Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.6336
Revised: January 9, 2014
Accepted: January 20, 2014
Published online: May 28, 2014
Processing time: 208 Days and 18 Hours
AIM: To investigate the prognostic and clinicopathological significance of glypican-3 (GPC3) overexpression in hepatocellular carcinoma (HCC).
METHODS: Publications were searched using PubMed, EMBASE, the Cochrane Library and the Chinese Biomedical Literature Database up to March 2013. Inclusion and exclusion criteria were established to screen eligible studies for meta-analysis. The hazard ratios (HRs) of the eligible studies were pooled using RevMan 5.2 software to evaluate the impact of GPC3 overexpression on overall survival (OS) and disease-free survival (DFS) in HCC patients. The correlation between GPC3 expression and clinicopathological parameters of HCC was also analyzed.
RESULTS: A total of five studies with 493 patients were included in the meta-analysis. The combined HRs indicated that GPC3 overexpression can predict poor OS (n = 362 in 3 studies, HR = 2.18, 95%CI: 1.47-3.24, Z = 3.86, P = 0.0001) and DFS (n = 325 in 3 studies, HR = 2.05, 95%CI: 1.43-2.93, Z = 3.94, P < 0.0001) in HCC patients without heterogeneity. Egger’s and Begg’s tests were applied to detect publication bias, and the results showed that there was no evidence of publication bias detected in the OS studies (the P value for Egger’s test was 0.216) or DFS studies (the P value for Egger’s test was 0.488). The combined odds ratios (ORs) suggested that GPC3 expression tends to be associated with tumor vascular invasion (OR = 2.74, 95%CI: 1.15-6.52, P = 0.02), hepatic cirrhosis (OR = 2.10, 95%CI: 1.31-3.36, P = 0.002), poor tumor differentiation (OR = 0.22, 95%CI: 0.13-0.40, P < 0.00001) and advanced TNM stage (OR = 0.31, 95%CI: 0.18-0.51, P < 0.00001).
CONCLUSION: From this study, we conclude that GPC3 overexpression tends to be associated with a poor prognosis (poor OS or DFS) in HCC.
Core tip: Glypican-3 (GPC3) is known to be a specific and available molecular marker for the diagnosis of hepatocellular carcinoma (HCC). However, the prognostic value of GPC3 overexpression in patients with HCC is less researched and remains controversial. We performed the meta-analysis including six eligible studies and demonstrated that GPC3 overexpression was associated with poor overall survival and disease-free survival in HCC. We also found that GPC3 expression was closely related with tumor vascular invasion, hepatic cirrhosis, tumor grade and tumor stage in HCC.
- Citation: Li J, Gao JZ, Du JL, Wei LX. Prognostic and clinicopathological significance of glypican-3 overexpression in hepatocellular carcinoma: A meta-analysis. World J Gastroenterol 2014; 20(20): 6336-6344
- URL: https://www.wjgnet.com/1007-9327/full/v20/i20/6336.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i20.6336
Hepatocellular carcinoma (HCC) is the third most frequent cause of malignant tumor-related death worldwide and the second most frequent cause in China; its incidence rate is continuously increasing[1]. Advances in surgical and adjuvant techniques have greatly improved the results of hepatic resection, liver transplantation and transcatheter arterial chemoembolization (TACE) for HCC treatment. Nonetheless, long-term overall survival (OS) rates after clinical treatments remain unsatisfactory due to the high incidence of recurrence and metastasis[2]. The 5-year survival rate after surgery for HCC has been reported to be 25%-39%[3]. Therefore, it is essential to identify biological markers for diagnosing HCC in its early stage and predicting the prognosis, recurrence and metastasis of HCC after treatments. This identification may also be helpful in the selection of personalized therapeutic strategies and may further improve the survival of HCC patients.
Glypican-3 (GPC3), which encodes cell-surface heparan sulfate proteoglycans, belongs to the glypican family and plays an important role in cell proliferation, differentiation and invasion[4]. A large number of studies have reported that GPC3 increases in HCC tissue and is released into the serum during hepatic carcinogenesis[5-8]. Moreover, subsequent studies indicated that GPC3 can be regarded as a specific diagnostic molecular marker for HCC and that its diagnostic sensitivity and specificity are superior to those of alpha fetoprotein (AFP)[9-14]. Further research suggested that GPC3 may play a role as an oncogene in HCC that can be partially activated by c-Myc[15] and that can then promote tumor cell proliferation and stimulate HCC growth by activating the Wnt[16] and the insulin-like growth factor signaling pathways[17]. Additionally, GPC3 can inhibit apoptosis by inducing dysfunction in the Bax/Bcl-2/cytochrome c/caspase-3 signaling pathway, thereby regulating cell proliferation and playing an important role in the tumorigenesis and progression of HCC[18].
In recent years, aberrant GPC3 expression has been discovered to be related to tumor progression, metastasis and other malignant biological behaviors in a variety of other tumors, such as lung squamous cell carcinoma[19], gastrointestinal and pancreatic epithelial neoplasms[20] and malignant melanoma[21]. Nevertheless, conflicting results have been reported regarding the ability of GPC3 to predict tumor progression, recurrence and metastasis; OS; and disease-free survival (DFS) in HCC. Therefore, it was necessary to perform a meta-analysis to comprehensively and systematically summarize and explore the prognostic significance of GPC3 in HCC.
In this study, we extracted and pooled the results of the included studies and attempted to objectively evaluate GPC3 expression in HCC tissues and its association with OS and DFS in patients with HCC. The relationship between GPC3 expression and tumor vascular invasion or other clinicopathological characteristics (such as hepatic cirrhosis, tumor grade and TNM stage) were also analyzed. The results of this meta-analysis will help us to design a personalized therapeutic schedule for each patient and to provide a closer follow-up for patients with GPC3 overexpression. Furthermore, based on our understanding of the effect and function of GPC3 in HCC, GPC3 may become a target for the treatment of HCC patients and deserves further research and clinical application.
The electronic databases PubMed, EMBASE, the Chinese Biomedical Literature Database (CBM) and the Cochrane Library were searched for publications assessing the prognostic significance and clinicopathological characteristics of GPC3 in HCC up to March 2013. The search terms were as follows: (liver cell carcinoma or carcinoma, hepatocellular or hepatocellular carcinoma or HCC or hepatoma) AND (GPC3 or glypican-3).
Studies eligible for inclusion in this meta-analysis had to meet the following criteria: (1) articles published as a full-text paper in English; (2) original research articles that directly explored GPC3 expression in primary HCC tissues and paired para-tumor tissues by the method of immunohistochemistry (IHC); (3) studies that demonstrated a relationship between GPC3 expression and HCC prognosis or clinicopathological parameters; (4) studies that provided sufficient information to estimate the hazard ratio (HR) and 95% confidence interval (CI); and (5) if multiple studies reused the same sample of patients, the article with more detailed data was included. Articles were excluded from the analyses based on the following criteria: (1) non-English-language papers; (2) case reports, reviews articles or letters; and (3) articles containing insufficient data for calculating the HR and 95%CI of OS or DFS.
All data were extracted from eligible studies by two independent reviewers (Li J and Gao JZ) to minimize bias. The following survival and clinicopathological characteristics were documented from the primary articles: the first author’s name; the name of the journal; the publication year of the article; the sample size; the strategy for clinical treatment; the test method and cut-off level used; hepatic cirrhosis; tumor vascular invasion, differentiation and stage; and GPC3 expression-related OS or DFS. As the cut-off level of GPC3 expression varied among the selected studies, we defined GPC3 high- and low-expression groups with reference to the original articles. For articles in which the survival data were not directly published except in Kaplan-Meier curves, the software Engauge Digitizer version 4.1 (http://sourceforge.net/projects/digitizer/) was used to read the curves for data extraction.
The Newcastle-Ottawa quality assessment scale for cohort studies[22] was developed to assess the quality of nonrandomized studies, with its design, content and ease of use directed toward the task of incorporating quality assessments in the interpretation of meta-analysis results. This scale was used to assess the quality of the available studies, which were graded independently by two investigators (Li J and Gao JZ). Each study was judged from three broad perspectives: (1) the selection of the study groups; (2) the comparability of the groups; and (3) the ascertainment of either the exposure or the outcome of interest in cohort studies[23]. A study could be awarded a maximum of one star for each numbered item within the Selection and Outcome categories. A maximum of two stars was given for Comparability. The total number of stars was then summed, with more stars representing better methodological quality. The details of the scale can be found online (URL: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf).
All statistical analyses were performed using RevMan 5.2 and Stata 10.0 software (http://www.cochrane.org). For the quantitative evaluation of OS or DFS results, HRs with their 95%CIs were extracted from each article and combined to estimate the relationship between GPC3 overexpression and the survival conditions of patients. When the HR and its variance were not directly provided in an article, they were calculated from available numerical data according to the methods reported by Parmar et al[24].
We also extracted and combined data on GPC3 expression and several clinicopathological parameters, including tumor differentiation (well/moderate and poor), TNM stage (I-II/III-IV), vascular invasion (present/absent), hepatic cirrhosis (positive/negative), HBV infection (positive/negative), HCV infection (positive/negative) and tumor size (≥ 5/< 5), associated with HCC in each article. For these data, the odds ratios (ORs) and their 95%CIs were calculated and combined to provide the effective value.
In the process of data pooling, we used the χ2 test to estimate the heterogeneity, and the I2 statistic was applied to measure the extent of inconsistency among the results. A fixed-effect model could be used when there was no heterogeneity (P > 0.1); otherwise, a random-effect model was selected. A value for I2 exceeding 50% could be regarded as an indicator of significant heterogeneity[25]. A significant two-way P value for comparing results from different articles was defined as P < 0.05. Detection of publication bias was performed using Egger’s and Begg’s tests, with significant publication bias defined as P < 0.05.
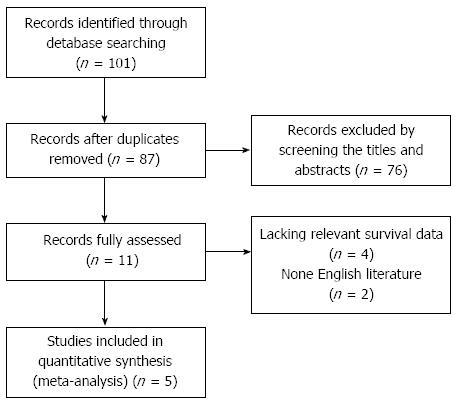
From our initial literature search, we identified 101 studies with titles regarding the association of GPC3 and HCC; after manually screening the titles and abstracts, 90 articles were excluded (review articles, abstracts, experimental research, duplicate reports and studies irrelevant to the current research objectives). The remaining 11 relevant studies were selected for full-text review and full assessment, further reducing our meta-analysis to a total of five eligible studies[26-30] published from 2009-2012 (Figure 1). A total of 493 patients from the five studies were used to study the relationship between GPC3 expression and OS/DFS or clinicopathological features. Two studies were performed in China[26,28]; two, in Japan[27,29]; and one, in Taiwan[30]. Surgical resection as the preferred treatment for HCC was reported in four studies[26,27,29,30], and liver transplantation (LT) was reported in one study[28]. The sample size in each study varied from 31-194, with the male population varying from 29-142. All studies had a follow-up period exceeding 3 years, with four studies with follow-up exceeding 5 years. All studies used IHC to detect tissue-based GPC3 expression. The methodological quality of the studies, as assessed by the Newcastle-Ottawa quality assessment scale, varied from 6-7 (with a mean of 6.4); a higher score indicates better methodological quality. HRs and their 95%CIs were extracted from each study or calculated using the methods described above. The basic characteristics and clinicopathological parameters of the five studies are summarized in Table 1.
| Ref. | Year | Sample sizen(M/F) | Mean age (yr) | Treatment | Tumor vascular invasion (yes/no) | Tumor stage (I-II/III-IV) | Tumor differentiation (well/moderate-to-poor) | Study quality (points) | GPC3 detection method | Survival analysis | Hazard ratio | Counting method in immunohistochemistry | Cut-off level of “high” GPC3 | Number of patients with “high” GPC3 (n) |
| Shirakawa et al[27] | 2009 | 107 (85/22) | NR | S | 47/60 | NR | 12/95 | 7/9 | IHC | OS | Reported in text | Percentage of positive cells | > 10% | 87 |
| Yorita et al[29] | 2010 | 194 (142/52) | NR | S | 108/86 | 82/112 | 56/138 | 7/9 | IHC | OS/DFS | Reported in text | Positive area score + Sp-Cm | ≥ 2-point score | 96 |
| Ning et al[26] | 2012 | 61 (55/6) | 48 | S | NR | 45/16 | NR | 6/9 | IHC | OS | Reported in text | Positive area score × staining intensity score | > ‘‘+” (2-point score) | 32 |
| Yu et al[30] | 2012 | 100 (90/10) | 51 | S | 23/77 | NR | NR | 6/9 | IHC | DFS | Reported in text | NR | NR | NR |
| Wang et al[28] | 2012 | 31 (29/2) | 49 | LT | 16/15 | 12/19 | 6/25 | 6/9 | IHC | DFS | Estimated | Percentage of positive cells | > 10% | 21 |
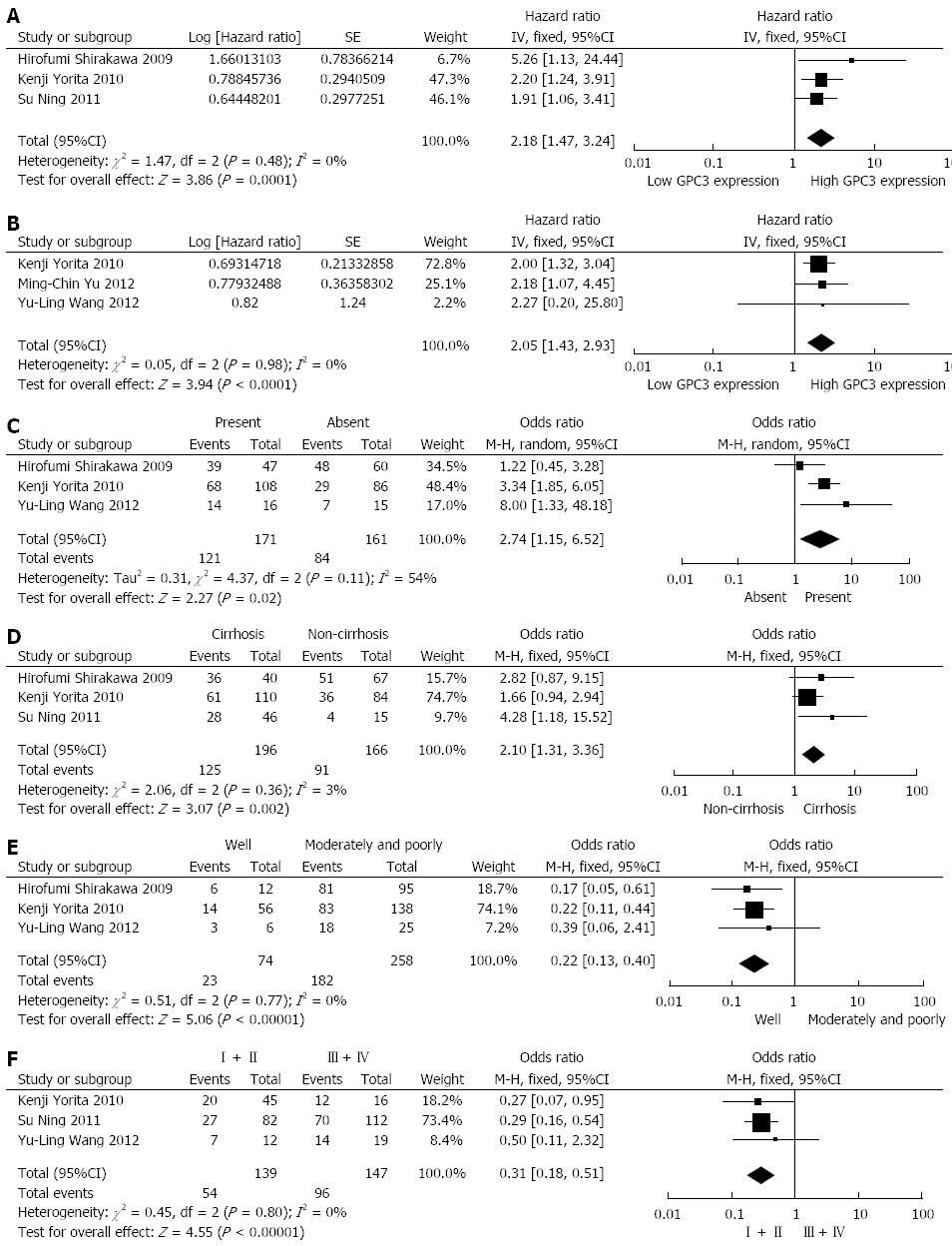
Among the included studies, three reported data on GPC3 expression and OS in HCC[26,27,29], involving a total of 362 HCC patients. All of the three studies had a maximum follow-up period exceeding 5 years. High GPC3 expression was associated with poor OS in the three studies, and all associations were statistically significant. Combined data from all three studies showed that increased GPC3 levels were significantly correlated with poorer OS. The pooled HR was 2.18 (95%CI: 1.47-3.24, Z = 3.86, P = 0.0001; Figure 2A). A fixed-effect model was used in the meta-analysis, as there was no heterogeneity in the data (χ2=1.47, I2=0.0%, P = 0.48).
The data from three studies (325 patients) showed a relationship between GPC3 expression and DFS in HCC[28-30]. Among the three studies, two provided data on GPC3 expression and DFS in HCC initially treated by surgical resection, which is still the standard therapeutic approach[29,30]. High GPC3 expression was associated with poor DFS in all three studies, and statistical significance was observed in two studies[29,30]. Combined data from the three studies indicated that elevated GPC3 levels were significantly correlated with reduced DFS. The pooled HR was 2.05 (95%CI: 1.43-2.93, = 3.94, P < 0.0001; Figure 2B). A fixed-effect model was used in the meta-analysis, as there was no heterogeneity in the data (χ2=0.05, I2=0.0%, P = 0.98). Subgroup analysis illustrated that increased GPC3 expression was significantly associated with DFS in HCC patients who underwent surgical resection (HR: 2.04, 95%CI: 1.43-2.93, P = 0.0001), without significant heterogeneity in the data (χ2 = 0.04, I2 = 0.0%, P = 0.84).
Three studies evaluated the correlation of GPC3 expression with tumor vascular invasion in 332 patients[27-29]. High GPC3 expression seemed to be correlated with the presence of tumor vascular invasion in all three studies, but statistical significance was achieved in only two studies. The combined OR for the three studies was 2.74 (95%CI: 1.15-6.52, Z = 2.27, P = 0.02), with evident heterogeneity (χ2 = 4.37, I2 = 54%, P = 0.11), indicating that GPC3 expression was associated with tumor vascular invasion in HCC (Figure 2C). The relationship between GPC3 expression and hepatic cirrhosis was reported in three articles, covering 362 patients[26,27,29]. A positive correlation between GPC3 expression and hepatic cirrhosis was observed in all three studies, but only one study reported a significant result. However, by combining the data from the three studies, we found a significant trend toward a correlation between high GPC3 expression and the presence of hepatic cirrhosis (OR = 2.10, 95%CI: 1.31-3.36, Z = 3.07, P = 0.002; Figure 2D), with nearly no heterogeneity (χ2 = 2.06, I2 = 3%, P = 0.36).
Three studies reported data on GPC3 expression and tumor histological differentiation in HCC, covering 332 patients[27-29]. High GPC3 levels seemed to be correlated with poorer histological differentiation (moderate and poor) in all three studies, and statistical significance was reported in two of these studies. The pooled OR was 0.22 (95%CI: 0.13-0.40, Z = 5.06, P < 0.00001), without heterogeneity (χ2 = 0.51, I2 = 0%, P = 0.77), suggesting a correlation between GPC3 expression and poorer tumor differentiation (Figure 2E). Three studies presented research on GPC3 expression and tumor TNM stage in 286 HCC patients[26,28,29]. All three studies suggested that high GPC3 expression was associated with high tumor stage (III-IV), and statistical significance was observed in two articles. Combining the data from the three studies, the pooled OR was 0.31 (95%CI: 0.18-0.51, Z = 4.55, P < 0.00001), without heterogeneity (χ2 = 0.45, I2 = 0%, P = 0.80), demonstrating a correlation between high GPC3 expression and advanced tumor TNM stage (Figure 2F).
The data on other clinicopathological parameters, including HBV infection, HCV infection and tumor size, were extracted and pooled from the included studies. The results showed that there was no significant association between the expression level of GPC3 and HBV infection, HCV infection or tumor size. The combined ORs were 1.47 (95%CI: 0.53-4.05, Z = 0.74, P = 0.46), 0.69 (95%CI: 0.42-1.13, Z = 1.48, P = 0.14) and 1.15 (95%CI: 0.72-1.86, Z = 0.59, P = 0.56), respectively (Table 2).
| Ref. | Clinicopathological feature | Total | Events (GPC3-positive) | OR | 95%CI | P value for OR | P value for heterogeneity |
| Shirakawa et al[27] | HBV (+/-) | 29/78 | 26/61 | 1.47 | 0.53-4.05 | 0.46 | 0.10 |
| Yorita et al[29] | 60/134 | 38/59 | |||||
| Ning et al[26] | 53/8 | 26/6 | |||||
| Shirakawa et al[27] | HCV (+/-) | 62/45 | 50/37 | 0.69 | 0.42-1.03 | 0.14 | 0.54 |
| Yorita et al[29] | 83/111 | 36/61 | |||||
| Yorita et al[29] | Tumor size (≥ 5/< 5) | 78/116 | 41/56 | 1.15 | 0.72-1.86 | 0.56 | 0.95 |
| Ning et al[26] | 37/24 | 20/12 | |||||
| Yu et al[30] | 15/16 | 10/11 |
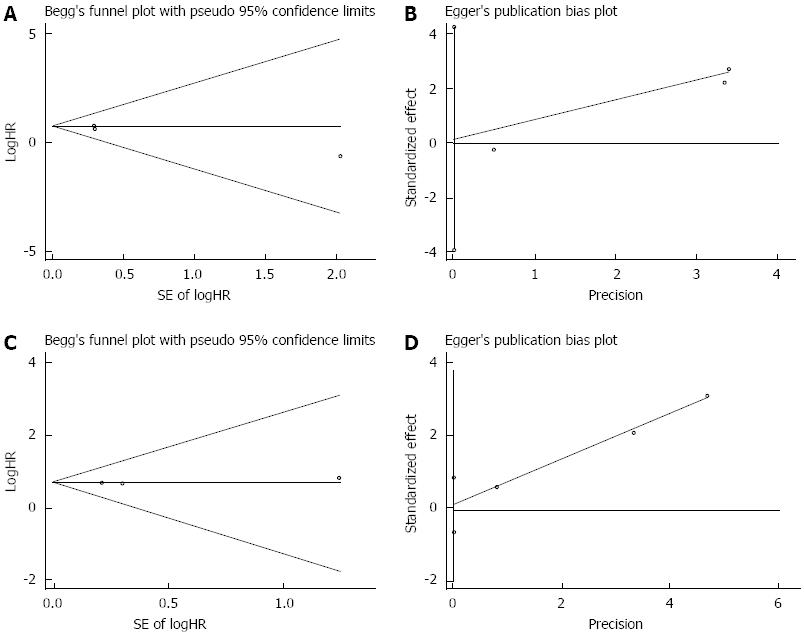
The main use of publication bias is to evaluate the reliability of meta-analysis results, and especially those with statistical significance[31]. Egger’s and Begg’s tests were applied to detect publication bias. The results showed that there was no evidence of publication bias detected in the OS studies (the P value for Egger’s test was 0.216) or DFS studies (the P value for Egger’s test was 0.488), with no funnel plot asymmetry found in either study (Figure 3).
Tumor recurrence and metastasis are the primary causes of poor prognosis in HCC patients, and cancer cell invasion is the key step in the process of HCC recurrence and metastasis. For years, many researchers have been devoted to a search for the most useful molecular markers of HCC progression, spread and survival, which may offer guidance for clinical treatment and improve the outcome of HCC patients. GPC3, a member of the glypican family, is highly expressed in a large proportion of HCCs and is regarded as a useful serological and histochemical marker for the diagnosis of HCC[32,33]. Sun et al[34] reported that the suppression of GPC3 in Huh7 and HepG2 cells inhibited proliferation and cell cycle progression, increased TGF-β2 expression and enhanced apoptosis, which suggested an oncogenic function for GPC3 in HCC. Additionally, Ruan et al[35] found that the downregulation of GPC3 expression can significantly decrease the proliferative and invasive abilities of MHCC97-H human hepatocellular carcinoma cells. We speculated that GPC3 overexpression may be closely associated with postoperative recurrence/metastasis and the prognosis of HCC patients. However, studies exploring the relationship between GPC3 expression and survival in HCC have been infrequent and controversial.
Meta-analysis is a quantitative approach that pools the results of randomized controlled trials (RCTs) and has been successfully used for the evaluation of prognostic biomarkers in patients with malignant tumors[36,37]. In the present study, we included five studies concerning the effects of the overexpression of GPC3 on HCC clinicopathological parameters and patients’ OS or DFS. In this meta-analysis, we pooled the results of eligible studies and demonstrated that high GPC3 expression was closely related to poor OS and DFS in HCC patients (P = 0.0001 and P < 0.0001, respectively). Additionally, subgroup analysis indicated that elevated GPC3 expression could predict poor DFS in patients who underwent surgical resection (P = 0.0001). Although the results were positive and statistically significant, we still do not know the exact reasons leading to the differences in survival among HCC patients with different expression levels of GPC3. Further in-depth research is needed. We also demonstrated that GPC3 overexpression was associated with tumor vascular invasion, hepatic cirrhosis, poorer tumor differentiation and advanced TNM stage, which were strong risk factors for the poor prognosis of HCC patients.
Heterogeneity is an important factor in meta-analysis that must be dealt with strictly. Otherwise, this factor may affect the accuracy and reliability of meta-analysis results. In the present study, the χ2 test was used to estimate heterogeneity. Although the majority of the results showed nearly no heterogeneity in our study, because of the different clinical treatments, detection methods, lengths of follow-up and the inconsistency of clinicopathological features, much heterogeneity was still detected while analyzing the relationship between GPC3 expression and tumor vascular invasion. In this case, a random-effect model was selected.
In addition, there are certain limitations in this meta-analysis that should be discussed. First, in this analysis, we searched for studies using limited databases, so the total number of eligible studies was relatively small. Second, we only enrolled suitable English-language literature reports, so there may have been certain bias due to the language criteria. Third, if HRs and their 95%CIs were not directly obtained from articles, they were calculated using the available data or Kaplan-Meier survival curves from each study according to the methods mentioned above or with statistical software. In this process, unavoidable errors could have been generated, and the reliability of the results may have been decreased. Fourth, all patients in the selected studies were from Asia (China, Japan and Taiwan), where HCC patients are predominantly HBV infected. Thus, extending and applying the present results to HCC patients from Western countries (who are primarily HCV infected) is not valid or reliable. Fifth, all studies included in this analysis were positive for GPC3 expression in tissues, but serum GPC3 expression levels could be more easily detected by a noninvasive method. Whether serum-based GPC3 expression levels are better suited to measurement than tissue-based levels remains to be investigated. Lastly, the follow-up periods in the selected studies were inconsistent.
In conclusion, based on our meta-analysis, GPC3 overexpression in tumor specimens was associated with poor OS and DFS in HCC patients. We also found that GPC3 expression was correlated with tumor vascular invasion, cirrhosis, tumor grade and tumor stage in HCC. GPC3 expression alone or in combination with other biomarkers may provide useful information about HCC progression and prognosis for the guidance of clinical treatment. Our study also indicated that GPC3 may be regarded as a potential molecular target for clinical therapy for HCC. An anti-GPC3 monoclonal antibody (GC33) has shown anticancer activity in vitro and in vivo, and a humanized IgG version is currently in clinical trials studying safety and tolerability in patients with HCC[38,39]. Further studies will be needed to develop new drugs targeting GPC3 to dramatically improve the clinical outcome in HCC, with optimistic prospects.
We thank all of the clinical investigators and patients who were involved in the studies selected in this meta-analysis.
Glypican-3 (GPC3) is known to be a specific and available molecular marker for the diagnosis of hepatocellular carcinoma (HCC). Certain studies have even reported that the diagnostic sensitivity and specificity of GPC3 are superior to those of alpha fetoprotein, which reveals a close relationship between GPC3 and HCC. However, the prognostic value of GPC3 overexpression in patients with HCC is less researched and remains controversial.
GPC3, which belongs to the glypican family, encodes cell-surface heparan sulfate proteoglycans and plays an important role in cell proliferation, differentiation and invasion. Based on the relationship between GPC3 and HCC, the research hotspot is whether the expression level of GPC3 is closely correlated with the prognosis and survival of HCC patients.
As a known available molecular marker of HCC, GPC3 has garnered increasing attention regarding its prognostic significance in HCC in recent studies. Several studies have demonstrated that the overexpression of GPC3 can predict poorer overall survival (OS) or disease-free survival (DFS) in HCC patients. However, the results are conflicting, and the sample size of each research study was limited. In the present meta-analysis, the authors synthesized different articles investigating the prognostic significance of GPC3 in HCC. These studies differed in sample size, research region and population, the characteristics of the patients included (age, histological type, differentiation and disease stage), therapeutic method and the duration of follow-up, among other factors. Data from each study were extracted and pooled to calculate the hazard ratio (HR) and the odds ratio (OR), which are the main evaluation indexes. Because of its strong objectivity, the meta-analysis enhanced the cogency of the results showing that GPC3 may be regarded as a prognostic factor in HCC.
The study suggests that GPC3 is a candidate prognostic indicator of HCC and is a potential therapeutic target that could be used in preventing the progression of HCC and improving the prognosis.
OS: Overall survival is a term that denotes the likelihood of staying alive for patients suffering from cancer. DFS: Disease-free survival is a term that denotes the length of time after the end of primary treatment for cancer during which a patient survives any signs or symptoms of that cancer. TNM staging: TNM staging is a cancer staging system that describes the extent of a person’s cancer. HR: The hazard ratio is the ratio of the hazard rates corresponding to the conditions described by two levels of an explanatory variable. OR: As a measure of association between an exposure and an outcome, the odds ratio represents the odds that an outcome will occur given a particular exposure compared with the odds of the outcome occurring in the absence of that exposure.
This is an effective meta-analysis in which the authors investigate the prognostic and clinicopathological significance of GPC3 overexpression in HCC patients. The paper is well written, and the conclusion of this study may be valuable in developing drugs for the targeted treatment of HCC.
P- Reviewers: Jothy S, Lee YJ, Nagai H S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Shiraha H, Yamamoto K, Namba M. Human hepatocyte carcinogenesis (review). Int J Oncol. 2013;42:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J, Liu Z. Repeat hepatectomy for recurrent hepatocellular carcinoma: a local experience and a systematic review. World J Surg Oncol. 2010;8:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001;108:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [PubMed] |
| 6. | Hippo Y, Watanabe K, Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y, Nakano K. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64:2418-2423. [PubMed] |
| 7. | Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16-25. [PubMed] |
| 8. | Wang XY, Degos F, Dubois S, Tessiore S, Allegretta M, Guttmann RD, Jothy S, Belghiti J, Bedossa P, Paradis V. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 2006;37:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Kandil DH, Cooper K. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Libbrecht L, Severi T, Cassiman D, Vander Borght S, Pirenne J, Nevens F, Verslype C, van Pelt J, Roskams T. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan YF, Li N, Ding HG. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410-4415. [PubMed] |
| 12. | Ozkan H, Erdal H, Koçak E, Tutkak H, Karaeren Z, Yakut M, Köklü S. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. J Clin Lab Anal. 2011;25:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, Kongtawelert P. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 2010;25:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Xu C, Yan Z, Zhou L, Wang Y. A comparison of glypican-3 with alpha-fetoprotein as a serum marker for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, Liu K, Li N, Chen D. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology. 2012;56:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Gao W, Ho M. The role of glypican-3 in regulating Wnt in hepatocellular carcinomas. Cancer Rep. 2011;1:14-19. [PubMed] |
| 17. | Cheng W, Tseng CJ, Lin TT, Cheng I, Pan HW, Hsu HC, Lee YM. Glypican-3-mediated oncogenesis involves the Insulin-like growth factor-signaling pathway. Carcinogenesis. 2008;29:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Liu S, Li Y, Chen W, Zheng P, Liu T, He W, Zhang J, Zeng X. Silencing glypican-3 expression induces apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2012;419:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Lin Q, Xiong LW, Pan XF, Gen JF, Bao GL, Sha HF, Feng JX, Ji CY, Chen M. Expression of GPC3 protein and its significance in lung squamous cell carcinoma. Med Oncol. 2012;29:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Mounajjed T, Zhang L, Wu TT. Glypican-3 expression in gastrointestinal and pancreatic epithelial neoplasms. Hum Pathol. 2013;44:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Komori H, Beppu T, Baba H, Nakatsura T, Nishimura Y. Assessment of serum GPC3 as a tumor marker for hepatocellular carcinoma and malignant melanoma. Nihon Rinsho. 2010;68 Suppl 7:833-836. [PubMed] |
| 22. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12616] [Article Influence: 841.1] [Reference Citation Analysis (0)] |
| 23. | Wells GA, OâConnell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 24. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [PubMed] |
| 25. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25764] [Article Influence: 1120.2] [Reference Citation Analysis (0)] |
| 26. | Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, Fang-yin Z, Da-yong Z, Rong-cheng L. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Wang YL, Zhu ZJ, Teng DH, Yao Z, Gao W, Shen ZY. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol. 2012;18:2408-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Yorita K, Takahashi N, Takai H, Kato A, Suzuki M, Ishiguro T, Ohtomo T, Nagaike K, Kondo K, Chijiiwa K. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int. 2011;31:120-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee WC, Chen MF, Tsai CN. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann Surg Oncol. 2012;19 Suppl 3:S455-S463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | E Y, He N, Wang Y, Fan H. Percutaneous transluminal angioplasty (PTA) alone versus PTA with balloon-expandable stent placement for short-segment femoropopliteal artery disease: a metaanalysis of randomized trials. J Vasc Interv Radiol. 2008;19:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Honsová E, Lodererová A, Franková S, Oliverius M, Trunecka P. Glypican-3 immunostaining significantly improves histological diagnosis of hepatocellular carcinoma. Cas Lek Cesk. 2011;150:37-40. [PubMed] |
| 33. | Zhang L, Liu H, Sun L, Li N, Ding H, Zheng J. Glypican-3 as a potential differential diagnosis marker for hepatocellular carcinoma: a tissue microarray-based study. Acta Histochem. 2012;114:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-β2. Neoplasia. 2011;13:735-747. [PubMed] |
| 35. | Ruan J, Liu F, Chen X, Zhao P, Su N, Xie G, Chen J, Zheng D, Luo R. Inhibition of glypican-3 expression via RNA interference influences the growth and invasive ability of the MHCC97-H human hepatocellular carcinoma cell line. Int J Mol Med. 2011;28:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Li C, Li Z, Zhu M, Zhao T, Chen L, Ji W, Chen H, Su C. Clinicopathological and prognostic significance of survivin over-expression in patients with esophageal squamous cell carcinoma: a meta-analysis. PLoS One. 2012;7:e44764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Zhang LQ, Wang J, Jiang F, Xu L, Liu FY, Yin R. Prognostic value of survivin in patients with non-small cell lung carcinoma: a systematic review with meta-analysis. PLoS One. 2012;7:e34100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Allegretta M, Filmus J. Therapeutic potential of targeting glypican-3 in hepatocellular carcinoma. Anticancer Agents Med Chem. 2011;11:543-548. [PubMed] |
| 39. | Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer. 2011;47:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |