The studies also generally involve the sphincter as a whole, with no investigation of the individual characteristics or separate contributions of the sling or clasp fibers to the function of the sphincter. This is the first report of the binding characteristics of CCK-8S to CCK receptor subtypes in isolated human sling and clasp fibers.
Binding of CCK to CCK-2 receptors modulates motility in the upper gut (including the esophagus, lower esophageal sphincter and the stomach). CCK antagonists may have therapeutic value in the prevention or treatment of gastro-esophageal reflux disease including esophagitis, Barrett’s esophagus and esophageal adenocarcinoma.
The results are interesting and suggest that CCK binding modulates the contractile function of the lower esophageal sphincter through differential binding to the CCK-A receptor on the sling and clasp fibers.
P- Reviewers: Maher MM, Milone M, Misra SP S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 466] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Liebermann-Meffert D, Allgöwer M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31-38. [PubMed] |
| 3. | Braverman AS, Vegesna AK, Miller LS, Barbe MF, Tiwana M, Hussain K, Ruggieri MR. Pharmacologic specificity of nicotinic receptor-mediated relaxation of muscarinic receptor precontracted human gastric clasp and sling muscle fibers within the gastroesophageal junction. J Pharmacol Exp Ther. 2011;338:37-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Tian ZQ, Liu JF, Wang GY, Li BQ, Wang FS, Wang QZ, Cao FM, Zhang YF. Responses of human clasp and sling fibers to neuromimetics. J Gastroenterol Hepatol. 2004;19:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Uc A, Oh ST, Murray JA, Clark E, Conklin JL. Biphasic relaxation of the opossum lower esophageal sphincter: roles of NO., VIP, and CGRP. Am J Physiol. 1999;277:G548-G554. [PubMed] |
| 6. | Liu JF, Gao LP, Wen SW, Lu HL, Zhang J, Sun J, Zhang SW, Wang QZ. Responses of human sling and clasp fibers to cholecystokinin (CCK) and gastrin through CCK receptors. J Gastroenterol Hepatol. 2008;23:1608-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:G749-G761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | González AA, Farré R, Monés J, Capellà G, Clavé P. Pharmacological and molecular characterization of muscular cholecystokinin receptors in the human lower oesophageal sphincter. Neurogastroenterol Motil. 2000;12:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Reubi JC, Waser B, Läderach U, Stettler C, Friess H, Halter F, Schmassmann A. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology. 1997;112:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Trudgill NJ, Hussain FN, Moustafa M, Ajjan R, D’Amato M, Riley SA. The effect of cholecystokinin antagonism on postprandial lower oesophageal sphincter function in asymptomatic volunteers and patients with reflux disease. Aliment Pharmacol Ther. 2001;15:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Hirsch DP, Tiel-Van Buul MM, Tytgat GN, Boeckxstaens GE. Effect of L-NMMA on postprandial transient lower esophageal sphincter relaxations in healthy volunteers. Dig Dis Sci. 2000;45:2069-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Smith E, Kelly JJ, Ruskiewicz AR, Sullivan T, Jamieson GG, Drew PA. The effect of long-term control of reflux by fundoplication on aberrant deoxyribonucleic acid methylation in patients with Barrett esophagus. Ann Surg. 2010;252:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Orlando RC. Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. 2008;42:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Sternini C, Wong H, Pham T, De Giorgio R, Miller LJ, Kuntz SM, Reeve JR, Walsh JH, Raybould HE. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology. 1999;117:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA. 1993;90:10365-10369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 317] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10214] [Cited by in RCA: 10609] [Article Influence: 204.0] [Reference Citation Analysis (0)] |
| 18. | D'Amato M, Stamford IF, Bennett A. Studies of three non-peptide cholecystokinin antagonists (devazepide, lorglumide and loxiglumide) in human isolated alimentary muscle and guinea-pig ileum. Br J Pharmacol. 1991;102:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Makovec F, Bani M, Cereda R, Chisté R, Pacini MA, Revel L, Rovati LA, Rovati LC, Setnikar I. Pharmacological properties of lorglumide as a member of a new class of cholecystokinin antagonists. Arzneimittelforschung. 1987;37:1265-1268. [PubMed] |
| 20. | Revel L, Mennuni L, Garofalo P, Makovec F. CR 2945: a novel CCKB receptor antagonist with anxiolytic-like activity. Behav Pharmacol. 1998;9:183-194. [PubMed] |
| 21. | Boulant J, Fioramonti J, Dapoigny M, Bommelaer G, Bueno L. Cholecystokinin and nitric oxide in transient lower esophageal sphincter relaxation to gastric distention in dogs. Gastroenterology. 1994;107:1059-1066. [PubMed] |
| 22. | Ledeboer M, Masclee AA, Biemond I, Lamers CB. Effect of medium- and long-chain triglycerides on lower esophageal sphincter pressure: role of CCK. Am J Physiol. 1998;274:G1160-G1165. [PubMed] |
| 23. | Masclee AA, Jansen JB, Rovati LC, Lamers CB. Effect of cholestyramine and cholecystokinin receptor antagonist CR1505 (loxiglumide) on lower esophageal sphincter pressure in man. Dig Dis Sci. 1993;38:1889-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Minashi K, Kusano M. [Esophageal peristalsis, lower esophageal function, and the methods of their evaluation]. Nihon Rinsho. 2000;58:1827-1831. [PubMed] |
| 25. | Salapatek AM, Hynna-Liepert T, Diamant NE. Mechanism of action of cholecystokinin octapeptide on cat lower esophageal sphincter. Am J Physiol. 1992;263:G419-G425. [PubMed] |
| 26. | Preiksaitis HG, Tremblay L, Diamant NE. Cholinergic responses in the cat lower esophageal sphincter show regional variation. Gastroenterology. 1994;106:381-388. [PubMed] |