Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.5987
Revised: February 21, 2014
Accepted: April 1, 2014
Published online: May 28, 2014
Processing time: 258 Days and 10.4 Hours
Hepatocellular carcinoma (HCC) is a malignant disease that substantially affects public health worldwide. It is especially prevalent in east Asia and sub-Saharan Africa, where the main etiology is the endemic status of chronic hepatitis B. Effective treatments with curative intent for early HCC include liver transplantation, liver resection (LR), and radiofrequency ablation (RFA). RFA has become the most widely used local thermal ablation method in recent years because of its technical ease, safety, satisfactory local tumor control, and minimally invasive nature. This technique has also emerged as an important treatment strategy for HCC in recent years. RFA, liver transplantation, and hepatectomy can be complementary to one another in the treatment of HCC, and the outcome benefits have been demonstrated by numerous clinical studies. As a pretransplantation bridge therapy, RFA extends the average waiting time without increasing the risk of dropout or death. In contrast to LR, RFA causes almost no intra-abdominal adhesion, thus producing favorable conditions for subsequent liver transplantation. Many studies have demonstrated mutual interactions between RFA and hepatectomy, effectively expanding the operative indications for patients with HCC and enhancing the efficacy of these approaches. However, treated tumor tissue remains within the body after RFA, and residual tumors or satellite nodules can limit the effectiveness of this treatment. Therefore, future research should focus on this issue.
Core tip: The pivotal role of radiofrequency ablation (RFA) has recently been established among the various treatment strategies for hepatocellular carcinoma (HCC), primarily due to its excellent local tumor control. RFA may be complementary to the other treatments with curative intent for HCC and its beneficial outcomes in patients have been demonstrated in several clinical studies.
- Citation: Feng K, Ma KS. Value of radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Gastroenterol 2014; 20(20): 5987-5998
- URL: https://www.wjgnet.com/1007-9327/full/v20/i20/5987.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i20.5987
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third most frequent cause of cancer-related death worldwide; in 2008 alone, more than 700000 cases of HCC were diagnosed[1]. For most patients HCC develops secondary to hepatic cirrhosis, and is the leading cause of death among this population[2]. The various geographical incidence of HCC worldwide reflects the presence of different risk factors among different regions. The majority of HCC cases (80%) have occurred in east Asia and sub-Saharan Africa, where the main etiologies are chronic infection with the hepatitis B virus (HBV) and exposure to the fungal aflatoxin B1[1,3]. In particular, the incidence of HCC in China alone accounts for 55% of all cases worldwide[4]. In contrast, hepatitis C virus infection and alcoholism are the major risk factors for HCC in North America, Europe, and Japan[5].
HCC can be cured by liver transplantation, liver resection (LR), or local ablation therapy[6]. Liver transplantation is theoretically the best therapeutic choice for patients who meet the Milan criteria[7]. However, this technique is severely undermined by the shortage of donor organs and hepatectomy remains the first-line treatment for HCC. Nevertheless, only 9% to 29% of patients are able to undergo this procedure because of either poor hepatic reserve secondary to underlying chronic liver disease or a multifocal distribution of tumor nodules[8-10]. Various local ablation techniques have been increasingly applied as alternative HCC treatments to overcome this clinical challenge. Among them, radiofrequency ablation (RFA) is the most widely used method because of its technical ease, safety, cost-effectiveness, and minimal invasiveness[11-15].
Continuous improvements are being made in RFA devices and operation strategies. As a result, the application of RFA has far outreached its original objective as palliative HCC treatment[16] and it has gradually expanded into the therapeutic fields for other diseases[17]. Specifically, RFA demonstrates significant advantages in local tumor control, making it particularly beneficial in the treatment of HCC. As a relatively new technique, few randomized controlled trials evaluating RFA have been reported, highlighting the lack of definitive, good quality data in the publicly available literature. This article reviews the application of RFA in various treatments for HCC.
HCC is the only solid cancer that can be treated by liver transplantation. This surgical approach can simultaneously cure the tumor and underlying cirrhosis, thereby minimizing the chance of recurrence. Moreover, liver transplantation is not affected by the degree of liver function impairment[18]. Mazzaferro et al[19] reported a four-year survival rate of 75% and recurrence rate of < 15% after treatment by deceased-donor liver transplantation in patients with one HCC nodule ≤ 5 cm in diameter or up to three nodules ≤ 3 cm in diameter without vascular invasion or extrahepatic spread. The efficacy of the treatment performed in this landmark study was comparable to that of liver transplantation for cirrhosis and initiated the application of liver transplantation for early HCC. These criteria have become known as the Milan criteria and have been adopted as the standard criteria worldwide for the selection of patients with HCC who are eligible for liver transplantation[6,20-22]. In 2001, investigators from the University of California, San Francisco (UCSF) argued in favor of loosening these criteria to enable greater numbers of patients with HCC to become eligible for liver transplantation[23]. Their study revealed that patients with HCC who met the following criteria achieved treatment efficacy equivalent to patients meeting the Milan criteria (one- and five-year survival rates of 90.0% and 75.2%, respectively): (1) solitary tumor ≤ 6.5 cm in diameter or a maximum of three nodules ≤ 4.5 cm in diameter; and (2) a total tumor diameter ≤ 8 cm. Subsequent studies corroborated the therapeutic outcome of liver transplantation using the two aforementioned sets of criteria[24,25]. In addition, with the development of living-donor liver transplantation, the enlistment criteria for eligible patients were further loosened and impressive treatment results have been demonstrated[26-29].
Despite the successful outcomes of liver transplantation, the insufficient availability of donor organs severely restricts the global application of this technique. Patients with HCC often must wait a long period of time to undergo liver transplantation. During this waiting period, the tumors continue to progress and eventually prevent transplantation[30]. Unfortunately, the average waiting time before cadaveric liver transplantation is more than one year in most countries[31]. The time spent on the transplant waiting list is as long as six to twelve months in Europe and the United States, with dropout rates of 30% to 40% per year[32,33]. Therefore, if the waiting time is more than six months, locoregional strategies [e.g., transarterial chemoembolization (TACE), percutaneous ethanol injection, or RFA] are needed to slow the tumor progression[32]. RFA demonstrates a better effect on local tumor control than TACE, especially for tumors < 3 cm in diameter. As a result, RFA is more frequently used in downsizing therapy prior to liver transplantation.
As early as 2001, RFA was used to delay tumor progression prior to liver transplantation with satisfactory outcomes[34]. Many investigators subsequently reported the use of RFA as a pretransplantation bridge therapy[34-40] (Table 1). According to these studies, preoperative RFA might slow down tumor progression and extend the average waiting time beyond six to ten months. However, as these were retrospective studies with insufficient data, a well-designed randomized trial with sufficient size is necessary for validation. A common belief is that the underestimation of tumor lesions in radiologic studies and the presence of partial tumor necrosis limit the role of RFA as a bridge treatment prior to liver transplantation[41,42]. However, most studies have not supported this conclusion. Brillet et al[39] reported that after an average waiting period of 11.9 mo, 76% of preoperative RFA patients were able to undergo liver transplantation regardless of the fact that only 75% of these tumors were completely ablated and that satellite lesions were identified in 44% of the specimens.
| Ref. | n | Tumor size (cm) | RFA→LT interval (mo) | Dropout | Radiologic response necrosis | Pathologic response necrosis | Satellites found in explants | Follow-up after LT (mo) | Survival | |
| 1-yr | 3-yr | |||||||||
| Pulvirenti et al[34] | 14 | 3.50 | 8.0 | 0.0% | 90.7% | 86.4% | 57.0% | 16.0 | 100.0% | 100.0% |
| Fontana et al[35] | 33 | 3.60 | 7.9 | 21.7% | 66.0% | - | - | 26.9 | 85.0% | 85.0% |
| Mazzaferro et al[36] | 50 | 2.75 | 9.5 | 0.0% | 70.0% | 55.0% | 28.0% | 22.0 | 95.0% | 83.0% |
| Pompili et al[37] | 40 | 2.80 | 8.6 | 0.0% | 75.0% | 46.7% | 14.0% | 34.4 | 91.9% | 85.4% |
| Lu et al[38] | 52 | 2.50 | 8.7 | 5.8% | 89.6% | 70.3% | 24.0% | 14.9 | 85.0% | 76.0% |
| Brillet et al[39] | 21 | 2.40 | 11.9 | 24.0% | 76.0% | 75.0% | 44.0% | 25.0 | - | - |
| DuBay et al[40] | 77 | 2.50 | 9.5 | 21.0% | 83.0% | - | - | 30.0 | -1 | -1 |
The Milan criteria remain a worldwide standard for identifying appropriate candidates for liver transplantation. However, according to the above-mentioned results, patients whose tumors meet these radiographic criteria may have excellent survival outcomes from locoregional therapy alone, while others with similar baseline imaging findings develop distant metastatic disease despite transplantation and have a poor prognosis. These differences occur because of the heterogeneity of HCC[43-48]. Hence, investigators have been dedicated to seeking alternative therapeutics with an efficacy comparable to that of liver transplantation to save precious donor resources. Nkontchou et al[49] found no significant difference in prognosis between patients undergoing RFA before salvage liver transplantation with those undergoing liver transplantation before RFA. Thus, the authors proposed a two-step therapeutic strategy for patients eligible for liver transplantation: RFA should be performed first and, if relapse occurs, salvage liver transplantation should then be performed. Although some inadequacies were present in the design and analysis, Nkontchou et al[50] did provide a novel concept of the “test of time” using a hypothesis that originated from another UCSF study involving 61 patients who were treated by RFA and/or TACE to downsize tumors. Approximately 30% of these patients were unable to undergo liver transplantation because of tumor progression. The remaining patients successfully underwent liver transplantation after an average waiting period of 8.2 mo and demonstrated a satisfactory four-year survival rate of 69.3% (intention-to-treat analysis). These results suggest that the strategy of “ablation and waiting” potentially facilitates the identification of patients with unfavorable tumor biology who demonstrate progression despite downsizing strategies and who are unlikely to benefit from transplantation. Such a strategy may, to a certain degree, compensate for the inadequacies of the Milan criteria with respect to excessive reliance on imaging examination results. Undoubtedly, the outcome of preoperative RFA is closely related to the complete tumor necrosis rate. Advancement of the modern RFA technique has enabled a one-session complete ablation rate of > 90% for tumors < 5 cm in diameter. Hence, tumors in peculiar locations and small lesions unidentifiable by preoperative imaging severely restrict the efficiency of this technique. Laparoscopic RFA provides an effective solution to these problems[51].
LR remains the first-line curative treatment for many patients with HCC, especially those in the early stage of the disease. Worldwide, this strategy has achieved a five-year survival rate of > 50% for patients with good hepatic functional reserve and a low operative mortality of 0.0% to 6.4%[52-56]. Unfortunately, only 5% to 15% of patients with malignant liver cancer are eligible for resection because of various contraindications such as multicentric tumor occurrence, unresectable tumor locations, and insufficient hepatic reserve[57]. Therefore, RFA has become a pivotal method for HCC treatment because of its excellent local tumor control and minimal invasiveness.
Comparison between the efficacy of RFA and that of LR has been a source of long-standing controversy, especially in the treatment of small HCC. Anatomic resection of centrally situated small HCC sacrifices a large volume of functional liver parenchyma, contributing to a high rate of complications and surgical mortality[58-60]. However, RFA provides excellent local control and has achieved an efficacy equivalent to that of surgical resection in the treatment of small HCC[13,57,61]. Moreover, RFA requires a shorter hospitalization, fewer blood transfusions, and has a lower rate (0.0% to 8.5%) of major complications, including gastrointestinal bleeding or perforation, serious infection, biliary duct injury, persistent jaundice, hepatic failure and death[61-65]. RFA has been written into the international liver cancer treatment guidelines established by the American Association for the Study of Liver Diseases as a curative treatment for early HCC[6]. However, Imai et al[66] proposed that surgical resection is superior to RFA for the treatment of small solitary HCC, as demonstrated by increased five-year overall (87.5% vs 59%) and disease-free survival rates (46.8% vs 23.9%) for tumors with a diameter of 2 to 3 cm. Though Peng et al[67] argued that RFA should be the first choice for early HCC tumors ≤ 3 cm in diameter, reporting one-, three- and five-year overall survival rates of 94.2%, 82.6% and 67.5% compared to 90.1%, 65.0% and 55.1%, respectively, for resection. In addition, they report corresponding RFA-associated recurrence-free survival rates of 85.5%, 69.1% and 40.7%, compared to resection-associated rates of 82.2%, 40.1% and 31.8%, respectively, especially for tumors located in the central liver parenchyma.
Advancements in RFA ablation devices and technologies, particularly secondary to the emergence of various radiofrequency electrodes, have made it feasible to use a single probe to achieve greater necrosis in a large area. As a result, RFA has been used to treat tumor nodules of greater sizes. Many scientists have attempted to expand the treatment indications for RFA by conducting numerous clinical studies, although the conclusions have often been inconsistent and occasionally conflicting[68-72]. Most of these were retrospective studies with insufficient probative value of evidence-based medicine. From this viewpoint, it is also important to consider three recently published randomized controlled trials. One of these trials, performed by Chen et al[61], showed for the first time that RFA and surgical resection demonstrate indistinguishable efficacy for single-nodule HCC < 5 cm in diameter. However, in a study examining patients who met the Milan criteria, Huang et al[65] discovered that hepatectomy is superior to RFA in terms of both postoperative survival and disease-free survival. Given that these two contradictory studies were each characterized by an evidence level of I, it is difficult to draw a firm conclusion regarding surgical treatment versus RFA for patients with HCC. Although these two reports had different inclusion criteria, the conclusion obtained by Huang et al[65] was opposite that obtained by Chen et al[61] from the analyses of patients with single tumor nodules. In this regard, many retrospective analyses tend to support the finding that RFA yields a significantly higher postoperative recurrence rate than LR. This is particularly true with respect to the short-term postoperative recurrence rate, whereas the long-term rate varies considerably. Interestingly, a recent meta-analysis revealed that RFA generates a higher recurrence rate in the previous site than does surgical resection, whereas surgical resection generates a higher recurrence rate in new areas[69]. In addition, two randomized controlled trials showed that RFA produced complete tumor necrosis rates of 91.5% and 100.0% based on post-RFA imaging examination results[61,65]. In comparison, only approximately 70% of tumors were completely ablated in explants from post-RFA liver transplantation patients, and the occurrence rate of satellite lesions was as high as 44%. Whether the post-RFA recurrence is relapse or simply the regrowth of residual tumors from the previous operation remains unclear. To address this question, we used the ratio change of Lens culinaris agglutinin-reactive alpha-fetoprotein to identify all possible patients with postoperative residual tumors. This strategy allowed us to minimize the skewing of experimental results by this factor. Our results showed that when small HCC tumors were completely ablated, neither RFA nor LR showed significant differences in postoperative recurrence or overall survival[73]. Moreover, even in cases of short-term postoperative recurrence involving residual tumors, no significant difference was found in the three-year survival rate between the two strategies. These findings suggest that proactive therapeutic measures after recurrence may avoid reductions in post-RFA survival. Although this controversy has not been settled, the results of most studies on this issue are summarized along with some recommendations in Table 2.
| Tumors (n) | Tumor size (cm) | Child-Pugh class | Tumor characteristics | Recommended strategy |
| 1 | ≤ 2 | A | M0, subcapsular, adjacent to intrahepatic vessel trunk or extrahepatic organs | LR |
| B | M0, central location | RFA | ||
| > 2 to ≤ 4 | A | M0 | LR or RFA | |
| M0, subcapsular, adjacent to intrahepatic vessel trunk or extrahepatic organs | LR | |||
| B | M0, central location | RFA | ||
| > 4 | A | M0 | LR | |
| 2-3 | ≤ 3 | A | M0, bilobar disease | LR and/or RFA |
| M0, unilobar disease | LR | |||
| B | M0 | RFA |
In addition to postoperative recurrence and survival rates, the potential differences between these approaches (with respect to their other aspects) have been compared. Given the prominent feature of minimal invasiveness, RFA is associated with a markedly lower rate of postoperative complications and a better economic benefit than LR[74]. It is also well established that HBV reactivation is an independent risk factor for postoperative HCC recurrence. A study by Dan et al[75] reported that LR is more likely to cause postoperative HBV reactivation than RFA. Meredith et al[76] performed a study using a mouse model and revealed that LR led to increased secretion of hepatocyte growth factor and basic fibroblast growth factor, thereby promoting tumor growth. However, the levels of these two factors decreased after RFA. In contrast to LR, RFA leaves the treated tumor tissue in the body instead of removing it from the body, which may induce innate and adaptive immune responses[77-79]. Zerbini et al[80,81] recently demonstrated that RFA can activate tumor-specific T cells and enhance the ability of natural killer cells to kill HCC cells. Many other important aspects of the influence of RFA on the tumor biology of HCC are also evident. For example, residual tumors display markedly enhanced growth and invasive ability after RFA. This likely occurs because the RFA transition zone provides a special microenvironment for the locally remaining tumor cells[82-85]. In this type of secondary persistent anoxic environment, tumor cells undergo heat shock-resistant apoptosis and upregulate the expression of various cytokines (e.g., proliferating cell nuclear antigen, matrix metalloproteinase 9, vascular endothelial growth factor, hepatocyte growth factor, interleukin 6, etc.), resulting in increased proliferation and invasiveness of the tumor cells[86]. Moreover, Cheng et al[87] reported that sublethal heat can stimulate the transition of hepatoma cells into epithelial mesenchymal-like cells and augment their invasive capacity.
Taken together, the evidence suggests that improved therapeutic efficacy of HCC treatment, either by LR or RFA, principally requires minimizing residual tumors during the procedure, close follow-up, and active treatment once recurrence develops. In addition, complete removal of HCC tissue comprising more than two nodules does not guarantee curative resection because of the multicentric nature of HCC. Surgical resection of single-nodule HCC tissue without vascular invasion results in an excellent prognosis even if the nodule is larger than that recommended in the Milan criteria[88]. Therefore, the Milan criteria, which are mainly based on preoperative imaging analyses, cannot precisely determine whether patients with similar baseline imaging findings will develop distant metastatic disease. Such patients often cannot benefit from LR, RFA, or even liver transplantation.
The presence of multicentric tumor lesions is a key factor that limits the efficacy of surgical treatment for HCC. Hepatectomy cannot remove all tumor lesions due to cirrhotic impairment of the functional reserve, as is particularly evident for small HCC foci situated deep in the liver parenchyma. Elias et al[89,90] used RFA to ablate microscopic lesions outside of the main tumor tissue during hepatectomy and achieved excellent outcomes. In addition, Choi et al[91] reported that the combination of RFA and LR to treat multifocal HCC yielded one-, three- and five-year survival rates of 87%, 80% and 55%, respectively, which were comparable to rates from simple surgical removal. Interestingly, these authors also revealed that the resected tumor size was a significant prognostic predictor of long-term survival. Cheung et al[92] found that a combined surgery group had one- and three-year survival rates of 88.8% and 62.6%, respectively, compared to 88.9% and 51.8%, respectively, in a simple surgery group. Moreover, the postoperative recurrence rate in the combined surgery group was higher than that in the simple surgery group (63.2% vs 50.0%), though this difference was not statistically significant. No operative deaths occurred in these studies, as individuals with relatively good hepatic functional reserve were recruited. Although RFA has a far lower probability of causing liver failure than does extended hepatectomy, RFA in patients with multiple tumor nodules may result in morbidity[91,93]. Therefore, suitable patients should be carefully selected. Those with < 10% indocyanine green retention at 15 min are good candidates for lobectomy[94].
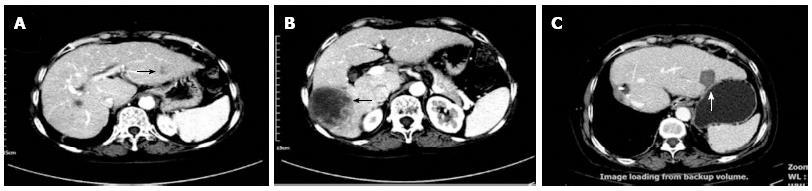
Repeat hepatectomy to treat recurrent HCC reportedly yields three- and five-year survival rates of 56% to 83% and 40% to 52%, respectively[95-98]. However, repeat hepatectomy is not feasible in a majority of patients because of substantial hepatic dysfunction or multiplicity of recurrent HCC. In the past, patients with recurrence who were intolerant to repeat hepatectomy were mainly treated by TACE and ethanol ablation[99]. In contrast, RFA is now more often performed to treat recurrent tumors in the remnant liver after hepatectomy and has shown good outcomes (Table 3)[100-108]. A recent report showed that three- and five-year survival rates reached satisfactory levels of 65.7% and 51.6%, respectively, which are very similar to those of repeat hepatectomy[105]. Such impressive advances in efficacy may be closely associated with the tremendous innovations in RFA devices and operation strategies. Figure 1 shows the results of RFA combined with hemihepatectomy for the treatment of multifocal HCC in a 69-year-old woman.
| Ref. | n | Tumors (n) | Tumor size (cm) | Radiologic response necrosis | PLR→RFA interval (mo) | Follow-up after RFA (mo) | Overall survival | Disease-free survival | Main findings | ||||
| 1-yr | 3-yr | 5-yr | 1-yr | 3-yr | 5-yr | ||||||||
| Nicoli et al[100] | 5 | - | - | - | 43 (31.0-61.0) | 25.5 (-) | - | 60.0% | - | - | 20.0% | - | RFTA is the first-choice treatment in the management of intrahepatic recurrence |
| Choi et al[101] | 45 | 53 | 2.1 (0.8-4.0) | 87.0% (46.0/53.0) | 23 (10.0-40.0) | 18.0 (2.0-47.0) | 82.0% | 54.0% | - | 57.0% | 34.0% | - | Percutaneous RFA is effective and safe for intrahepatic recurrent HCC after hepatectomy. Serum alpha-fetoprotein level before RFA and resected tumor size were significant prognostic predictors of long-term survival |
| Lu et al[102] | 72 | 124 | 2.4 (0.9-7.0) | 96.0% (119.0/124.0) | 27.9 (2.0-75.9) | 21.0 (1.0-215.2) | 70.0% | 55.0% | 28.0% | 22.0% | 95.0% | 83.0% | Percutaneous thermal ablative therapies were particularly suitable for recurrent HCC and improved long-term survival |
| Schindera et al[103] | 35 | 61 | 1.7 (0.5-5.3) | 85.5% (54.0/61.0) | 18 (1.0-65.0) | - | 76.0% | 45.0% | - | - | - | - | Percutaneous RFA is effective and safe for recurrent HCC after hepatectomy, with a good overall patient survival rate |
| Yang et al[104] | 41 | 76 | 3.8 (2.0-6.6) | 93.4% (71.0/76.0) | - | 24.5 (1.0-96.0) | 73.0% | 41.0% | - | 46.0% | 24.0% | - | Percutaneous RFA is effective and safe for recurrent hepatic tumors after previous partial hepatectomy |
| Choi et al[105] | 102 | 119 | 2.0 (0.8-5.0) | 93.3% (111.0/119.0) | 35.6 (7.0-83.0) | 22.3 (1.3-125.7) | 93.9% | 65.7% | 51.6% | 52.2% | 21.3% | 7.2% | RFA is effective and safe for recurrent HCC after hepatectomy and is more effective in late than in early recurrence |
| Liang et al[106] | 66 | 88 | - | 93.9% (62.0/66.0) | 21.1 (2.4-69.4) | - | 76.6% | 48.6% | 39.9% | - | - | - | Percutaneous RFA is as effective as repeat hepatectomy for recurrent small HCC. Percutaneous RFA has an advantage over repeat hepatectomy in terms of being less invasive |
| Chan et al[107] | 45 | - | 2.2 (0.8-6.0) | 87.0% (46.0/53.0) | 35.6 (7.0-83.0) | - | 83.7% | 43.1% | 29.1% | 32.2% | 12.4% | 9.3% | Repeat resection and RFA attained similar survival benefits in the management of recurrent HCC after hepatectomy. The high repeatability of RFA and its ability to be delivered percutaneously render it a preferred treatment option for selected patients |
| Eisele et al[108] | 27 | - | 2.8 (-) | - | - | 21.0 (-) | 96.0% | 62.0% | 32.0% | 51.0% | 30.0% | 11.0% | Overall survival and disease-free survival were not significantly different between patients treated by RFA and repeat resection. There was, however, a tendency toward longer tumor-free survival in the resected patients |
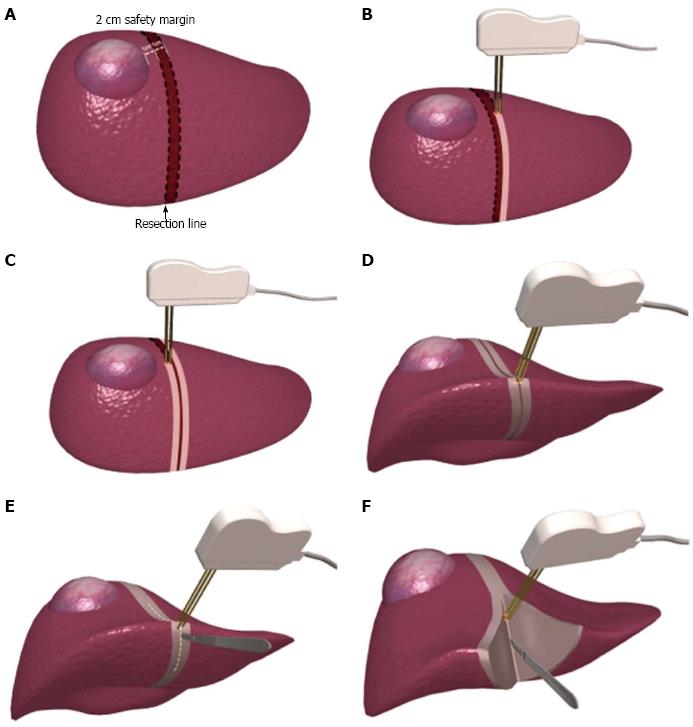
The heat generated by RFA may result in coagulative necrosis of liver tissues, in turn clogging the sinusoids and bile duct. Therefore, Weber et al[109] creatively introduced RFA to the hepatectomy approach in 2002. This modification markedly reduced surgical blood loss, decreased the number of portal triad clampings, and achieved excellent efficacy. Subsequently, this approach was adopted for LR by many groups and gave rise to the concept of “bloodless LR”[110-116]. However, this method is still accompanied by operative death and severe postoperative complications, mainly liver failure, bile leakage, and intraperitoneal bile collection[117]. The standard procedure of this technique is to use a bipolar radiofrequency electrode to burn two continuous ablation zones, 1 to 2 cm in width, flanking the predetermined resection line on the liver surface; this is followed by severance of the liver parenchyma (Figure 2)[118]. However, such an approach may damage a considerable number of functional hepatocytes, particularly in cirrhotic livers, and significantly increase the likelihood of liver failure. Such an ablation strategy undoubtedly elevates the occurrence of liver failure in patients with concurrent HCC and liver cirrhosis, especially for patients in Asian and South African regions[119]. Therefore, adjustments and alterations of this operative technique are necessary to minimize the loss of functional liver parenchyma.
Advantages of RFA over LR include minimal invasiveness and almost no postoperative intra-abdominal adhesion. These factors produce favorable conditions for subsequent liver transplantation. Patients in the waiting period can receive the most suitable treatment via an extended “test time.” This strategy not only provides patients with therapeutic benefits, but also saves precious donor resources. Studies demonstrating mutual interactions between RFA and hepatectomy have expanded the operative indications for patients with HCC and have enhanced the efficacy of these approaches. Nevertheless, the novel strategy of radiofrequency-assisted LR requires strict adherence to individual patient-specific indications to avoid the occurrence of severe complications. Most importantly, the outcome of RFA in HCC treatment is inevitably linked to the development of residual tumors. Therefore, the goal of future research is to minimize residual tumors and suppress their growth.
P- Reviewers: Berlakovich GA, Chau GY, Mizuguchi T, Peck-Radosavljevic M, Tanaka K S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 2. | Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 4. | Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 353] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 7. | Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Duffy JP, Hiatt JR, Busuttil RW. Surgical resection of hepatocellular carcinoma. Cancer J. 2008;14:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Ikai I, Kudo M, Arii S, Omata M, Kojiro M, Sakamoto M, Takayasu K, Hayashi N, Makuuchi M, Matsuyama Y, Monden M. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2010;40:1043-1059. [RCA] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Curley SA. Radiofrequency ablation leads to excellent local tumor control and durable longterm survival in specific subsets of early stage HCC patients confirming to the Milan criteria. Ann Surg. 2010;252:913-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 17. | Zhang B, Moser M, Zhang E, Zhang WJ. Radiofrequency ablation technique in the treatment of liver tumours: review and future issues. J Med Eng Technol. 2013;37:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3595] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 19. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5308] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 20. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 21. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3243] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 22. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 23. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 24. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 25. | Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, Lipshutz G, Yersiz H, Lu DS, Lassman C. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502-59; discussion 502-59;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 27. | Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Tamura S, Sugawara Y, Kokudo N. Living donor liver transplantation for hepatocellular carcinoma: the Japanese experience. Oncology. 2011;81 Suppl 1:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Ransford R, Gunson B, Mayer D, Neuberger J, Christensen E. Effect on outcome of the lengthening waiting list for liver transplantation. Gut. 2000;47:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, Rodés J, Bruix J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 33. | Fisher RA, Maluf D, Cotterell AH, Stravitz T, Wolfe L, Luketic V, Sterling R, Shiffman M, Posner M. Non-resective ablation therapy for hepatocellular carcinoma: effectiveness measured by intention-to-treat and dropout from liver transplant waiting list. Clin Transplant. 2004;18:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Pulvirenti A, Garbagnati F, Regalia E, Coppa J, Marchiano A, Romito R, Schiavo M, Fabbri A, Burgoa L, Mazzaferro V. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, Rudich S, McClure LA, Arenas J. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 37. | Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Brillet PY, Paradis V, Brancatelli G, Rangheard AS, Consigny Y, Plessier A, Durand F, Belghiti J, Sommacale D, Vilgrain V. Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: a prospective study with histopathologic comparison. AJR Am J Roentgenol. 2006;186:S296-S305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 40. | DuBay DA, Sandroussi C, Kachura JR, Ho CS, Beecroft JR, Vollmer CM, Ghanekar A, Guba M, Cattral MS, McGilvray ID. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford). 2011;13:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Schroeder T, Sotiropoulos GC, Molmenti EP, Kuehl H, Cicinnati VR, Schmitz KJ, Kóbori L, Paul A, Mathé Z. Changes in staging for hepatocellular carcinoma after radiofrequency ablation prior to liver transplantation as found in the explanted liver. Hepatogastroenterology. 2011;58:2029-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Panaro F, Piardi T, Audet M, Gheza F, Woehl-Jaegle ML, Portolani N, Cinqualbre J, Wolf P. Laparoscopic ultrasound-guided radiofrequency ablation as a bridge to liver transplantation for hepatocellular carcinoma: preliminary results. Transplant Proc. 2010;42:1179-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 44. | Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Takahashi S, Kudo M, Chung H, Inoue T, Nagashima M, Kitai S, Tatsumi C, Minami Y, Ueshima K, Fukunaga T. Outcomes of nontransplant potentially curative therapy for early-stage hepatocellular carcinoma in Child-Pugh stage A cirrhosis is comparable with liver transplantation. Dig Dis. 2007;25:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Ioannou GN, Perkins JD, Carithers RL. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 48. | Kelley RK, Yao F. Salvage liver transplantation for recurrent hepatocellular carcinoma after radiofrequency ablation: a new strategy? J Hepatol. 2012;56:14-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | N’Kontchou G, Aout M, Laurent A, Nahon P, Ganne-Carrié N, Grando V, Baghad I, Roulot D, Trinchet JC, Sellier N. Survival after radiofrequency ablation and salvage transplantation in patients with hepatocellular carcinoma and Child-Pugh A cirrhosis. J Hepatol. 2012;56:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 51. | Di Benedetto F, Tarantino G, Montalti R, Ballarin R, D’Amico G, Di Sandro S, Gerunda GE. Laparoscopic radiofrequency ablation in the caudate lobe for hepatocellular carcinoma before liver transplantation. J Laparoendosc Adv Surg Tech A. 2012;22:400-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Ishizawa T, Mise Y, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. Surgical technique: new advances for expanding indications and increasing safety in liver resection for HCC: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [PubMed] |
| 54. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-79; discussion 790-79;. [PubMed] |
| 55. | Esnaola NF, Mirza N, Lauwers GY, Ikai I, Regimbeau JM, Belghiti J, Yamaoka Y, Curley SA, Ellis LM, Nagorney DM. Comparison of clinicopathologic characteristics and outcomes after resection in patients with hepatocellular carcinoma treated in the United States, France, and Japan. Ann Surg. 2003;238:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P’eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2005;92:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: Current status. World J Radiol. 2010;2:417-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 59. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1792] [Article Influence: 85.3] [Reference Citation Analysis (2)] |
| 60. | Kaibori M, Matsui Y, Hijikawa T, Uchida Y, Kwon AH, Kamiyama Y. Comparison of limited and anatomic hepatic resection for hepatocellular carcinoma with hepatitis C. Surgery. 2006;139:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1103] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 62. | Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology. 2008;55:2171-2174. [PubMed] |
| 64. | Guo WX, Sun JX, Cheng YQ, Shi J, Li N, Xue J, Wu MC, Chen Y, Cheng SQ. Percutaneous radiofrequency ablation versus partial hepatectomy for small centrally located hepatocellular carcinoma. World J Surg. 2013;37:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 641] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 66. | Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, Nitta H, Ishiko T, Takamori H, Baba H. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Peng ZW, Liu FR, Ye S, Xu L, Zhang YJ, Liang HH, Lin XJ, Lau WY, Chen MS. Radiofrequency ablation versus open hepatic resection for elderly patients (> 65 years) with very early or early hepatocellular carcinoma. Cancer. 2013;119:3812-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 69. | Liu Z, Zhou Y, Zhang P, Qin H. Meta-analysis of the therapeutic effect of hepatectomy versus radiofrequency ablation for the treatment of hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2010;20:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Qi X, Tang Y, An D, Bai M, Shi X, Wang J, Han G, Fan D. Radiofrequency Ablation Versus Hepatic Resection for Small Hepatocellular Carcinoma: A Meta-analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2014;48:450-457. [PubMed] |
| 71. | Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9:e84484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013;11:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 597] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 74. | Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 75. | Dan JQ, Zhang YJ, Huang JT, Chen MS, Gao HJ, Peng ZW, Xu L, Lau WY. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013;39:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 76. | Meredith K, Haemmerich D, Qi C, Mahvi D. Hepatic resection but not radiofrequency ablation results in tumor growth and increased growth factor expression. Ann Surg. 2007;245:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Waitz R, Solomon SB. Can local radiofrequency ablation of tumors generate systemic immunity against metastatic disease? Radiology. 2009;251:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 79. | Greten TF, Korangy F. Radiofrequency ablation for the treatment of HCC--maybe much more than simple tumor destruction? J Hepatol. 2010;53:775-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 81. | Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, Cerioni S, Fagnoni F, Soliani P, Ferrari C. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138:1931-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 82. | Portolani N, Tiberio GA, Ronconi M, Coniglio A, Ghidoni S, Gaverini G, Giulini SM. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology. 2003;50:2179-2184. [PubMed] |
| 83. | Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137-1140. [PubMed] |
| 84. | Nijkamp MW, van der Bilt JD, de Bruijn MT, Molenaar IQ, Voest EE, van Diest PJ, Kranenburg O, Borel Rinkes IH. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg. 2009;249:814-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Nijkamp MW, Hoogwater FJ, Steller EJ, Westendorp BF, van der Meulen TA, Leenders MW, Borel Rinkes IH, Kranenburg O. CD95 is a key mediator of invasion and accelerated outgrowth of mouse colorectal liver metastases following radiofrequency ablation. J Hepatol. 2010;53:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Ke S, Ding XM, Kong J, Gao J, Wang SH, Cheng Y, Sun WB. Low temperature of radiofrequency ablation at the target sites can facilitate rapid progression of residual hepatic VX2 carcinoma. J Transl Med. 2010;8:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Cheng J, Li M, Lv Y. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2014;59:1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Yeh CN, Lee WC, Chen MF. Hepatic resection and prognosis for patients with hepatocellular carcinoma larger than 10 cm: two decades of experience at Chang Gung memorial hospital. Ann Surg Oncol. 2003;10:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 89. | Elias D, Debaere T, Muttillo I, Cavalcanti A, Coyle C, Roche A. Intraoperative use of radiofrequency treatment allows an increase in the rate of curative liver resection. J Surg Oncol. 1998;67:190-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 90. | Elias D, Goharin A, El Otmany A, Taieb J, Duvillard P, Lasser P, de Baere T. Usefulness of intraoperative radiofrequency thermoablation of liver tumours associated or not with hepatectomy. Eur J Surg Oncol. 2000;26:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Choi D, Lim HK, Joh JW, Kim SJ, Kim MJ, Rhim H, Kim YS, Yoo BC, Paik SW, Park CK. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14:3510-3518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Cheung TT, Ng KK, Chok KS, Chan SC, Poon RT, Lo CM, Fan ST. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: prognosis and outcomes. World J Gastroenterol. 2010;16:3056-3062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Kim YS, Rhim H, Lim HK, Choi D, Lee WJ, Jeon TY, Joh JW, Kim SJ. Intraoperative radiofrequency ablation for hepatocellular carcinoma: long-term results in a large series. Ann Surg Oncol. 2008;15:1862-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Choi D, Lim HK, Rhim H. Concurrent and subsequent radiofrequency ablation combined with hepatectomy for hepatocellular carcinomas. World J Gastrointest Surg. 2010;2:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Shuto T, Kinoshita H, Hirohashi K, Kubo S, Tanaka H, Tsukamoto T, Okuda T. Indications for, and effectiveness of, a second hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology. 1996;43:932-937. [PubMed] |
| 96. | Sugimachi K, Maehara S, Tanaka S, Shimada M, Sugimachi K. Repeat hepatectomy is the most useful treatment for recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Arii S, Teramoto K, Kawamura T, Okamoto H, Kaido T, Mori A, Imamura M. Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience. J Hepatobiliary Pancreat Surg. 2001;8:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Nagasue N, Kohno H, Hayashi T, Uchida M, Ono T, Yukaya H, Yamanoi A. Repeat hepatectomy for recurrent hepatocellular carcinoma. Br J Surg. 1996;83:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222. [PubMed] |
| 100. | Nicoli N, Casaril A, Marchiori L, Mangiante G, Hasheminia AR. Treatment of recurrent hepatocellular carcinoma by radiofrequency thermal ablation. J Hepatobiliary Pancreat Surg. 2001;8:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Choi D, Lim HK, Kim MJ, Lee SH, Kim SH, Lee WJ, Lim JH, Joh JW, Kim YI. Recurrent hepatocellular carcinoma: percutaneous radiofrequency ablation after hepatectomy. Radiology. 2004;230:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Lu MD, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, Kuang M, Zheng YL. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg. 2005;92:1393-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Schindera ST, Nelson RC, DeLong DM, Clary B. Intrahepatic tumor recurrence after partial hepatectomy: value of percutaneous radiofrequency ablation. J Vasc Interv Radiol. 2006;17:1631-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Yang W, Chen MH, Yin SS, Yan K, Gao W, Wang YB, Huo L, Zhang XP, Xing BC. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early- and late-phase recurrence. AJR Am J Roentgenol. 2006;186:S275-S283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, Lau WY. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 107. | Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, Lo CM. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 108. | Eisele RM, Chopra SS, Lock JF, Glanemann M. Treatment of recurrent hepatocellular carcinoma confined to the liver with repeated resection and radiofrequency ablation: a single center experience. Technol Health Care. 2013;21:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 110. | Ferko A, Lesko M, Subrt Z, Melichar B, Hoffman P, Dvorák P, Vacek Z, Liao LR, Habib NA, Kocí J. A modified radiofrequency-assisted approach to right hemihepatectomy. Eur J Surg Oncol. 2006;32:1209-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 111. | Zacharoulis D, Tzovaras G, Rountas C, Poultsidis A, Katsogridakis E, Sioka E, Hatzitheofilou C. Modified radiofrequency-assisted liver resection: a new device. J Surg Oncol. 2007;96:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Sandonato L, Cipolla C, Fulfaro F, Lo Re G, Latteri F, Terranova A, Mastrosimone A, Bova V, Cabibbo G, Latteri MA. Minor hepatic resection using heat coagulative necrosis. Am Surg. 2009;75:1213-1219. [PubMed] |
| 113. | Wagman LD, Lee B, Castillo E, El-Bayar H, Lai L. Liver resection using a four-prong radiofrequency transection device. Am Surg. 2009;75:991-994. [PubMed] |
| 114. | Curro G, Bartolotta M, Barbera A, Jiao L, Habib N, Navarra G. Ultrasound-guided radiofrequency-assisted segmental liver resection: a new technique. Ann Surg. 2009;250:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 115. | Taibbi A, Furlan A, Sandonato L, Bova V, Galia M, Marin D, Cabibbo G, Soresi M, Bartolotta TV, Midiri M. Imaging findings of liver resection using a bipolar radiofrequency electrosurgical device--initial observations. Eur J Radiol. 2012;81:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Akyildiz HY, Morris-Stiff G, Aucejo F, Fung J, Berber E. Techniques of radiofrequency-assisted precoagulation in laparoscopic liver resection. Surg Endosc. 2011;25:1143-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Pai M, Spalding D, Jiao L, Habib N. Use of bipolar radiofrequency in parenchymal transection of the liver, pancreas and kidney. Dig Surg. 2012;29:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Pai M, Frampton AE, Mikhail S, Resende V, Kornasiewicz O, Spalding DR, Jiao LR, Habib NA. Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol. 2012;38:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Mitsuo M, Takahiro T, Yasuko T, Masayasu A, Katsuya O, Nozomi S, Yoshihide O, Isamu K. Radiofrequency (RF)-assisted hepatectomy may induce severe postoperative liver damage. World J Surg. 2007;31:2208-2212; discussion 2208-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |