Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.6293
Revised: January 27, 2014
Accepted: February 17, 2014
Published online: May 28, 2014
Processing time: 255 Days and 20.9 Hours
AIM: To study the pattern of cancer incidence and determine the incidence rates in Eastern Libya (for the first time in a decade).
METHODS: A hospital-based registry of cancer patients was formed using records from the primary oncology center in eastern Libya - focusing on those diagnosed in the year 2012.
RESULTS: The most common malignancies in men were cancers of the colon (22.3%, n = 90), lung (20.3%, n = 82), prostate (16.1%, n = 65), pancreas (4.2%, n = 17) and liver (4.2%, n = 17). For women, they were found to be cancers of the breast (41.5%, n = 213), colon (16.4%, n = 84), uterus (8%, n = 41), ovary (5.5%, n = 28) and pancreas (3.1%, n = 16). Additionally age-standardized rates (ASR) were determined for Libya. The different cities and towns in eastern Libya were compared for any variation. The city of Beida in particular was found to have a remarkably high incidence of gastric cancer. The different findings were discussed and comparisons were made with past literature as well as the incidence rates for neighbouring countries. The incidence rates given for the eastern region showed differences from previously reported values (i.e., the rate of colon cancer was the highest in North Africa whereas other malignancies occurred less frequently). Potential explanations for the urban-rural difference as well as the difference in incidence rates were put forth. The significance of this study is that it establishes a baseline of cancer incidence which should be the backbone for any future national cancer plan in Libya.
CONCLUSION: Proper surveillance programs need to be in place and healthcare policy should be adjusted to take into account the more prevalent and pressing cancers in society.
Core tip: The cancer incidence in Libya has changed greatly since the last time it was determined, nearly a decade ago. The most common cancers are breast, colorectal and lung cancers. Libya has the highest rate of colorectal cancer in North Africa. Late presentation was found to be a major problem in the Libyan case. Clear urban-rural differences were seen when the different districts were analyzed. Different hypotheses were put forth to explain these variations. Proper surveillance programs need to be in place and healthcare policies should be adjusted to take into account the more prevalent and pressing cancers in society.
- Citation: Bodalal Z, Azzuz R, Bendardaf R. Cancers in Eastern Libya: First results from Benghazi Medical Center. World J Gastroenterol 2014; 20(20): 6293-6301
- URL: https://www.wjgnet.com/1007-9327/full/v20/i20/6293.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i20.6293
Cancer is an important health concern in Libya, especially in the setting of an aging population and limited healthcare facilities. The Benghazi Cancer Registry began to collect data and produce results from 2003 till the year 2004[1,2]. Since then, different obstacles prevented the registry from functioning effectively and no reports have been produced.
Despite the importance of the issue, data concerning Libya is notoriously scarce and the last time age standardized rates (ASRs) were determined for this region was nearly a decade ago. In order to establish proper cancer control programs, accurate statistics for cancer incidence are absolutely vital[3-5].
Using patient records from the only oncology center in eastern Libya, a hospital based registry was formed and the cancer incidence was determined.
Libya is a North African country categorized under the Eastern Mediterranean Regional Office (EMRO) within the WHO classification. According to the 2006 census, over 5.5 million people lived in Libya, with 28.5% (n = 1613749) residing in the eastern part of the country. Benghazi is the largest city in eastern Libya with over 670000 inhabitants. The area under study covered eight major locations, namely, Ajdabia, Beida, Benghazi, Derna, Kufra, Marj, Tobruk, and Wahat. The districts consisted of urban, suburban and rural populations (as shown in Figure 1) and patients were classified under these main municipalities.
The study was approved by the Biomedical Ethics Committee of the Libyan International Medical University. All personal identifiers were stripped from the data and only medically significant parameters were analyzed.
Data were obtained from the patient records of those who were diagnosed in the Department of Oncology at the Benghazi Medical Center (BMC) from January 1, 2012 to December 31, 2012. The department receives effectively all the cancer cases in Benghazi and the overwhelming majority of the malignancy patients in eastern Libya (being the only oncological center in the region). The patients were diagnosed through various techniques particularly microscopic verification (MV) and clinically/radiologically diagnosis, however due to clerical difficulties, this parameter (i.e., the method of diagnosis) could not reliably be collected for all patients and was hence excluded from the analysis. These data serve as a good indicator for eastern Libya in general and Benghazi in particular.
Hematological malignancies were not included in this study since such patients are recorded at the Department of Hematology and their data were not made available.
Different parameters were recorded for each patient specifically, including age, gender, city, type of cancer, subtype, and stage. In the light of clerical errors, a number of cases were set aside for certain parameters but used for others. The patients were filtered by city of origin to include only patients residing in the eastern part and not referrals.
The data was computerized in a data sheet and organized as per ICD-O (International Classification of Diseases for Oncology). An SPSS-based model was designed that spanned the collected data and descriptive statistics were performed (t tests and χ2 tests).
The 2012 Libyan population was determined using the 2006 Libyan census, taking into consideration the appropriate population growth. Age-specific incidence and ASRs were calculated via the direct method using the standard population distribution[6] arranged by site of malignancy (ICD-O). This method uses the nation’s population and standardizes it to a world population distribution that was determined by the WHO. It is the standardized method used worldwide and is most prominently displayed in the “Cancer in Five Continents” series[6].
A total of 953 cases were recorded at the Department of Oncology in the BMC during the year 2012. Thirty-six cases were referrals from outside the eastern region seeking services in Benghazi and were excluded from this study, leaving 917 eligible cases. Among them, 44.1% (n = 404) were male and 55.9% (n = 513) were female. Twenty-two cases did not have a recorded date of birth, so they were included in the incidence but were excluded from any calculations involving age or age distribution (leaving 895 patients with full data).
The most common malignancies in men were cancers of the colon (22.3%, n = 90), lung (20.3%, n = 82), prostate (16.1%, n = 65), pancreas (4.2%, n = 17) and liver (4.2%, n = 17). For women, they were found to be cancers of the breast (41.5%, n = 213), colon (16.4%, n = 84), uterus (8%, n = 41), ovary (5.5%, n = 28) and pancreas (3.1%, n = 16). This ranking is based on percentage of patients, however it was found to be identical when ASRs were compared. Comparison between the genders on the basis of ASRs or percentages yielded the same findings.
The overall average age of cancer patients at presentation was 57.6 ± 15 years, ranging from 14 to 94 years. For male patients, the average age was found to be 61.9 ± 14.2 years, while for female patients the mean age of presentation was 54.3 ± 14.6 years. Male patients tend to present at significantly later ages than average (t = 5.89, P < 0.001), whereas female patients seek care earlier (t = -5.11, P < 0.001).
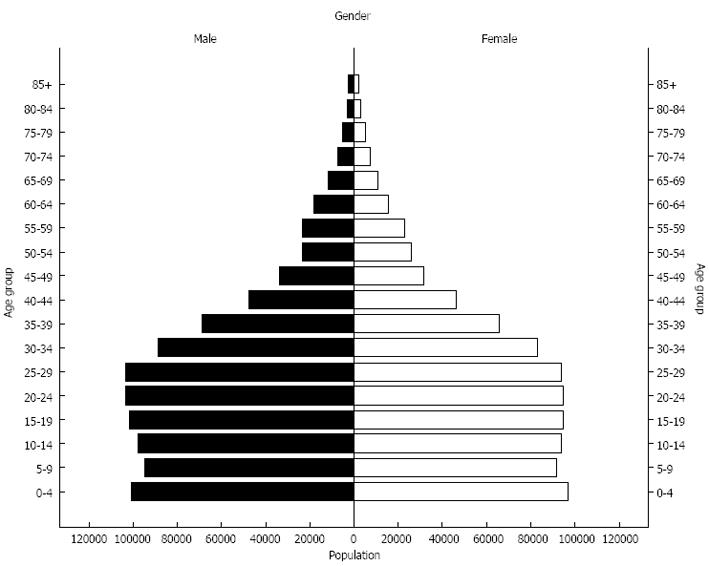
The population distribution of eastern Libya is shown in Figure 2. Table 1 shows the average age at presentation for the various forms of cancers that were studied.
| Type | n (%) | Age | ||
| Minimum | Maximum | mean ± SD | ||
| Bladder | 20 (2.2) | 38 | 78 | 62.2 ± 11.5 |
| Bone Tumour | 7 (0.9) | 17 | 74 | 48.1 ± 26.2 |
| Brain | 23 (2.5) | 15 | 81 | 47.5 ± 17.1 |
| Breast | 214 (23.7) | 23 | 87 | 51.3 ± 12.7 |
| Cervix | 13 (1.5) | 43 | 81 | 57.5 ± 11.8 |
| Colon | 170 (19.0) | 21 | 89 | 58.7 ± 13.4 |
| CUP | 18 (2.1) | 36 | 87 | 61.2 ± 16.5 |
| Esophagus | 4 (0.4) | 45 | 86 | 59.0 ± 18.7 |
| Gallbladder | 13 (1.5) | 36 | 77 | 60.5 ± 12.5 |
| Kidney | 21 (2.4) | 34 | 82 | 61.4 ± 13.2 |
| Larynx | 14 (1.6) | 26 | 89 | 67.6 ± 15.4 |
| Liver | 22 (2.4) | 48 | 84 | 66.7 ± 9.7 |
| Lung | 93 (10.3) | 27 | 85 | 62.4 ± 11.9 |
| Misc. | 13 (1.4) | 25 | 82 | 54.5 ± 21.2 |
| Nasopharyngeal | 16 (1.7) | 20 | 64 | 46.3 ± 10.4 |
| Ovary | 26 (3.1) | 32 | 86 | 54.7 ± 13.6 |
| Pancreas | 33 (3.6) | 29 | 83 | 61.4 ± 13.4 |
| Prostate | 65 (7.1) | 50 | 91 | 72.9 ± 9.0 |
| Salivary | 4 (0.4) | 39 | 74 | 55.3 ± 14.9 |
| Skin | 10 (1.1) | 29 | 92 | 63.0 ± 18.0 |
| Soft Tissue | 28 (3.2) | 14 | 84 | 45.1 ± 20.9 |
| Stomach | 22 (2.7) | 34 | 82 | 57.6 ± 13.0 |
| Thyroid | 7 (0.8) | 34 | 71 | 50.1 ± 12.7 |
| Uterus | 39 (4.5) | 20 | 80 | 56.8 ± 13.8 |
| Overall | 895 (100.0) | 14 | 92 | 57.6 ± 15.0 |
Benghazi contributed the largest proportion of cancer cases in eastern Libya (60.5%, n = 555), followed by Beida (10.1%, n = 93) and Marj (8.3%, n = 76). The average age of presentation was determined for each city. It was found that only Marj (t = 3.569, P = 0.001) and Tobruk (t = -2.109, P = 0.039) had any statistically significant difference from Benghazi in terms of age. The features of the different cities are shown in Table 2, along with the three most common cancers in those areas.
| City | n (%) | Avg age | SD | No 1. cancer | No 2. cancer | No 3. cancer |
| Ajdabia | 55 (6.0) | 56.2 | 16.7 | Lung | Breast | Colon |
| Beida | 93 (10.1) | 58.5 | 14.2 | Breast | Colon | Stomach |
| Benghazi | 555 (60.5) | 57.5 | 15.0 | Breast | Colon | Lung |
| Derna | 45 (4.9) | 57.4 | 13.8 | Breast | Colon | - |
| Kufra | 16 (1.7) | 50.1 | 18.9 | Breast | Colon | Prostate |
| Marj | 76 (8.3) | 63.2 | 13.8 | Colon | Prostate | Breast |
| Tobruk | 66 (7.2) | 53.8 | 14.0 | Breast | Colon | Lung |
| Wahat | 11 (1.2) | 60.2 | 14.7 | Breast | Prostate | - |
| Overall | 917 (100.0) | 57.6 | 15.0 | Breast | Colon | Lung |
The overwhelming majority of the oncological patients were Libyans (97.1%, n = 890) as opposed to foreign nationals (2.9%, n = 27). The latter were under-represented among cancer patients (χ2 = 17.644, P < 0.001).
The most common cancer (overall) was found to be breast cancer (23.7%, n = 217), followed by colon cancer (19%, n = 174) and lung cancer (10.3%, n = 94) as shown in Table 2. However, within certain cities the distributive ranking of the types of malignancies varied (Table 2).
The gender predominance was observed for the cancer cases and is presented in Table 3. In addition, the specific distribution of patients by the site of the cancer and their age group is depicted in the table. The age-specific distribution and age-standardized rates are put forth in Table 4. These values represent the incidence rates per 100000 people and a comparison is made with previously reported values for Libya as well as the figures reported by cancer registries in neighbouring countries (i.e., Egypt, Tunisia and Algeria).
| 0-14 yr | 15-24 yr | 25-34 yr | 35-44 yr | 45-54 yr | 55-64 yr | 65+ yr | Total | |||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Bladder (C67) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 5 | 7 | 2 | 11 | 8 |
| Bone (C40-41) | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 6 |
| Brain (C70-72) | 0 | 0 | 0 | 1 | 2 | 1 | 4 | 4 | 1 | 2 | 3 | 0 | 4 | 1 | 14 | 9 |
| Breast (C50) | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 52 | 2 | 67 | 0 | 46 | 2 | 31 | 4 | 210 |
| Cervix uteri (C53) | - | 0 | - | 0 | - | 0 | - | 2 | - | 4 | - | 2 | - | 5 | - | 13 |
| Colon (C18-21) | 0 | 0 | 0 | 0 | 5 | 3 | 8 | 9 | 17 | 18 | 27 | 24 | 29 | 30 | 86 | 84 |
| Esophagus (C15) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 3 | 1 |
| Gallbladder etc. (C23-24) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | 4 | 2 | 1 | 7 | 6 |
| Kidney (C64) | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 2 | 3 | 6 | 4 | 12 | 9 |
| Larynx (C32) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4 | 0 | 7 | 2 | 11 | 3 |
| Liver (C22) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 2 | 12 | 2 | 17 | 5 |
| Lung (C33-34) | 0 | 0 | 0 | 0 | 0 | 2 | 6 | 2 | 11 | 1 | 26 | 4 | 35 | 3 | 78 | 12 |
| Nasopharynx (C11) | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 1 | 4 | 1 | 3 | 0 | 0 | 0 | 14 | 2 |
| Ovary (C56) | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 11 | 0 | 4 | 0 | 6 | 0 | 24 |
| Pancreas (C25) | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 1 | 2 | 0 | 10 | 12 | 3 | 17 | 16 |
| Prostate (C61) | 0 | - | 0 | - | 0 | - | 0 | - | 1 | - | 10 | - | 54 | - | 65 | - |
| Salivary (C07-08) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 1 | 4 | 0 | 8 | 2 |
| Soft tissue (C47+49) | 0 | 2 | 2 | 2 | 1 | 1 | 1 | 4 | 6 | 0 | 2 | 1 | 4 | 2 | 16 | 12 |
| Stomach (C16) | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 2 | 3 | 1 | 1 | 6 | 4 | 13 | 9 |
| Thyroid (C73) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 2 | 5 |
| Uterus (C54) | - | 0 | - | 0 | - | 2 | - | 4 | - | 9 | - | 12 | - | 12 | - | 39 |
| Miscellaneous (O and U and skin etc.) | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 3 | 1 | 4 | 1 | 7 | 9 | 10 | 15 | 26 |
| Total (excluding missing cases) | 0 | 2 | 2 | 4 | 13 | 33 | 41 | 86 | 53 | 127 | 90 | 128 | 195 | 121 | 394 | 501 |
| Site | Benghazi (2012) | Benghazi (2003)[2] | Benghazi (Globocan est. 2002)[5] | Western Libya (2007)[36] | Algeria[6] | Egypt[6] | Tunis[6] | |||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Bladder (C67) | 3.3 | 1.1 | 11.7 | 1.3 | 22.4 | 2.7 | 4.8 | 1.2 | 4.5 | 0.5 | 27.9 | 5.4 | 19.0 | 2.2 |
| Bone (C40-41) | 0.3 | 1.1 | - | - | - | - | - | - | 1.4 | 1.2 | 1.5 | 1.0 | 0.4 | 0.5 |
| Brain (C70-72) | 2.3 | 1.3 | 5.3 | 2.1 | 2.1 | 1.1 | 4.8 | 0.7 | 0.7 | 1.6 | 4.0 | 3.1 | 3.7 | 1.8 |
| Breast (C50) | 0.9 | 37.4 | - | 22.9 | - | 17.4 | 0.5 | 31.1 | 0.6 | 18.8 | 0.8 | 42.5 | 0.9 | 29.8 |
| Cervix uteri (C53) | - | 2.8 | - | 5.3 | - | 8.2 | - | 2.2 | - | 11.6 | - | 2.1 | - | 7.1 |
| Colon (C18-21) | 17.5 | 17.2 | 11.6 | 8.8 | 4.9 | 2.5 | 14.2 | 12.0 | 6.6 | 6.8 | 6.9 | 4.8 | 12.0 | 9.2 |
| Others and Unspec. | 1.8 | 2.2 | - | - | - | - | - | - | 2.7 | 2.1 | 10.3 | 7.3 | 5.7 | 4.3 |
| Esophagus (C15) | 0.5 | 0.2 | 1.5 | 0.0 | 2.4 | 1.0 | 1.3 | 2.8 | 0.2 | 0.2 | 1.7 | 0.9 | 0.5 | 0.2 |
| Gallbladder, etc. (C23-24) | 1.3 | 1.3 | 1.6 | 3.6 | - | - | - | - | 2.1 | 10.0 | 1.2 | 1.0 | 1.8 | 3.1 |
| Kidney (C64) | 2.7 | 1.8 | 3.9 | 2.8 | 1.8 | 0.9 | 2.6 | 1.7 | 0.7 | 1.2 | 2.5 | 1.5 | 2.6 | 1.6 |
| Larynx (C32) | 2.5 | 0.6 | 6.1 | 0.3 | 3.7 | 0.5 | - | - | 2.8 | 0.1 | 4.2 | 0.3 | 5.7 | 0.2 |
| Liver (C22) | 4.0 | 1.0 | 3.3 | 3.1 | 4.8 | 1.6 | 5.1 | 1.9 | 1.1 | 0.8 | 21.9 | 4.4 | 2.2 | 0.7 |
| Lung (C33-34) | 18.0 | 2.2 | 24.8 | 2.0 | 10.4 | 1.5 | 19.3 | 3.9 | 19.9 | 1.7 | 14.0 | 3.6 | 37.1 | 1.7 |
| Nasopharynx (C11) | 2.2 | 0.3 | 1.9 | 0.8 | 2.2 | 0.9 | - | - | 5.4 | 1.7 | 1.2 | 0.4 | 4.6 | 1.9 |
| Ovary (C56) | - | 4.9 | - | 4.0 | - | 1.9 | - | 3.0 | - | 2.1 | - | 5.1 | - | 3.3 |
| Pancreas (C25) | 3.8 | 3.3 | 4.4 | 1.6 | 1.5 | 0.5 | 1.9 | 0.6 | 0.5 | 0.3 | 4.0 | 2.3 | 2.5 | 1.9 |
| Prostate (C61) | 14.8 | - | 11.4 | - | 5.6 | - | 19.1 | - | 7.5 | - | 8.5 | - | 14.1 | - |
| Salivary (C07-08) | 0.1 | 0.6 | - | - | - | - | - | - | 0.3 | 0.2 | 0.7 | 0.6 | 0.9 | 0.4 |
| Soft tissue (C47+49) | 2.8 | 1.6 | - | - | - | - | - | - | 0.8 | 0.5 | 3.3 | 2.7 | 1.4 | 1.3 |
| Stomach (C16) | 2.7 | 1.9 | 4.4 | 3.4 | 4.1 | 1.5 | 1.4 | 2.6 | 7.1 | 3.1 | 3.3 | 2.0 | 5.1 | 2.5 |
| Thyroid (C73) | 0.4 | 0.8 | - | - | - | - | 0.8 | 1.7 | 1.4 | 3.6 | 1.1 | 2.6 | 1.3 | 3.1 |
| Uterus (C54) | - | 8.0 | - | 8.0 | - | 1.6 | - | - | - | 1.1 | - | 2.6 | - | 3.4 |
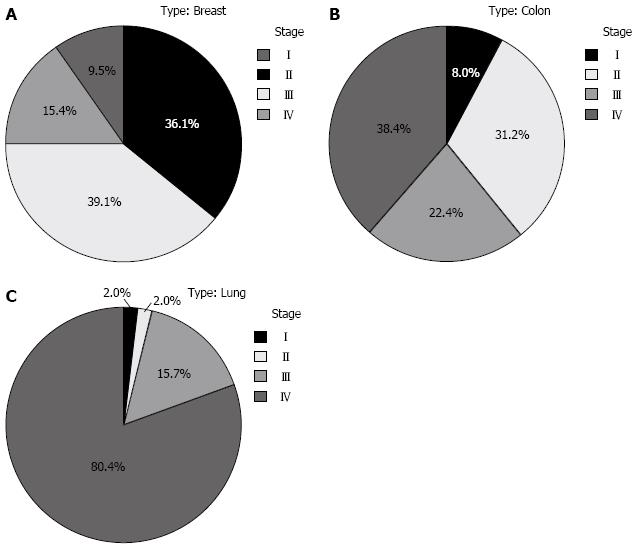
The three most common malignancies (i.e., breast, colon, and lung cancers) were studied to determine the clinical stage at presentation. For breast cancer, more than half (54.5%, n = 92) were diagnosed at advanced stages (III/IV). The situation was worse in colon cancer cases (60.8%, n = 76), with the majority of patients presenting at stage IV (38.4%, n = 48). However, the worst situation was in lung cancer where 96.1% were at late stages with 80.4% presenting at stage IV. This is further illustrated in Figure 3.
The fourth most common malignancy, prostate cancer, was studied in terms of its differentiation (being a better indicator of prognosis than clinical stage). Sixty-five cases were recorded, however, 25 had missing data for grade (due to clerical errors). Of the remaining patients (63%, n = 41), around 9.8% (n = 4), 53.7% (n = 22), and 36.6% (n = 15) were graded with well, moderate and poor differentiation, respectively.
Comprehensive studies in the field of cancer epidemiology are inherently limited in developing countries by the dearth of tools for disease control and cancer surveillance[7]. This is further compounded in the Libyan scenario by the lack of a proper documentation system, absent digitalization of patient records and no central authority to follow cancer patients on a long term basis (i.e., surveillance).
Previous reports have been made for Libya in terms of cancer incidence[2,8], however, the last published report done in Benghazi was nearly a decade ago. This is the second time that ASRs were ever calculated for Libya. It is desirable to see what changes may have occurred in that period of time. Moreover, being based in the only oncology department in the region, the percentage of cancer patients in eastern Libya who seek its services is very high.
In certain cancers, the incidence was found to be much higher than the surrounding countries or even past figures for Benghazi. This is especially true for breast cancer (in females) and colon cancer (for both sexes). A potential explanation for our divergence from the GLOBOCAN estimate is that they are often based on rates in neighbouring countries[9].
While on the whole, women formed the majority of oncological patients, they were predominantly in the form of breast cancer. Hence, their representation was found to be greatest till the age of 60, after which men formed the bulk of malignancy patients. This reflects the nature of cancers affecting the different sexes. It was observed that the major malignancies in males tend to present at later ages. Another potential factor is that men tend to hide symptoms and delay seeking medical care for the sake of bravado (especially in developing countries).
The new incidence rates show a change from previous values calculated for Benghazi. Males presented more often with colon and lung cancers than any other malignancies. The incidence rate for colon cancer in Libya was found to be the highest in its region. We suspect that there is strong association between this high rate of colon cancer and the dietary and lifestyle habits of the Libyan population. This sharp increase in the incidence rate of colorectal carcinoma may be explained by the greater availability of endoscopic techniques and better detection.
While the overall incidence of colon cancer was fairly close between the sexes, the same cannot be said for lung cancer. The male to female ratio of lung cancer was found to be 6.8, a large decrease from values reported in past literature (18.6:1 ratio)[10].
Eastern Libya had the lowest rates for lung cancer in the region. Men are eight times more likely to have lung malignancies than women. This is similar to the situation in other surrounding areas where heavy smoking among males would lead to these figures[11]. While smoking is on the decline in the developed nations[12], it is expected to rise in our part of the world. Prostate cancer was also common; however, there was an underestimation for the incidence in previous literature. They also formed the oldest patients (on average) which increased the overall average age of male patients.
The incidence of breast cancer among Libyan women was found to be high (the second highest in the region). Furthermore, the average age of our breast cancer patients is low by international standards. Patients under the age of 51 year formed 57% (n = 122) of the cases. Despite presenting at younger ages, the clinical stage at diagnosis is usually advanced. It was reported that the median reported age for Libyan breast cancer patients was 7.5 mo after symptoms[13]. Late presentation of women for breast cancer can be due to a number of factors such as not knowing the major signs of breast cancer[14], non-disclosure of symptoms to others[15], low risk perception[16], fears and negative outlook about treatment[17-19] and fatalistic attitude[20].
Libyan men were also more likely to be affected by breast cancer than their counterparts from the other North-African nations. African males themselves have been found to be more at risk of breast cancer than others[21]. Usually male breast cancer patients present at later ages and more advanced stages[22].
An interesting finding was that the ASR’s calculated for Benghazi in 2012 now resemble the values in western Libya (with a few differences). It was previously thought that the rate of malignancies in the eastern region was significantly higher.
Benghazi is the largest city in the eastern region of Libya and is the main center of high level healthcare. Naturally, it would produce the greatest number of patients with a cancer pattern similar to that of an urban center[23,24]. However, it is when we compare between the cities that interesting observations can be made.
Long term studies are required to determine the ASR for the various districts individually. This would serve to confirm/refute the existence of a statistically significant rural-urban difference similar to other countries[25-27]. Ajdabia formed only 6% of the patients overall, but 13.8% of lung cancer patients came from that town (i.e., over-representation). Moreover, the most common malignancy in that area was lung cancer. In Libya, Ajdabia is well known for having frequent Ghibli (dust storms) which would increase the inspiration of inorganic particles (respirable particles). This is mostly sand and soil and might induce a sort of pneumoconiosis-like condition[28]. Additionally, it is worth mentioning that Ajdabia is situated near the main oil and gas centers in Libya. These factors may be related to the elevated rank of lung cancer in that region.
Similarly, Wahat recorded prostate cancer as the second most common malignancy (as opposed to colon cancer). Another good example is the case of Beida where the third most common malignancy is gastric cancer. Despite forming only 10% of the recorded patients, over one third (36%) of gastric cancer cases were from the town. This may be due to their diet (which largely depends on locally grown produce), the use of insecticide or perhaps an increased incidence of Helicobacter pylori infection among the inhabitants of the city[29]. Further studies in this topic are needed in order to make a sure statement.
North African countries have traditionally had higher rates of nasopharyngeal cancers[30], however, the figures presented in the Libyan scenario were lower than the neighbouring nations. This could be explained by a lower prevalence of certain risk factors such as alcohol and excessively spicy food.
Due to the descriptive nature of this study, certain caveats seem appropriate. For example, the data was collected retrospectively in the setting of poor-quality patient record keeping. The lack of a central mortality centre in North African countries has traditionally prevented them from providing mortality figures for their cancer patients[7,31,32], and Libya is no exception to this trend[33]. Furthermore, due to difficulties in obtaining data from the cemetery facilities, death certificate only (DCO) cases were not included in this study, however, only nine percent of cancer cases in eastern Libya fall under this category[2]. Additionally, hematological malignancies were not covered in this study since the data from the Department of Hematology were not made available.
Previous studies have warned that the values calculated for incidence may be overestimated due to the misclassification of a significant number of prevalent cases (i.e., diagnosed in previous years)[2,34]. This limitation does not exist in this study since we only included cancer cases diagnosed within the specified time interval (namely the year 2012). Hospital based series may also overestimate the easily diagnosed malignancies due to the availability to facilities (i.e., mammography and endoscopy) and under-report the more difficult cancers which are sent abroad[35].
Nonetheless, these data are a good representation of the cancer pattern and incidence in the region.
The scenario presented in these results correlate well to the experience of oncologists in Benghazi. It is the sincere hope of the authors that this article is used to establish a baseline and highlight the areas where the Libyan healthcare system needs to focus. Particular emphasis needs to be placed on cancer awareness since late presentation is a scourge in Libya. Cancer is a preventable form of mortality, especially with manageable malignancies such as breast and colon. Cancer surveillance plans need to be set in place in Libya in order to monitor the trends and incidence rates. With these establishments, we can finally hope to make a change in our country and raise the level of healthcare in our facilities.
Cancer is rapidly becoming a major cause of morbidity and mortality worldwide, especially in the light of the aging populations in the developing world. Despite the growing malignancy problem in third world countries, cancer epidemiology is mostly based on values from developed countries. Cancer incidence for Libya has not been studied for nearly a decade for different reasons.
National cancer surveillance plans cannot be set in place without first determining recent baseline incidence rates.
For the first time in ten years, all the cancer cases in eastern Libya (an area covering a population of around two million) were gathered and subsequently studied. Using population data from the 2006 Libyan census with projections for future years, the age standardized incidence (ASR) was calculated. The ranking of different cancers was seen and interestingly, breast cancer was found to be the most common malignancy. Incidence rates were determined and compared to previously reported values for Libya and for neighbouring countries. For instance, it was found that Libya had the highest incidence rate for colorectal carcinoma in North Africa. Various parameters were gathered for the patients, including age, gender, nationality, site of malignancy and clinical stage. The geographical distribution of cancer patients in Libya was also studied for the first time.
Based on the values in this study, the health authorities in Libya will be able to design and set into action a national cancer plan. The more prevalent forms of cancer should receive greater attention either at the government or media level. Improved patient awareness and removal of the stigma/fatalism of a cancer diagnosis are critical in order to improve the prognosis for the patients. Certain regions contributed more in terms of patient load and hence more focus needs to be placed there.
ASR is an internationally used measure of new cancer cases relative to the standard world population (as stated in the Cancer in Five Continents series).
This paper is really interesting.
P- Reviewer: Patelarou E S- Editor: Song XX L- Editor: Wang TQ E- Editor: Ma S
| 1. | El Mistiri M, Verdecchia A, Rashid I, El Sahli N, El Mangush M, Federico M. Cancer incidence in eastern Libya: the first report from the Benghazi Cancer Registry, 2003. Int J Cancer. 2007;120:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 2. | El Mistiri M, Pirani M, El Sahli N, El Mangoush M, Attia A, Shembesh R, Habel S, El Homry F, Hamad S, Federico M. Cancer profile in Eastern Libya: incidence and mortality in the year 2004. Ann Oncol. 2010;21:1924-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. National cancer control programmes. Policies and managerial guidelines. 2nd ed. Geneva: World Health Organization 2002; Available from: http://www.hqlibdoc.who.int/hq/2002/9241545577.pdf. |
| 4. | Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11837] [Article Influence: 845.5] [Reference Citation Analysis (4)] |
| 6. | Curado M, Edwards B, Shin H, Storm H, Ferlay J, Heanue M, Boyle P, editors . Cancer Incidence in Five Continents. Lyon: IARC Scientific Publications 2008; . |
| 7. | Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, Yazdanbod A, Shokoohi B, Mashayekhi A, Arshi S. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. In: Ferlay J, Bray F, Pisani P, Parkin D, editors. IARC cancer base No 5. Lyon: IARC Press 2004; . |
| 9. | Nasseri K, Mills PK, Allan M. Cancer incidence in the Middle Eastern population of California, 1988-2004. Asian Pac J Cancer Prev. 2007;8:405-411. [PubMed] |
| 10. | Akhtar SS, Abu Bakr MA, Dawi SA, Huq IU. Cancer in Libya--a retrospective study (1981-1985). Afr J Med Med Sci. 1993;22:17-24. [PubMed] |
| 11. | Baili P, De Angelis R, Casella I, Grande E, Inghelmann R, Francisci S, Verdecchia A, Capocaccia R, Meneghini E, Micheli A. Italian cancer burden by broad geographical area. Tumori. 2007;93:398-407. [PubMed] |
| 12. | Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 680] [Cited by in RCA: 697] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 13. | Ermiah E, Abdalla F, Buhmeida A, Larbesh E, Pyrhönen S, Collan Y. Diagnosis delay in Libyan female breast cancer. BMC Res Notes. 2012;5:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Burgess C, Hunter MS, Ramirez AJ. A qualitative study of delay among women reporting symptoms of breast cancer. Br J Gen Pract. 2001;51:967-971. [PubMed] |
| 15. | Burgess CC, Ramirez AJ, Richards MA, Love SB. Who and what influences delayed presentation in breast cancer? Br J Cancer. 1998;77:1343-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Meechan G, Collins J, Petrie K. Delay in seeking medical care for self-detected breast symptoms in New Zealand women. N Z Med J. 2002;115:U257. [PubMed] |
| 17. | Bish A, Ramirez A, Burgess C, Hunter M. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Burgess CC, Potts HW, Hamed H, Bish AM, Hunter MS, Richards MA, Ramirez AJ. Why do older women delay presentation with breast cancer symptoms? Psychooncology. 2006;15:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Norsa’adah B, Rampal KG, Rahmah MA, Naing NN, Biswal BM. Diagnosis delay of breast cancer and its associated factors in Malaysian women. BMC Cancer. 2011;11:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Gullatte MM, Brawley O, Kinney A, Powe B, Mooney K. Religiosity, spirituality, and cancer fatalism beliefs on delay in breast cancer diagnosis in African American women. J Relig Health. 2010;49:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Ndom P, Um G, Bell EM, Eloundou A, Hossain NM, Huo D. A meta-analysis of male breast cancer in Africa. Breast. 2012;21:237-241. [PubMed] |
| 22. | El-Habbash MM, Alwindi AA. Male breast cancer in Tripoli, Libya. Saudi Med J. 2009;30:1060-1062. [PubMed] |
| 23. | Dey S, Soliman AS, Hablas A, Seifeldein IA, Ismail K, Ramadan M, El-Hamzawy H, Wilson ML, Banerjee M, Boffetta P. Urban-rural differences in breast cancer incidence in Egypt (1999-2006). Breast. 2010;19:417-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Mohagheghi MA, Mosavi-Jarrahi A, Malekzadeh R, Parkin M. Cancer incidence in Tehran metropolis: the first report from the Tehran Population-based Cancer Registry, 1998-2001. Arch Iran Med. 2009;12:15-23. [PubMed] |
| 25. | Dey S, Zhang Z, Hablas A, Seifeldein IA, Ramadan M, El-Hamzawy H, Soliman AS. Geographic patterns of cancer in the population-based registry of Egypt: Possible links to environmental exposures. Cancer Epidemiol. 2011;35:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Dey S, Hablas A, Seifeldin IA, Ismail K, Ramadan M, El-Hamzawy H, Wilson ML, Banerjee M, Boffetta P, Harford J. Urban-rural differences of gynaecological malignancies in Egypt (1999-2002). BJOG. 2010;117:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Valerianova Z, Gill C, Duffy SW, Danon SE. Trends in incidence of various cancers in Bulgaria, 1981-1990. Int J Epidemiol. 1994;23:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Archer JD, Cooper GS, Reist PC, Storm JF, Nylander-French LA. Exposure to respirable crystalline silica in eastern North Carolina farm workers. AIHA J (Fairfax, Va). 2002;63:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Elzouki AN, Buhjab SI, Alkialani A, Habel S, Sasco AJ. Gastric cancer and Helicobacter pylori infection in the eastern Libya: a descriptive epidemiological study. Arab J Gastroenterol. 2012;13:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Sobrato I, Busso P, Zanetti R. [What are we learning from the new data on cancer incidence in North Africa]. Epidemiol Prev. 2010;34:23-26. [PubMed] |
| 31. | Mans DR, Mohamedradja RN, Hoeblal AR, Rampadarath R, Joe SS, Wong J, Ramautar P, Mahabier R, Vrede MA. Cancer incidence in Suriname from 1980 through 2000 a descriptive study. Tumori. 2003;89:368-376. [PubMed] |
| 32. | Gibson TN, Blake G, Hanchard B, Waugh N, McNaughton D. Age-specific incidence of cancer in Kingston and St Andrew, Jamaica, 1998-2002. West Indian Med J. 2008;57:81-89. [PubMed] |
| 33. | Zanetti R, Tazi MA, Rosso S. New data tells us more about cancer incidence in North Africa. Eur J Cancer. 2010;46:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Jensen O, Parkin D, MacLennan R, Muir C, Skeet R. Cancer registration: principles and methods. Lyon: IARC Press 2003; 304 p Available from: http://www.iarc.fr. |
| 35. | Awadelkarim KD, Mariani-Costantini R, Elwali NE. Cancer in the Sudan: an overview of the current status of knowledge on tumor patterns and risk factors. Sci Total Environ. 2012;423:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | National Oncology Institute. First Annual Report: Population Based Cancer Registry. Sibratha: Sibratha Cancer Registry 2008; Available from: http://www.ncisabratha.ly/nci/filesystem/uploads/REPORT1.pdf. |