Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.6159
Revised: January 26, 2014
Accepted: March 19, 2014
Published online: May 28, 2014
Processing time: 212 Days and 7 Hours
Liver transplantation (LT) is the best treatment for end-stage hepatic failure, with an excellent survival rates over the last decade. Biliary complications after LT pose a major challenge especially with the increasing number of procured organs after circulatory death. Ischaemic cholangiopathy (IC) is a set of disorders characterized by multiple diffuse strictures affecting the graft biliary system in the absence of hepatic artery thrombosis or stenosis. It commonly presents with cholestasis and cholangitis resulting in higher readmission rates, longer length of stay, repeated therapeutic interventions, and eventually re-transplantation with consequent effects on the patient’s quality of life and increased health care costs. The pathogenesis of IC is unclear and exhibits a higher prevalence with prolonged ischaemia time, donation after circulatory death (DCD), rejection, and cytomegalovirus infection. The majority of IC occurs within 12 mo after LT. Prolonged warm ischaemic times predispose to a profound injury with a subsequently higher prevalence of IC. Biliary complications and IC rates are between 16% and 29% in DCD grafts compared to between 3% and 17% in donation after brain death (DBD) grafts. The majority of ischaemic biliary lesions occur within 30 d in DCD compared to 90 d in DBD grafts following transplantation. However, there are many other risk factors for IC that should be considered. The benefits of DCD in expanding the donor pool are hindered by the higher incidence of IC with increased rates of re-transplantation. Careful donor selection and procurement might help to optimize the utilization of DCD grafts.
Core tip: Biliary complications after liver transplantation represent a major challenge. Ischaemic cholangiopathy is a set of disorders characterized by multiple diffuse intrahepatic strictures. Ischaemic cholangiopathy can cause a late graft loss. It becomes more evident after the widespread usage of grafts after circulatory deaths. Awareness of predisposing factors, presentation, diagnosis, and management is mandatory. Prophylaxis is essential by controlling risk factors. Management varies from simple radiological interventions to re-transplantation.
- Citation: Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol 2014; 20(20): 6159-6169
- URL: https://www.wjgnet.com/1007-9327/full/v20/i20/6159.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i20.6159
Liver transplantation (LT) has achieved excellent 5-year survival rates in the last decade. One of the current challenges is the high incidence of biliary complications after transplantation. Biliary complications after LT represent a major surgical problem with incidence of up to 30%[1-3]. They include a variety of functional and structural biliary abnormalities that alter the hepatic function and contribute to graft dysfunction leading eventually to biliary reconstruction and/or re-transplantation[4,5]. The classification of biliary complications should refer to their aetiology. Many factors are identified as predisposing to post-transplant biliary complications which include: hepatic artery thrombosis/stenosis, immunological disorders, rejection, ischaemia-reperfusion injury (IR), and surgical reconstructive technique[6,7]. These factors can lead to ischaemic-related biliary complications that are seen with the increased use of donation after circulatory death (DCD) grafts[8,9].
Ischaemic cholangiopathy (IC) is a wide spectrum pathological entity characterized by multiple diffuse intra-hepatic strictures in the absence of hepatic artery thrombosis or stenosis[10-12]. The identification of factors associated with ischaemic biliary damage remains a challenge especially with increased the related-mortality by 8%-15%[1,13]. IC also impacts on the quality of life and has as a significant economic effect. Patients with IC experience higher readmission rates, longer length of stay (LOS), repeated radiologic-guided procedures and higher incidence of re-transplantation as a final definite treatment[14,15]. Jay et al[16] showed that, patients with IC receive a median of 12 invasive biliary procedures (range, 0-21) in the first 2 years following transplantation and this is associated with higher costs reaching around 54% over and above the first post-transplant year costs[17]. In addition, poor graft survival is significantly related to the development of IC and this is evident among DCD recipients[18,19].
We have reviewed the current literature on incidence, risk factors, and management of ischaemic cholangiopathy after liver transplantation with attention to the proposed precautions that could be taken during the liver procurement and transplantation to decrease its occurrence.
Post-transplant biliary strictures are classified into two types: (1) anastomotic strictures (AS) which are defined as strictures at the site of bile duct anastomosis (choledochocholedochostomy or choledochojejunostomy); and (2) non-anastomotic biliary strictures (NAS) which may be either extrahepatic or intrahepatic[20].
The incidence of AS after LT is between 5% and 10%[13]. They are often isolated, short in length, and are a result of fibrotic healing at the site of a biliary anastomosis within the first year after transplantation[21,22]. The pathogenesis of AS are attributed to many factors that appear early in the post-transplant period. Technical issues appear to be the most important factor which include: improper surgical technique, small calibre of the bile ducts, a mismatch in size between the donor and recipient bile ducts, inappropriate suture material, tension at the anastomosis, excessive use of electrocauterization for control of bile duct bleeding, and infection[13,23,24]. Management of AS is mainly by endoscopic management, percutaneous therapy, surgical revision which is now reserved for patients who have failed the previous modalities, and re-transplantation is the final option when all else fails[25,26].
The NAS are due to hepatic artery thrombosis, stenosis, or IC which account for 10%-25% of all stricture complications after LT[13,23,27-29]. The majority are due to hepatic artery thrombosis. The diffuse NAS after liver transplantation in the presence of a patent hepatic artery have been termed ischaemic cholangiopathy, it initially described by Sanchez-Urdazpal et al[10] at the Mayo Clinic, and Li et al[30] at the University of Nebraska. The incidence of IC was noticed between 2% and 20% of patients, localizing proximal to the anastomotic site affecting only the donor biliary tree. However, they were indistinguishable on cholangiography from strictures caused by hepatic artery thrombosis or stenosis[7,13]. The pathogenesis of IC remains unclear. It occurs either secondary to microangiopathic injury (IR injury, prolonged cold and warm ischaemia times, DCD grafts, prolonged donor ITU stay and use of dopamine), or secondary to immunological causes (ABO-incompatibility, rejection, and CMV infection)[26,31-33]. The majority of IC occurs within 12 mo following transplantation, and their prevalence continues to increase with time after LT[5].
Based on prognostic and therapeutic implications, NAS were classified into extrahepatic lesions (type I), intrahepatic (type II) or a combination of intrahepatic and extrahepatic abnormalities[34,35]. As the success of therapy in IC depends on the extent of intrahepatic biliary lesions, Buis et al[36] have suggested a classification of the involved intrahepatic zones A to D. The extrahepatic common bile duct, including the hilar bifurcation (zone A), the bile ducts between first and second order branches (zone B), the bile ducts between second and third order branches (zone C), and bile ducts in the periphery of the liver (zone D). In addition, the location of the strictures was categorized as left sided, right sided or bilateral. Involvement of zone C seems to predict a more severe clinical course, because of therapeutic difficulties. Zone D, representing the small peripheral ducts, is mainly involved in late forms of NAS with an immunological pathogenesis.
The majority of IC manifests between 1 to 6 mo after LT with involvement of both intrahepatic and extrahepatic bile ducts, while a few develop only extrahepatic or intrahepatic lesions[4,37]. Patients appear to present with similar non-specific symptoms as patients with anastomotic strictures[5]. IC may be asymptomatic during the initial period of the disease, the diagnosis being made when abnormal biochemical liver tests are discovered, usually elevated levels of serum alkaline phosphatase and gamma-glutamyl transferase. Progressive cholestasis and cholangitis are the two major presenting features. Later presentation of the disease includes itching, jaundice, and hepatocellular failure. However, some patients may have a very minor form of the disease and will never develop such complications[38]. As a result of cholestasis, formation of gallstones, sludge, and casts in the biliary tree is common and predisposes to recurrent attacks of cholangitis[39]. These most likely arise from ongoing sloughing of the biliary epithelium as a result of the underlying ischaemic or immunologic injury[40]. Patients with IC may be admitted many times to manage acute cholangitis with twice as long hospital stay compared to non-sufferers[15]. IC may present as bile duct necrosis, bile leak, biloma, bile duct fibrosis or stenosis. Bile duct necrosis and bilomas are rare with IC and occur predominantly when there is a sudden and complete interruption of arterial blood supply as in hepatic artery thrombosis[41,42].
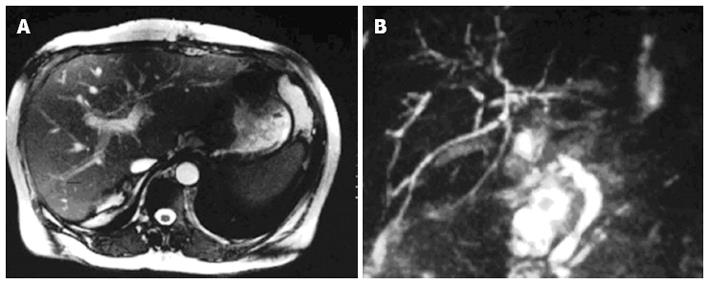
To diagnosis IC, patients need to have abnormal liver function tests and diffuse intrahepatic strictures on magnetic resonance imaging, endoscopic retrograde cholangiography or percutaneous transhepatic cholangiography. Cholangiographic findings include diffuse and multiple bile duct abnormalities (Figure 1). Bile ducts are affected in a pauci- or pluri-focal pattern. The middle third of the common bile duct is most commonly affected, followed by the hepatic duct confluence[43]. Biopsy specimens taken from patients with ischaemic cholangitis reveal biliary obstruction and ischaemic features in 90%, and occasionally cellular rejection in 50%[44].
The human biliary system is composed of extrahepatic and intrahepatic biliary systems. The latter includes two sets of small and large size bile ducts. The bile ducts are lined by biliary epithelium consisting of cholangiocytes[45,46]. Blood is supplied to the bile ducts through a network of arterioles and capillaries, called the peribiliary vascular plexus (PBP), arising from hepatic arteries (HAs)[47]. The PBP is arranged around both the extra- and intra-hepatic bile ducts in the normal liver[46]. In contrast to the liver parenchyma, which has dual arterial and portal blood supply, the biliary epithelium depends solely on blood flow from the HAs[48]. Hepatic artery damage during procurement or reconstruction, and any variables causing interference of the blood supply to biliary tract via the perivascular plexus or small arteries, can cause ischaemic-type biliary lesions and segmental strictures[48]. However, many authors postulate that the biliary system receives blood from the portal vein as well as from the PBP[49]. This was supported by the occurrence of IC in the absence of hepatic artery thrombosis or in the hepatic segments affected by partial portal vein thrombosis; hence the impact of portal venous blood cannot be ignored[50].
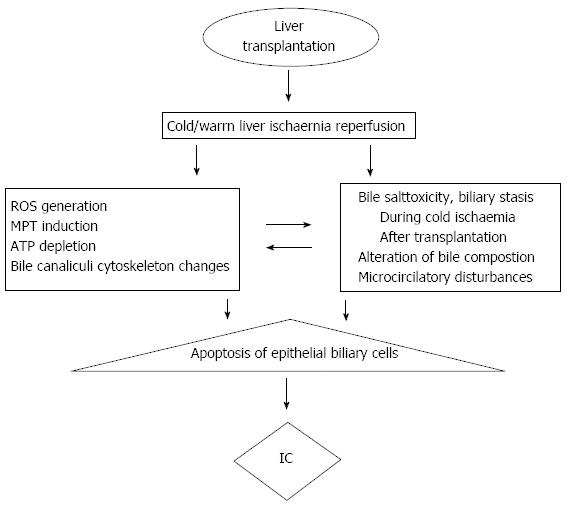
During the liver procurement, the whole blood flow to the liver is completely interrupted and it is several hours before re-establishment of circulation. Biliary ducts, especially cholangiocytes, seem to be more sensitive to ischaemia than hepatocytes or Kupffer cells. This has been observed in vitro studies which analyzed human graft specimens after LT where severe biochemical and histologic changes in the bile canaliculi associated with ischaemic injury were observed, indicating that the biliary system is very sensitive to ischaemic reperfusion injury[49,51]. A high incidence of the above complications is associated with prolonged cold ischaemic time[35,37], delayed re-arterialization of the graft[52] or transplants from DCD[53] indicating that IR injury plays the key-role in the pathogenesis of IC. IR injury during LT is mediated by thrombotic and ischaemic events due to endothelial activation[54-56] which include multiple pathophysiologic mechanisms such as impaired microcirculatory function, leukocyte adhesions, platelet aggregation, increased oxygen free radical production, lipid peroxidation, and cellular hypoxia[57,58].
Chan et al[19] found that a CIT exceeding 9 h was associated with increased incidence of IC (RR = 2.7; 95%CI: 2.6-2.8, P = 0.013). Dubbeld et al[59] found that an increasing cold ischaemia time (CIT) correlated with worse outcomes and this may be attributed to IC. However, ischaemia that lasts less than 90 min can still lead to some degree of liver injury, during the ischaemia, and more extensive lesions during the reperfusion[60,61]. The biliary epithelium is exposed to pro-inflammatory mediators derived not only from intrahepatic sources, but also from extrahepatic sources via arterial circulation[12,62]. Desquamated ductal cells together with polymorphonuclear leukocytes (PMNs) are discovered in the lumen of bile ducts and they appear in bile during the first few days after LT[63,64].
During cold ischaemia, rapid loss of high-energy molecules such as adenosine triphosphate (ATP) was observed due to mitochondrial dysfunction leading to cellular membrane damage manifested by loss of intracellular ions and intracellular accumulation of calcium and sodium[65,66]. In addition, this energy depletion leads to activation of proteolytic enzymes (proteases, phospholipases, and endonucleases), leading to production of xanthine oxidase from xanthine dehydrogenase and subsequent degradation of ATP to hypoxanthine[67]. During ischaemic cellular anoxia, cholangiocytes are significantly more resistant to cell death than hepatocytes[68]. The opposite occurs after reperfusion and re-oxygenation of the liver, when hepatocytes are more resistant to cell death than cholangiocytes.
After reperfusion in LT, re-oxygenation activates Kupffer cells and PMN, and produces microvascular disturbances, three processes collectively termed oxidative stress as shown in Figure 2[69]. Oxidative stress and production of free radicals lead to a biphasic pattern of reperfusion injury[70]. This is composed of: (1) an early phase (1-3 h) of activation of Kupffer cells with mild initial injury; and (2) a late phase (6-24 h) with more serious injury initiated by PMN activation. The severity of the liver IR is related to the length of CIT even more so than donor age[71]. Recently, Heidenhain et al[72] have shown that, CIT < 13 h is associated with only 7% risk of IC incidence; this percentage increases to 52% when the CIT was longer than 13 h, rising to 69% if it was longer than 15 h.
Additionally, experimental evidence in animals links irreversible intrahepatic strictures to the length of warm ischaemia time (WIT). This has been evidenced by the low incidence of intrahepatic biliary strictures when simultaneous hepatic artery and portal vein reperfusion is performed as compared to sequential vein followed by arterial reperfusion[53]. In animal models of DCD, irreversible biliary tract damage has been observed after 40 min of WIT, despite preservation of hepatocellular function[73].
Interest in DCD increased in late 1980s in order to expand the organ donor pool[74]. The total number of DCD transplants performed each year has increased more than 10-fold over the past decade and this has contributed inexpanding the liver transplant activity in many countries[75]. However, serious concerns have emerged regarding lower graft survival rates and higher biliary complication rates in comparison with DBD liver grafts[14,17]. As with all innovation in transplantation, there is a learning curve for the optimal utilization of DCD organs. The central issue surrounding DCD organs is the prolonged WIT which affects the graft function and organ damage is more profound[76].
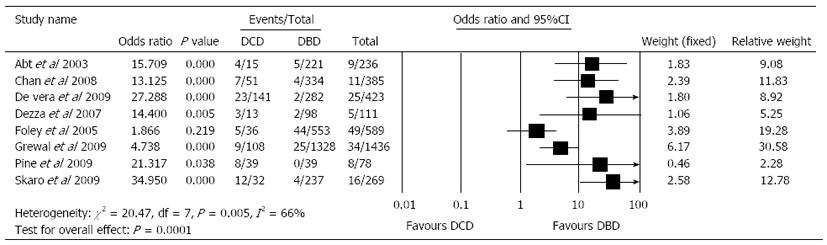
Pooled United Network for Organ Sharing data and several single-institution experiences have shown inferior graft survival in DCD liver transplant[15,17,77]. This is mostly related to IC which is the leading cause of graft loss and the complications that have prevented the widespread acceptance of DCD graft use by many transplant centres[78]. Livers from DCD have a second period of warm ischaemia when compared with DBD livers. This period of WIT results from the time from circulatory arrest until start of preservation, and results in, more ischaemic injury with subsequent high prevalence of IC[8]. Also the incidence of acute and chronic rejection, primary non-function and re-transplantation is higher with DCD livers[16,18,19,79]. The overall rate of biliary complications is 29% (range: 11%-53%) for DCD and 17% (9%-22%) for DBD recipients after LT[16]. D’Alessandro et al[80] from the University of Wisconsin have published 36 LTs after DCD, and also noted a higher rate of biliary complications after DBD (13.9% vs 8%, respectively). The IC rate is 16% (8%-38%) for DCD recipients and 3% (0%-8%) for DBD recipients (Figure 3)[8,15,16,81]. Chan et al[19] have shown that, the rate of IC in the DCD population compared to the DBD group was significantly higher (13.7% vs 1%) with 3-year follow-up, and all patients who developed IC did so in 120 d. Similarly, a retrospective case series by Abt et al[8] revealed that 4 out of the 15 patients receiving a DCD liver transplant developed IC. Ischaemic biliary lesions occur within 30 d in DCD compared to about 3 mo after transplantation in DBD grafts[4].
In our experience, since we started using livers from DCD in late 2004 (only small numbers until January 2007), the overall rate of biliary complications is 21% for DCD and 13% for DBD recipients after LT (P = NS). The IC rate is 8.3% for DCD recipients and 1.7% for DBD recipients (P = 0.001).
Many authors assume that, donor-warm ischaemia time (DWIT) which is the time between the asystole-to cross clamp was a risk factor for the development of IC. This leads the American Society of Transplant Surgeons to recommend a limit of the DWIT between 20 to 30 min[18,29,82]. It has been calculated that each minute of additional WIT increases the odds ratio for the development of ischemic cholangiopathy or hepatic necrosis by 16%[29]. Also, the donor haemodynamic status between the withdrawal of cardiopulmonary support and asystole in DCD is used to predict graft function after transplantation[83].
The identification ofthe risk factors of IC is important to optimize the greater use of different liver grafts, it is important for an experienced transplant surgeon to perform the procurement of DCD livers[29]. Donor age is a risk factor for the development of IC after LT[73,84]. Livers from older donors are more susceptible to warm and cold IR injury than young livers[85,86]. Aging may directly affect Kupffer cells resulting in tumour necrosis factor-α release and apoptosis[87]. Chan et al[19] have shown that, donor weight plays an important role in the development of IC. This may be explained by inadequate perfusion of preservation solution through the smaller arterioles that feed the biliary tree in a larger donor body weight. They also noticed that, donor body weight and age > 50 years appeared to be a strong predictors for the development of IC. In addition a female to male donor/recipient match is associated with late development of IC[36].
Development of early biliary complications is correlated with moderate macrovesicular steatosis[88]. Macrovesicular steatosis of the graft exceeding 25% is a significant predisposition for biliary complications[89,90].
Few preservation solutions have gotten widespread clinical use, such as Euro-Collins, Histidine-Tryptophan-Ketoglutarate (HTK), University of Wisconsin (UW) and Celsior solutions[91]. Euro-Collins has almost been abandoned because of the glucose disadvantage, and currently, Celsior is mainly used for cardiac preservation. HTK and UW are still being used for multiorgan preservation[92]. Despite trends were documented in some studies for the superiority of low viscosity HTK in biliary tract flush, prevention of biliary complications, and cost savings[2,93-95], many recent studies revealed no difference of effects between HTK and UW (high viscosity) regarding the incidence of post-transplant IC or survival[96-99].
Routine flushing of the donor biliary tree to remove stagnant bile salts is performed by most procurement surgeons, as bile can be cytotoxic to the ductal epithelium of allografts with long preservation times, resulting in intrahepatic stricture formation[10].
Immunologically related IC comprises injury to the biliary epithelium and/or vascular endothelium in the course of chronic rejection, CMV infection, recurrent sclerosing cholangitis and ABO-incompatible transplantation[6,100]. Although some studies did not show that graft rejection was a risk factor for development of IC following LT[101,102], others demonstrated that bile ducts are involved in acute and chronic rejection[103,104]. Two mechanisms have been suggested to cause bile duct loss (vanishing bile duct syndrome): (1) a direct immunological destruction of the biliary epithelium, the histologic appearance of cellular rejection is characterized by lymphocytic invasion and evidence of degenerative changes in the biliary epithelium[105]. Biliary inflammation is less severe in chronic rejection[106]. Lymphocyte cultures generated from liver allograft tissue undergoing rejection have demonstrated cytotoxic activity directed at donor major histocompatibility complex (MHC) antigens[107]. These MHC antigens are expressed strongly on bile ducts particularly during a rejection reaction[108]; and (2) an indirect, ischaemic damage through a process of chronic obliterative arteriopathy which is indicated by the absence of bile ducts in conjunction with arterial loss in specimens from chronically rejected human liver allografts[109,110]. The incidence of IC after ABO-incompatible LT in adults is much higher than in ABO-compatible LT[111]. Primary sclerosing cholangitis and autoimmune hepatitis as indications for LT are also associated with a higher incidence of IC[36,112].
CMV infection can damage the bile duct cells and this damage may be mediated by CMV infection of small arteries[113]. CMV infection mediates injury of endothelial cells of the PBP, with subsequent microthrombi formation and anoxic damage of the biliary epithelium, which causes ischaemic injury to the bile duct and development of IC[114,115]. Also this can be indirectly induced by an immune attack of the infected biliary epithelial cells as CMV infection is associated with rejection[114].
Baccarani et al[89] have shown that, the interval between portal and arterial reperfusion of the liver greatly affects the development of IC in case of sequential revascularization in LT. The delay of re-arterialization in sequential revascularization is associated with more pronounced microvascular disturbances, with subsequent graft dysfunction and a higher incidence of biliary complications following LT[116]. Dubbeld et al[59] found that sclerosing cholangitis (PSC) as underlying liver disease, and a trend for MELD-score were risk factors for non-anastomotic biliary strictures either in DBD or DCD.
IC does not appear to increase the risk of death, but leads to a significant increase in the morbidity associated with multiple dilatation procedures and hospitalizations from recurrent cholangitis[19]. IC is not reversible, and the management options are limited. Early recognition and the timely treatment of cholangitis, which is sometimes associated with intrahepatic bile duct necrosis, can save the graft function and prevent graft loss. IC tend to be diffuse and difficult to manage and may require endoscopic (ERCP) or percutaneous management with stricture dilatation and stent insertion, hepaticojejunostomy, or a permanent indwelling percutaneous transhepatic cholangiography-guided drainage in up to 50% of cases[40,101].
ERCP consists of removing biliary sludge and casts from the bile ducts, this followed by balloon dilation of all accessible strictures and placement of plastic stents with replacement every 3 mo[4]. In IC, strictures are less amenable to balloon dilation as they are diffuse, bilobar, and with high predilection for the smaller intrahepatic ducts. In addition, with recurrent cholangitis, rapid frequent stent occlusion is another challenge for physicians who need repeated admissions and management. Endoscopic therapy is the first line therapy for IC strictures and may occasionally be a definite solution, but appears to play a more prominent role as a bridge to liver re-transplantation[28,117].
Prophylaxis of IC might be valuable due to the complex nature of the disease and the high cost and high rate of readmissions with ultimately re-grafting in an era of donor pool shortage. Establishment of a sufficient biliary microcirculation during organ procurement is mandatory by arterial pressure perfusion[73,118], and also additional arterial back-table pressure perfusion has been suggested[31]. CIT should be kept ≤ 10 h. Bile salt residues are a further factor, which may potentiate the IR injury[119,120]. Thorough retrograde flushing of the bile ducts is therefore recommended. Also recipient characteristics like a Child-Pugh status C have been reported to significantly increase the incidence of NAS[72]. Simultaneous arterial and portal reperfusion reduced the biliary ischaemic time and subsequently also NAS as it decreases the WIT of the arterially perfused bile ducts[121]. The overall risk of IC might be balanced by matching of donor and recipient characteristics and avoidance of accumulation of risk factors. In the case of very high risk, e.g., after DCD, someauthors recommend additional secondary prophylaxis of NAS by insertion of a T-tube for earlier diagnosis and intervention[83].
Some pharmacological materials can be used to decrease the incidence of IC. The Wisconsin group showed that, administration of vitamin E, prostaglandin E1, and N-acetylcysteine to their DCD grafted patients has some experimental evidence to support this practice[74]. Similarly, researchers from Okayama, Japan have shown a protective effect of a vitamin E derivative in their DCD grafted patients which were indicated by rapid improvement of LFT after LT[122]. Others suggested a protective effect of platelet activating factor and some immunosuppressants like tacrolimus in preventing the IC[74].
The use of ischaemic preconditioning, during the procurement has shown a beneficial and protective effect especially with prolonged warm and cold ischaemia in experimental models[123]. This is carried out through 10 min portal vein clamping followed by 10 min reperfusion before starting the liver procurement. Cameron et al[74] showed excellent results using this protocol.
Uchiyama et al[124] and the Tokyo Medical College preserved the liver using machine perfusion through the hepatic artery with the use of pentoxifylline. This is particularly effective for graft conditioning in liver transplantation from a DCD, decreases the ischaemia time and therefore reduces the incidence of IC.
The widespread and successful use of DCD grafts has contributed to increased transplant activity. Ischaemic cholangiopathy is attributed to numerous causes, the incidence is high among DCD, and this can be managed by optimizing the utilization of this type of donors with proper donor selection and well trained procurement surgeons.
P- Reviewers: Lisotti A, Liaskou E, Lu WY, Martin M, Penkova-Radicheva MP, Raggi C S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003;23:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Verdonk RC, Buis CI, van der Jagt EJ, Gouw AS, Limburg AJ, Slooff MJ, Kleibeuker JH, Porte RJ, Haagsma EB. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Evans PC, Smith S, Hirschfield G, Rigopoulou E, Wreghitt TG, Wight DG, Taylor CJ, Alexander GJ. Recipient HLA-DR3, tumour necrosis factor-alpha promoter allele-2 (tumour necrosis factor-2) and cytomegalovirus infection are interrelated risk factors for chronic rejection of liver grafts. J Hepatol. 2001;34:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | DeOliveira ML, Jassem W, Valente R, Khorsandi SE, Santori G, Prachalias A, Srinivasan P, Rela M, Heaton N. Biliary complications after liver transplantation using grafts from donors after cardiac death: results from a matched control study in a single large volume center. Ann Surg. 2011;254:716-722; discussion 722-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 291] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Liu CL, Lo CM, Chan SC, Fan ST. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation. 2004;77:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant. 2012;2012:164329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Williams ED, Draganov PV. Endoscopic management of biliary strictures after liver transplantation. World J Gastroenterol. 2009;15:3725-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Ann Surg. 2008;248:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, Lyuksemburg V, Martin JA, Feinglass JM, Preczewski LB, Abecassis MM. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543-552; discussion 552-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Jay CL, Lyuksemburg V, Kang R, Preczewski L, Stroupe K, Holl JL, Abecassis MM, Skaro AI. The increased costs of donation after cardiac death liver transplantation: caveat emptor. Ann Surg. 2010;251:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | de Vera ME, Lopez-Solis R, Dvorchik I, Campos S, Morris W, Demetris AJ, Fontes P, Marsh JW. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center. Am J Transplant. 2009;9:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, Reyes JD, Larson AM, Levy AE. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Maguire D, Heaton ND. Biliary complications after orthotopic liver transplantation. Transplant reviews. 2002;16:220–240. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles’ heel. Liver Transpl. 2006;12:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Graziadei IW, Schwaighofer H, Koch R, Nachbaur K, Koenigsrainer A, Margreiter R, Vogel W. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006;12:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Ostroff JW. Management of biliary complications in the liver transplant patient. Gastroenterol Hepatol (N Y). 2010;6:264-272. [PubMed] |
| 27. | Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 357] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Tung BY, Kimmey MB. Biliary complications of orthotopic liver transplantation. Dig Dis. 1999;17:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Taner CB, Bulatao IG, Willingham DL, Perry DK, Sibulesky L, Pungpapong S, Aranda-Michel J, Keaveny AP, Kramer DJ, Nguyen JH. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Li S, Stratta RJ, Langnas AN, Wood RP, Marujo W, Shaw BW. Diffuse biliary tract injury after orthotopic liver transplantation. Am J Surg. 1992;164:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Moench C, Moench K, Lohse AW, Thies J, Otto G. Prevention of ischemic-type biliary lesions by arterial back-table pressure perfusion. Liver Transpl. 2003;9:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, Madariaga JR, Tzakis AG. Intrahepatic biliary strictures after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Alazmi WM, Fogel EL, Watkins JL, McHenry L, Tector JA, Fridell J, Mosler P, Sherman S, Lehman GA. Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy. 2006;38:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Hintze RE, Adler A, Veltzke W, Abou-Rebyeh H, Felix R, Neuhaus P. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepatogastroenterology. 1997;44:258-262. [PubMed] |
| 35. | Theilmann L, Küppers B, Kadmon M, Roeren T, Notheisen H, Stiehl A, Otto G. Biliary tract strictures after orthotopic liver transplantation: diagnosis and management. Endoscopy. 1994;26:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Fisher A, Miller CH. Ischemic-type biliary strictures in liver allografts: the Achilles heel revisited? Hepatology. 1995;21:589-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Sherlock S. Slerosing cholangitis. Diseases of the liver and biliary system. 11th ed Milan: Rotolito Lombarda 2002; 255–265. |
| 39. | Schlitt HJ, Meier PN, Nashan B, Oldhafer KJ, Boeker K, Flemming P, Raab R, Manns MP, Pichlmayr R. Reconstructive surgery for ischemic-type lesions at the bile duct bifurcation after liver transplantation. Ann Surg. 1999;229:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 41. | Nakanuma Y. Tutorial review for understanding of cholangiopathy. Int J Hepatol. 2012;2012:547840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Novellas S, Caramella T, Fournol M, Gugenheim J, Chevallier P. MR cholangiopancreatography features of the biliary tree after liver transplantation. AJR Am J Roentgenol. 2008;191:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Aldrighetti L, Arru M, Ronzoni M, Salvioni M, Villa E, Ferla G. Extrahepatic biliary stenoses after hepatic arterial infusion (HAI) of floxuridine (FUdR) for liver metastases from colorectal cancer. Hepatogastroenterology. 2001;48:1302-1307. [PubMed] |
| 44. | Ludwig J, Batts KP, MacCarty RL. Ischemic cholangitis in hepatic allografts. Mayo Clin Proc. 1992;67:519-526. [PubMed] |
| 45. | Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 286] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 48. | Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006;44:806-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Cutrin JC, Cantino D, Biasi F, Chiarpotto E, Salizzoni M, Andorno E, Massano G, Lanfranco G, Rizzetto M, Boveris A. Reperfusion damage to the bile canaliculi in transplanted human liver. Hepatology. 1996;24:1053-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Farid WR, de Jonge J, Slieker JC, Zondervan PE, Thomeer MG, Metselaar HJ, de Bruin RW, Kazemier G. The importance of portal venous blood flow in ischemic-type biliary lesions after liver transplantation. Am J Transplant. 2011;11:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Benkoël L, Dodero F, Hardwigsen J, Campan P, Botta-Fridlund D, Lombardo D, Le Treut YP, Chamlian A. Effect of ischemia-reperfusion on bile canalicular F-actin microfilaments in hepatocytes of human liver allograft: image analysis by confocal laser scanning microscopy. Dig Dis Sci. 2001;46:1663-1667. [PubMed] |
| 52. | Sankary HN, McChesney L, Frye E, Cohn S, Foster P, Williams J. A simple modification in operative technique can reduce the incidence of nonanastomotic biliary strictures after orthotopic liver transplantation. Hepatology. 1995;21:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Suárez F, Otero A, Solla M, Arnal F, Lorenzo MJ, Marini M, Vázquez-Iglesias JL, Gómez M. Biliary complications after liver transplantation from maastricht category-2 non-heart-beating donors. Transplantation. 2008;85:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Carles J, Fawaz R, Hamoudi NE, Neaud V, Balabaud C, Bioulac-Sage P. Preservation of human liver grafts in UW solution. Ultrastructural evidence for endothelial and Kupffer cell activation during cold ischemia and after ischemia-reperfusion. Liver. 1994;14:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Cosenza CA, Cramer DV, Cunneen SA, Tuso PJ, Wang HK, Makowka L. Protective effect of the lazaroid U74006F in cold ischemia-reperfusion injury of the liver. Hepatology. 1994;19:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 57. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |
| 58. | He XS, Ma Y, Ju WQ, Wu LW, Wu JL, Liang YJ, Hu RD, Chen GH, Huang JF. Dynamic microcirculatory changes in liver graft from non-heart-beating donor with warm ischemia injury in rat. Hepatobiliary Pancreat Dis Int. 2004;3:179-182. [PubMed] |
| 59. | Dubbeld J, Hoekstra H, Farid W, Ringers J, Porte RJ, Metselaar HJ, Baranski AG, Kazemier G, van den Berg AP, van Hoek B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 60. | Hasselgren PO. Prevention and treatment of ischemia of the liver. Surg Gynecol Obstet. 1987;164:187-196. [PubMed] |
| 61. | Frederiks WM, James J, Bosch KS, Schröder MJ, Schuyt HC. A model for provoking ischemic necrosis in rat liver parenchyma and its quantitative analysis. Exp Pathol. 1982;22:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Popper H. The relation of mesenchymal cell products to hepatic epithelial systems. Prog Liver Dis. 1990;9:27-38. [PubMed] |
| 63. | Kubota K, Ericzon BG, Reinholt FP. The correlation between cytological patterns in bile and histological findings in liver transplantation. Transplantation. 1992;53:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Oldhafer KJ, Gubernatis G, Ringe B, Pichlmayr R. Experience with bile cytology after liver transplantation. Transplant Proc. 1990;22:1524. [PubMed] |
| 65. | Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 66. | Gutierrez G. Cellular energy metabolism during hypoxia. Crit Care Med. 1991;19:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13:83-95. [PubMed] |
| 68. | Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg. 2000;63:336-347. [PubMed] |
| 70. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. [PubMed] |
| 71. | Briceño J, Marchal T, Padillo J, Solórzano G, Pera C. Influence of marginal donors on liver preservation injury. Transplantation. 2002;74:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | García-Valdecasas JC, Tabet J, Valero R, Deulofeu R, Taurá P, Rull R, Capdevila L, Cifuentes A, González FX, Net M. Evaluation of ischemic injury during liver procurement from non-heart-beating donors. Eur Surg Res. 1999;31:447-456. [PubMed] |
| 74. | Cameron AM, Busuttil RW. Ischemic cholangiopathy after liver transplantation. Hepatobiliary Pancreat Dis Int. 2005;4:495-501. [PubMed] |
| 75. | Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. 2007 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1997– 2006; Available from: http://www.srtr.org/annual_Reports/default.aspx. |
| 76. | Balupuri S, Buckley P, Snowden C, Mustafa M, Sen B, Griffiths P, Hannon M, Manas D, Kirby J, Talbot D. The trouble with kidneys derived from the non heart-beating donor: a single center 10-year experience. Transplantation. 2000;69:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 78. | Renz JF. Is DCD for liver transplantation DNR? Am J Transplant. 2008;8:485-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Abt PL, Desai NM, Crawford MD, Forman LM, Markmann JW, Olthoff KM, Markmann JF. Survival following liver transplantation from non-heart-beating donors. Ann Surg. 2004;239:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 275] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 80. | D’Alessandro AM, Fernandez LA, Chin LT, Shames BD, Turgeon NA, Scott DL, Di Carlo A, Becker YT, Odorico JS, Knechtle SJ. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant. 2004;9:68-71. [PubMed] |
| 81. | Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, Shames BD, Becker YT, Odorico JS, Knechtle SJ. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 82. | Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D’Alessandro A, Pomfret EA, Freeman RB, Markmann JF, Hanto DW. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 83. | Ho KJ, Owens CD, Johnson SR, Khwaja K, Curry MP, Pavlakis M, Mandelbrot D, Pomposelli JJ, Shah SA, Saidi RF. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation. 2008;85:1588-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Nakamura N, Nishida S, Neff GR, Vaidya A, Levi DM, Kato T, Ruiz P, Tzakis AG, Madariaga JR. Intrahepatic biliary strictures without hepatic artery thrombosis after liver transplantation: an analysis of 1,113 liver transplantations at a single center. Transplantation. 2005;79:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177-2187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 86. | Gouw AS, van den Heuvel MC, van den Berg AP, Slooff MJ, de Jong KP, Poppema S. The significance of parenchymal changes of acute cellular rejection in predicting chronic liver graft rejection. Transplantation. 2002;73:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Hilmer SN, Cogger VC, Le Couteur DG. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007;62:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, Pleass H, Crawford M, McCaughan GW, Verran DJ. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol. 2012;27:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 89. | Baccarani U, Isola M, Adani GL, Avellini C, Lorenzin D, Rossetto A, Currò G, Comuzzi C, Toniutto P, Risaliti A. Steatosis of the hepatic graft as a risk factor for post-transplant biliary complications. Clin Transplant. 2010;24:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 90. | Baccarani U, Adani GL, Lorenzin D, Donini A, Risaliti A. The role of steatosis of the liver graft in the development of post-transplant biliary complications. Transpl Int. 2010;23:239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Lee KW, Simpkins CE, Montgomery RA, Locke JE, Segev DL, Maley WR. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 92. | Lee KW, Cameron AM, Maley WR, Segev DL, Montgomery RA. Factors affecting graft survival after adult/child split-liver transplantation: analysis of the UNOS/OPTN data base. Am J Transplant. 2008;8:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Moench C, Otto G. Ischemic type biliary lesions in histidine-tryptophan-ketoglutarate (HTK) preserved liver grafts. Int J Artif Organs. 2006;29:329-334. [PubMed] |
| 94. | Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, Tector AJ. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 95. | Feng XN, Xu X, Zheng SS. Current status and perspective of liver preservation solutions. Hepatobiliary Pancreat Dis Int. 2006;5:490-494. [PubMed] |
| 96. | Feng L, Zhao N, Yao X, Sun X, Du L, Diao X, Li S, Li Y. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Rayya F, Harms J, Martin AP, Bartels M, Hauss J, Fangmann J. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in adult liver transplantation. Transplant Proc. 2008;40:891-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Avolio AW, Agnes S, Nure E, Maria G, Barbarino R, Pepe G, Castagneto M. Comparative evaluation of two perfusion solutions for liver preservation and transplantation. Transplant Proc. 2006;38:1066-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, Hay JE, Gores GJ, Charlton MR, Ludwig J. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Campbell WL, Sheng R, Zajko AB, Abu-Elmagd K, Demetris AJ. Intrahepatic biliary strictures after liver transplantation. Radiology. 1994;191:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Colonna JO, Shaked A, Gomes AS, Colquhoun SD, Jurim O, McDiarmid SV, Millis JM, Goldstein LI, Busuttil RW. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344-350; discussion 350-352. [PubMed] |
| 103. | Batts KP. Acute and chronic hepatic allograft rejection: pathology and classification. Liver Transpl Surg. 1999;5:S21-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 104. | Demetris AJ. Immune cholangitis: liver allograft rejection and graft-versus-host disease. Mayo Clin Proc. 1998;73:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Wight D, Portmann B. Pathology of liver transplantation. System pathol. 1987;11:543-596. |
| 106. | Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric post-transplant liver pathology. Pathol Annu. 1987;22 Pt 2:347-386. [PubMed] |
| 107. | Fung JJ, Zeevi A, Starzl TE, Demetris J, Iwatsuki S, Duquesnoy RJ. Functional characterization of infiltrating T lymphocytes in human hepatic allografts. Human immunol. 1986;16:182-199. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Whiteside T. Induction of DR/IA antigens in human liver allografts: an immunocytochemical and clinicopathologic analysis of twenty failed grafts. Transplantation. 1985;40:504. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Oguma S, Belle S, Starzl TE, Demetris AJ. A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology. 1989;9:204-209. [PubMed] |
| 110. | Martelius T, Krogerus L, Höckerstedt K, Bruggeman C, Lautenschlager I. Cytomegalovirus infection is associated with increased inflammation and severe bile duct damage in rat liver allografts. Hepatology. 1998;27:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Wu J, Ye S, Xu X, Xie H, Zhou L, Zheng S. Recipient outcomes after ABO-incompatible liver transplantation: a systematic review and meta-analysis. PLoS One. 2011;6:e16521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Ten Hove WR, Korkmaz KS, op den Dries S, de Rooij BJ, van Hoek B, Porte RJ, van der Reijden JJ, Coenraad MJ, Dubbeld J, Hommes DW. Matrix metalloproteinase 2 genotype is associated with nonanastomotic biliary strictures after orthotopic liver transplantation. Liver Int. 2011;31:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Martelius T, Krogerus L, Höckerstedt K, Mäkisalo H, Bruggeman C, Lautenschlager I. CMV causes bile duct destruction and arterial lesions in rat liver allografts. Transplant Proc. 1997;29:796-797. [PubMed] |
| 114. | Op den Dries S, Sutton ME, Lisman T, Porte RJ. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation. 2011;92:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 115. | Hoekstra H, Buis CI, Verdonk RC, van der Hilst CS, van der Jagt EJ, Haagsma EB, Porte RJ. Is Roux-en-Y choledochojejunostomy an independent risk factor for nonanastomotic biliary strictures after liver transplantation? Liver Transpl. 2009;15:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Post S, Palma P, Gonzalez AP, Rentsch M, Menger MD. Timing of arterialization in liver transplantation. Ann Surg. 1994;220:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Zajko AB, Campbell WL, Logsdon GA, Bron KM, Tzakis A, Esquivel CO, Starzl TE. Cholangiographic findings in hepatic artery occlusion after liver transplantation. AJR Am J Roentgenol. 1987;149:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Langrehr JM, Schneller A, Neuhaus R, Vogl T, Hintze R, Neuhaus P. [Etiologic factors and incidence of ischemic type biliary lesions (ITBL) after liver transplantation]. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:1560-1562. [PubMed] |
| 119. | Hertl M, Harvey PRC, Swanson PE, West DD, Howard TK, Shenoy S, Strasberg SM. Evidence of preservation injury to bile ducts by bile salts in the pig and its prevention by infusions of hydrophilic bile salts. Hepatology. 1995;21:1130-1137. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Hoekstra H, Porte RJ, Tian Y, Jochum W, Stieger B, Moritz W, Slooff MJ, Graf R, Clavien PA. Bile salt toxicity aggravates cold ischemic injury of bile ducts after liver transplantation in Mdr2+/- mice. Hepatology. 2006;43:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 121. | Baccarani U, Adani GL, Isola M, Avellini C, Lorenzin D, Rossetto A, Currò G, Comuzzi C, Toniutto P, Soldano F. Steatosis of the graft is a risk factor for posttransplantation biliary complications. Transplant Proc. 2009;41:1313-1315. [PubMed] |
| 122. | Yagi T, Sadamori H, Nakagawa H, Ishikawa T, Sasaki H, Ohta K, Ishine N, Inagaki M, Tanaka N. Hepatropic soluble vitamin E derivative EPC-K1 prevents warm ischemia/reperfusion injury of non-heart-beating donor liver transplantation in pigs. Transplant Proc. 1997;29:1390-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 123. | Berrevoet F, Schäfer T, Vollmar B, Menger MD. Ischemic preconditioning: enough evidence to support clinical application in liver surgery and transplantation? Acta Chir Belg. 2003;103:485-489. [PubMed] |
| 124. | Uchiyama M, Kozaki K, Nemoto T, Degawa H, Matsuno N, Kubota K, Takeuchi H, Sakurai E, Kozaki M, Ikeda T. Liver transplantation from non-heart-beating donors: effect of machine perfusion preservation and pentoxifylline. Transplant Proc. 1998;30:3798-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |