Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5889
Revised: November 12, 2013
Accepted: December 3, 2013
Published online: May 21, 2014
Processing time: 376 Days and 21.2 Hours
AIM: To determine the frequency of HER2/neu protein overexpression in gastric (group A), small intestine (group B), and colorectal (group C) adenocarcinoma.
METHODS: A descriptive, cross-sectional study was performed on 50 cases of gastrointestinal adenocarcinoma (stomach, small intestine, and colorectal); 11 from group A, 8 from group B, and 31 from group C. The samples were grossed and processed in the pathology department, and sections were stained with HE (hematoxylin and eosin stain) for histopathological confirmation of malignancy (well-differentiated, moderately-differentiated, and poorly-differentiated). The confirmed samples were processed for immunomarker study of HER2/neu.
RESULTS: HER2/neu protein overexpression was found in 33 (66%) patients overall (P = 0.000). Out of 33 HER2/neu positive subjects, 23 (69.6%) were from group C, while the remaining 10 (30%) were from group A. None of the patients from group B had positive HER2/neu protein overexpression. No protein overexpression or membrane staining in < 10% tumor cells was observed in 17 (34%) patients, which were labeled as score “0” and considered negative for HER2/neu protein overexpression. Faint/weak staining (in ≥ 10% of tumors cells) were observed in 8 (16%) patients and given the “1+” score. Similarly 13 (26%) patients reported moderate staining (in ≥ 10% tumor cells) and were thus labeled as “2+”, and strong staining (in ≥ 10% tumors cells), labeled as “3+”, was observed in 12 (24%) patients. Out of 50 patients, 26 (52%) were suffering from grade-II malignancy, 16 (32%) from grade-I, and 8 (16%) from grade-III. There was highly significant association between tumor grades and HER2/neu protein overexpression (P = 0.0000).
CONCLUSION: HER2/neu protein is credibly overexpressed in colon and gastric adenocarcinomas in immunohistochemistry. There is significant association between grade of tumor and HER2/neu protein overexpression.
Core tip: The role of HER2/neu was seen in 50 cases of gastrointestinal adenocarcinoma by immunohistochemistry. Overall, HER2/neu protein overexpression was found in 33 (66%) patients (P = 0.000), with 23 (69.6%) being colorectal carcinoma while the remaining 10 (30%) were gastric carcinoma. There was highly significant association between tumor grades and HER2/neu protein overexpression. HER2/neu protein was overexpressed in those adenocarcinomas showing a significant association between grade of tumor and HER2/neu protein overexpression. This discovery may improve treatment options for cases of gastric and colorectal carcinomas.
- Citation: Farzand S, Siddique T, Saba K, Bukhari MH. Frequency of HER2/neu overexpression in adenocarcinoma of the gastrointestinal system. World J Gastroenterol 2014; 20(19): 5889-5896
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5889
HER2/neu is an oncogene located on the chromosome 17q21. Similar to epidermal growth factor receptors HER1, HER3, and HER4, it is a member of the tyrosine kinase receptor family. Activation of HER2/neu leads to initiation of signaling pathways like MAPK/P13K/AKT, essential for cell proliferation and differentiation[1]. Overexpression of HER2/neu has been reported in many epithelial malignancies, including cancers of the lungs[2], prostate[3,4], bladder[5], pancreas[6], and osteosarcoma[7]. The role of HER2/neu directed therapy and its success in breast cancer patients has led to evaluation of protein overexpression, gene amplification, and anti-tumor activity of Herceptin in multiple tumor types, including colorectal and gastric adenocarcinomas[8,9].
The data available in the literature for HER2/neu positivity rates in gastric cancers vary from about 7%-43%. HER2/neu positive status in gastric cancer also appears to be associated with more aggressive disease, a poorer prognosis, and shorter survival. Recently, the EMEA (European Medicine Agency) has approved trastuzumab in combination with chemotherapy for use in patients with advanced gastric cancer[10-13].
In the West, colorectal cancer is one of the most common malignancies. Survival of patients with colorectal carcinoma has improved due to development of new cytotoxic agents (oxaliplatin and irinotecan) and advanced surgical techniques, but no further treatment options are available when patients become refractory to these modern chemotherapeutic regimens. Recently, however, the effects of these cytotoxic agents have been augmented by the development of monoclonal antibodies against vascular endothelial and epidermal growth factor receptors. Several studies have reported a variety of HER2/neu protein overexpression and gene amplification in colorectal adenocarcinoma. Data available about the prevalence of HER2/neu protein overexpression in colorectal cancer ranges from 0%-83%. However HER2/neu protein overexpression and its relation with clinicopathological features like Dukes classification and survival is conflicting[8,9].
This wide range of HER2/neu protein overexpression in colorectal adenocarcinoma reflects both differences in reagents used and methods, as well as study bias associated with patients selection (i.e., early versus advanced disease).
The study was carried out at the Department of Pathology of the King Edward Medical University’s Mayo Hospital, Lahore and the Nawaz Sharif Social Security Teaching Hospital, Multan Road, Lahore. Informed consent was obtained from patients or their guardians. Patient identities were concealed, and approval from the University Research Ethical Committee was obtained prior to conducting this study.
This was a descriptive, prospective cross-sectional study on 50 cases of gastrointestinal (GIT) adenocarcinoma (stomach, small intestine, and colorectal) collected from the Pathology Department of the King Edward Medical University’s Mayo Hospital, Lahore and the Nawaz Sharif Social Security Teaching Hospital, Multan Road, Lahore.
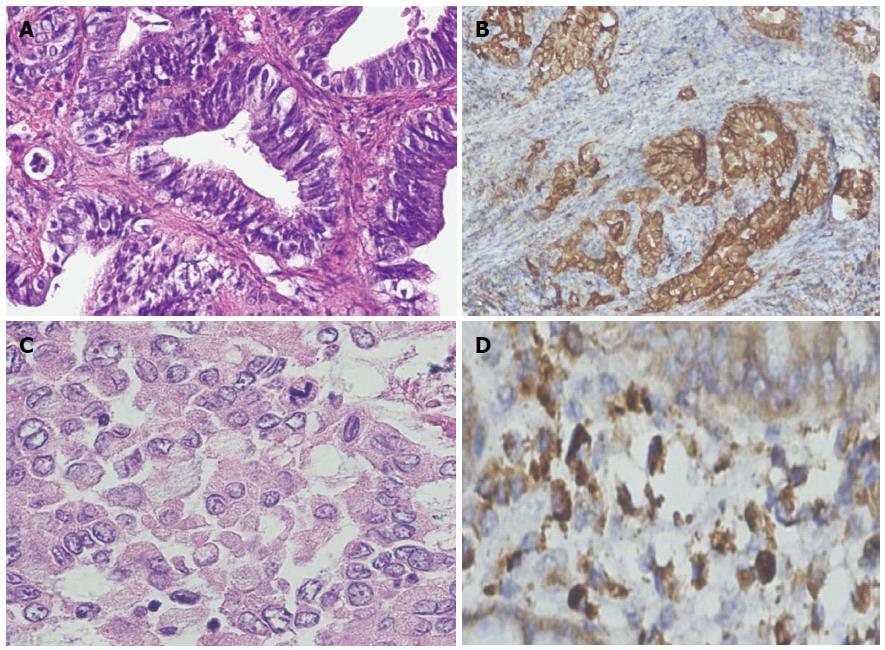
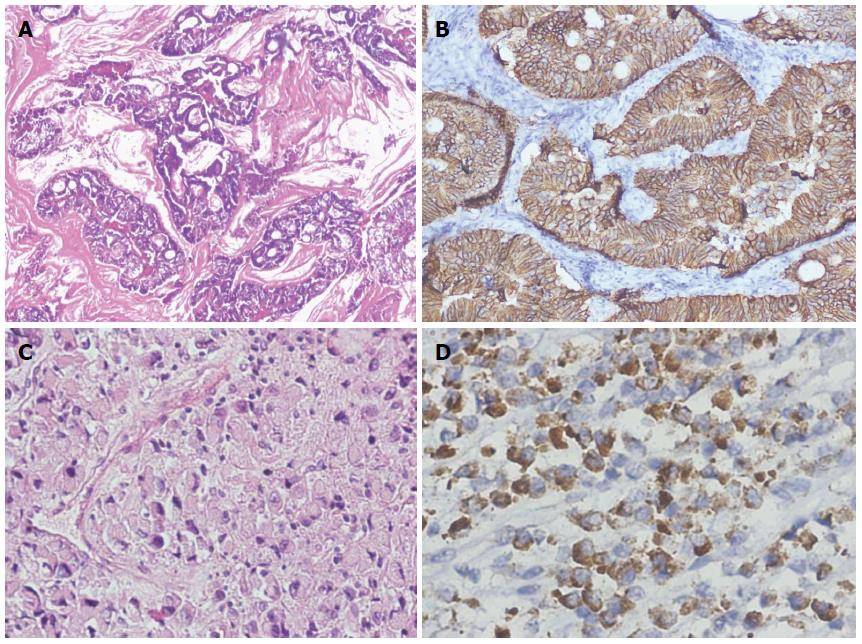
History and clinical findings of the patients were entered pro forma. Patient consent was obtained via their own signature, a thumb sign, or by the signature of a guardian. The samples were grossed and processed at the Department of Pathology, King Edward Medical University, Lahore. The sections were stained with HE (hematoxylin and eosin stain) for histopathological confirmation of malignancy. The histological grading of these tumors as well-differentiated (I), moderately-differentiated (II), and poorly-differentiated (III) was done according to the criterion of the American Joint Committee on Cancer[14,15]. The confirmed samples were processed for immunmarker study of HER2/neu. Known positive breast cancer cases were used as positive controls, with the omission of primary antibodies for the negative control. All sections were evaluated by two independent senior pathologists. Tumors with HER2/neu staining showing in ≥ 10% cancer cells were labeled as positive for HER2/neu protein overexpression. The results were reported as positive (cytoplasmic and membranous) or negative, and scored according to the intensity of the stain (weak, moderate, and severe) as 1+, 2+, or 3+.
The study was completed within 6 mo after the approval of final synopsis.
HER2/neu oncogene, a member of the tyrosine kinase receptor family, is essential for cell proliferation and differentiation, and its overexpression and/or amplification is associated with many types of tumors. Immunohistochemistry was performed by using a Hercep test kit to determine positive protein overexpression of HER2/neu in different grades of stomach, small intestine, and colorectal adenocarcinomas.
Fifty fresh cases of stomach, small intestine, and colorectal adenocarcinomas diagnosed via histopathology were included in this study. Patients of both sexes and a wide range of ages were taken in this study. Inadequate biopsies, autolysed specimens, and inflammatory lesions were excluded.
Results were evaluated according to the criteria recommended by the manufacturer by using scores from 0 to 3+. No staining at all or membrane staining in < 10% of tumor cells was given the score “0”. Faint staining in ≥ 10% of tumor cells was given the score “1+”, as well as in cells which were only stained in part of the membrane. Weak to moderate staining of the entire membrane in ≥ 10% of the tumor cells was given the score “2+”, and strong staining of the entire membrane in ≥ 10% tumor cells was given the score “3+”. Scores of 0 were labeled as negative tumors, while scores of 1+, 2+, and 3+ were labeled as weak, moderate, and strong positive expression of HER2/neu, respectively[16].
Evaluation of gastric cases was performed according to the modified gastric cancer testing protocol[17]. Accordingly, strong incomplete (basolateral) membranous staining was accepted as positive (3+), and the 10% cut-off for this group in biopsy cases was abolished (Hofmann M et al[17] 2008). In biopsy cases, a focus (clone) that is allowed to be scored should have at least 5 stained evaluable cells. Score 0 was considered as negative HER2/neu protein overexpression, while score 1+, 2+, and 3+ were considered as positive HER2/neu protein overexpression in our study.
The Institutional Review Board of the King Edward Medical University of Lahore approved the present study.
All information related to patients and their ailments was noted in a prescribed pro forma method. The data was analyzed by SPSS version 18. The comparison of HER2/neu protein overexpression in different grades was compared by chi-square test. A P value less than 0.5 was considered statistically significant.
The mean age of the studied patients was 48.5 ± 13.63 years (range: 17-75). The mean age of subjects in group A was found to be 52.82 ± 9.41 years (range: 40-70). In group B, the mean age observed was 50.25 ± 5.99 years (range: 45-60). The mean age in group C was 46.52 ± 15.92 years (range: 17-75) (Table 1).
| Intensity of HER2/neu staining | Study groups | |||
| Group A | Group B | Group C | Total | |
| No stain or membrane staining < 10% (0) | 1 | 8 | 8 | 17 |
| Weak staining ≥ 10% (1+) | 2 | 0 | 6 | 8 |
| Moderate staining ≥ 10% (2 +) | 6 | 0 | 7 | 13 |
| Strong staining ≥ 10% (3 +) | 2 | 0 | 10 | 12 |
| Total | 11 | 8 | 31 | 50 |
| Mean age (yr) | 52.81 ± 9.41 | 50.25 ± 9.41 | 46.51 ± 15.92 | 48.50 ± 13.62 |
| Minimum age (yr) | 40.00 | 45.00 | 17.00 | 17.00 |
| Maximum age (yr) | 70.00 | 60.00 | 75.00 | 75.00 |
In terms of gender, 23 males and 27 females were included in this study, with a male to female ratio of 1:1.13. The highest number of males was observed in group C (12, 52%) followed by group A (9, 39.1%), with the fewest in group B (2, 8.6%). There was a statistically insignificant association between gender and study groups (P = 0.078) (Figure 1).
HER2/neu protein overexpression was found in 33 (66%) patients overall. Out of 33 HER2 positive subjects, 23 (69.6%) were from group C, while the remaining 10 (30%) were from group A. None of the patients from group B had positive HER2/neu status. HER2/neu protein overexpression was not found in 17 (34%) patients, which was regarded as negative expression. Among these 17 HER2 negative patients, 8 (47%) were from group C, 1 (5.8%) was from group A, and 8 (47%) were from group B. There was a highly significant association between HER2/neu protein overexpression (positivity) status and the different study groups (P = 0.000) (Table 1). No expression or membrane staining (in < 10% of tumors cells) was observed in 17 (34%) of the patients who were scored as “0” and labeled negative. Out of these, 8 (47%) were in group C, 1 (5.8%) was from group A, and 8 (47%) were from group B. Faint/weak staining (in ≥ 10% of tumor cells) was observed in 8 (16%) of the patients (“1+” score), of which 6 (75%) were from group C and 2 (25%) were from group A. Similarly, 13 (26%) patients reported moderate staining (in ≥ 10% tumors cells) and were scored “2+”; 7 of these patients were from group C and 6 were from group A. Strong staining (in ≥ 10% of tumor cells) was scored “3+”, and was observed in 12 (24%) patients, of which 10 (83%) were from group C and 2 (16.6%) were from group A. Score “0’’ was regarded as negative while scores of 1+, 2+, and 3+ were regarded as positive for HER2/neu protein overexpression. Statistically, a highly significant association was seen between intensity of HER2/neu overexpression and the different study groups (P = 0.000) (Table 1).
Among 33 HER2/neu positive subjects, 18 (54.5%) were grade-II malignancy, 11 (33%) were grade-I, while 4 (12%) were grade III. Out of 17 HER2/neu negative subjects, 8 (47.05%) were grade-II, 5 (29.4%) were grade-I, and 4 (23.5%) were grade III. There was a highly significant association between tumor grades and HER2/neu overexpression (P = 0.0000) (Table 1). Among 9 subjects with lymph nodes metastasis, 8 (88.8%) were HER2/neu positive and 1 (11.1%) was HER2/neu negative. Out of 15 subjects with no lymph nodes metastasis, 12 (80%) were HER2/neu positive and 3 (20%) were HER2/neu negative. There is no significant association between lymph node involvement and HER2/neu overexpression (P = 0.572) (Table 2).
| Lymph nodes | HER2/neu status | Total | |
| +Ve | -Ve | ||
| Tumor involvement | 8 | 1 | 9 |
| No tumor involvement | 12 | 3 | 15 |
| Total | 20 | 4 | 24 |
Regarding the morphological types of adenocarcinomas positive for HER2/neu, we found 16 (48%) cases of adenocarcinoma (not otherwise specified) positive for HER2/neu overexpression, among which 13 (81.25%) were from group C and 3 (18%) were from group A. Five (15%) cases positive for HER2/neu overexpression were mucinous adenocarcinoma, out of which 4 (80%) were from group C and 1 (20%) was from group A. Five (15%) cases of positive HER2/neu overexpression were of the signet ring variant of adenocarcinoma; 2 (40%) from group C and 3 (60%) from group A. Both cases of adenocarcinoma with neuroendocrine differentiation were from group C and were strongly positive for HER2/neu overexpression (3+). Similarly, both cases of the adenocarcinoma papillary variant from group C were weak to moderately positive for HER2/neu status. All 3 cases of the intestinal variant of adenocarcinoma were from group A and moderately positive for HER2/neu status (2+). A highly significant association was observed between different morphological types and HER2/neu overexpression (P = 0.0000) (Table 3).
| Morphological type grade of tumor | HER2/neu status | ||
| Positive | Negative | Total | |
| Grade- | |||
| I | 11 | 5 | 16 |
| II | 18 | 8 | 26 |
| III | 4 | 4 | 8 |
| Total1 | 33 | 17 | 50 |
| Adenocarcinoma (NOS) | 16 | 13 | 29 |
| Mucinous adenocarcinoma | 5 | 2 | 7 |
| Adenocarcinoma (signet ring variant) | 5 | 1 | 6 |
| Adenocarcinoma (intestinal variant) | 3 | 0 | 3 |
| Papillary adenocarcinoma | 2 | 0 | 2 |
| Adenocarcinoma neuroendocrine differentiation | 2 | 0 | 2 |
| High grade neuroendocrine carcinoma | 0 | 1 | 1 |
| Total2 | 33 | 17 | 50 |
Tumor staging was not applicable (NA) on biopsy specimens 18 (36%); 6 (33%) were from group C, 7 (38%) from group A, and 5 (27%) from group B. Staging was only done on surgically resected specimens. In the tumor stage of modified Dukes, B2 was found to be most frequent [i.e., 19 (38%) group C patients], with C2 being the second most frequently observed tumor stage at 4 (8%), followed by Dukes C1 in 2 (4%). The remaining tumor stages were found in each study group, except for tumor stage-IIA in one patient in both group A and B. A highly significant association was found between all tumor stages and study groups (P = 0.000) (Table 3). Expression of HER2/neu is shown in Figures 1 and 2.
In our study, a total of 50 subjects were included; 11 from group A, 8 from group B, and 31 from group C. The mean age of overall patients was 48.5 ± 13.63 years (range: 17-75). The mean age in our study shows that presentation is more common in later life, which is an important focus in many studies based on HER2/neu overexpression. A comparative study in 2004 found older age to be statistically correlated with overexpression of HER2/neu in endometrial carcinoma[18]. Additionally, a total of 23 males and 27 females were included in our study, with a male to female ratio of 1:1.13. There was a very slight difference in male to female ratio in our setting, similar to another study in New York (2003), in which the male to female ratio was 1.03:1 (86 males and 83 females)[19]. A statistically insignificant association was found between gender and study groups (P = 0.074) in our setting. This insignificant association was compatible to another study conducted in 2006 by Schuell et al[16], which evaluated the frequency of HER2/neu protein overexpression in colorectal cancer. 77 cases of surgically resected malignant colorectal cancer lesions were evaluated via a similar mode of immunohistochemistry, as was used in our study. The relationship with gender was also found to be statistically insignificant in that study[16].
The tumor grades in our study varied with respect to the different study groups. Out of 26 (52%) patients who were suffering from Grade II malignancy, 20 (76.9%) were from group C, 2 (7.7%) were from group A, and 4 (15.3%) were from group B. The Grade I category was second commonest (16, 32%) and consisted of 10 (62.5%) from group C, 4 (25%) from group A, and 2 (12.5%) from group B. Very few patients (8, 16%) were observed to have a grade III tumor. Importantly, the prime focus of our study was to establish the obvious positivity status of HER2/neu protein overexpression by immunohistochemistry among different study groups. In our study, HER2/neu expression was found to be positive in 33 (66%) patients overall, whilst it was negative in 17 (34%) patients. Out of these 33 HER2 positive subjects, 23 (69.7%) were from group C, while the remaining 10 (30.3%) were from group A; none of the patients from group B had a positive HER2 status. A highly significant association was also observed between HER2/neu protein positivity status and the different study groups (P = 0.000). Among 33 HER2/neu positive patients in our study, 18 (54.5%) were of grade II malignancy, 11 (33%) were of grade I malignancy, and 4 (12%) were of grade III malignancy. A highly significant association between tumor grades and HER2/neu status was seen in our study. A study conducted in Iran in 2006 to evaluate the frequency and staining pattern of HER2/neu protein overexpression in colon carcinoma by immunohistochemistry found HER2/neu positivity to be as high as 59.4% of cases. The association between HER2/neu protein overexpression and tumor grades was also highly significant (P = 0.04) in this study; 66.7% cases were grade I, followed by 36% grade II malignancies. In higher grades, HER2/neu staining was decreased[20].
Half et al[21] conducted a study to assess HER2 expression in colon cancer. They documented cytoplasmic staining in 63.5% of primary tumors, with strong membranous staining observed in 5% of primary colorectal carcinomas. They also found significant correlation between HER2 positive overexpression (cytoplasmic staining) and tumor differentiation, as is the case in our study. The HER2 positivity in our study (66%) was much greater than that indicated in a study conducted in 2007, in which HER2/neu overexpression and gene amplification was assessed in 137 colorectal patients using immunohistochemistry and FISH. 47.4% subjects were determined to have overexpressed HER2/neu by immunohistochemistry. However, similar to our own study, a significant correlation of malignancy with HER2/neu overexpression was observed[22]. In a study which involved only gastric carcinoma, patients showed the even smaller percentage of only 18.8% patients with HER2 positive status, with a statistically insignificant association contrasting with our results in which a higher frequency, as well as significant association of HER2 positivity, was observed[23]. A Chinese study that included 145 patients with gastric carcinoma showed a total frequency of 32.4% positive HER2/neu protein overexpression, which was statistically significant. In this study, the evaluation of lymph nodes involvement was also assessed and found to be significantly associated with HER2/neu status[24].
Contrary to these results, our study showed an insignificant association of lymph node involvement and HER2/neu status (P = 0.572). Lymph nodes were identified in 24 subjects, of which 9 (37.5%) displayed lymph node metastasis. Among these, 6 (67%) were from group C, with the other 3 (33%) being from group A. Among the 9 subjects with lymph nodes metastasis, 8 (88.8%) were HER2 positive and 1 (11.1%) was HER2 negative. Of the 15 subjects with no lymph nodes metastases, 12 (80%) were HER2 positive and 3 (20%) were HER2 negative. In the above study, only gastric carcinoma patients were studied, and they were found to have significant association with lymph node involvement. In a Chinese study conducted in 2006, a significant association was found between HER2/neu protein overexpression, lymph node metastasis, and clinical stage[25]. Hence, lymph node involvement is generally considered in adenocarcinomas when HER2 protein overexpression is concerned. We were unable to establish this relationship statistically despite having a considerable frequency apparently involved with tumors. General cancer studies that were not GIT-related malignancies, particularly breast cancer, have also indicated possible involvement of HER2/neu in lymph node metastatic tumors[26].
McKay et al[27] studied HER2/neu protein overexpression in a large cohort of colorectal tumors and lymph node metastases. HER2/neu was expressed in 81.8% tumors. They did not find any correlation between HER2/neu protein overexpression and lymph node metastasis. The results were very similar to our study, especially with regards to the correlation between HER2/neu protein overexpression and lymph node metastasis.
Another study assessed how the expression of HER2/neu correlates with the stage of disease and survival in colorectal cancer. This study included 201 samples and found HER2 positivity status to be significantly associated with adenocarcinomas, similar to our study[28]. Ross et al[29] studied HER2/neu protein overexpression in gastrointestinal tract tumors. They found a wide range of HER2/neu protein overexpression in esophageal, gastric, and colon carcinomas. They concluded that either HER2/neu protein overexpression or gene amplification is associated with one-fourth of all GIT malignancies, and strategies should thus be designed to employ this marker in therapy selection. Among adenocarcinomas of the gastrointestinal system, colorectal and gastric carcinomas frequently present with protein overexpression of HER2/neu, and are reported to be affected by its presence as well[29].
From our study, it would appear that HER2/neu protein overexpression may play a crucial role in the therapeutic management of GIT adenocarcinomas, particularly in the colorectal region. Further studies are suggested to increase the knowledge in this area, assess other clinical dimensions of HER2/neu involvement, and to provide in depth impact evaluation of cancers. HER2/neu protein is credibly overexpressed in colorectal and gastric adenocarcinomas
We thank Dr. Shahida Niazi for her valuable suggestions and critical reading of this research article.
Colorectal carcinoma and carcinoma of the stomach are the most prevalent cancers worldwide as well as in Pakistan, where it has a poor prognosis. HER2/neu is an epidermal growth factor receptor 2, and its overexpression has been detected in gastric and colorectal carcinomas that are essential for understanding the biological behavior of HER2/neu on these cancers. Chemotherapy with Herceptin (trastuzumab) can improve the prognosis of patients, but a standardized HER2 scoring system is still required. This study on the role of HER2/neu in these malignancies, as well as its clinicopathological attributes, could help patient selection for clinicians performing targeted therapy using anti-HER2/neu drugs like Herceptin.
Anti-HER2/neu therapy is playing a significant role in breast carcinoma and as a new treatment option for gastrointestinal malignancies in suitable candidates. The Food and Drug Administration has approved the use of Herceptin for the treatment of gastric and colorectal carcinomas. Many researchers have been evaluating HER2/neu positive patients daily via the accurate and reliable Immunohistochemistry scoring system for selection of Herceptin targeted therapy.
In Pakistan, there have been limited studies concerning the role of HER2/neu expression in patients with gastrointestinal (GIT) malignancies. The results show HER2/neu protein overexpression in high grade tumors and lymph node metastases, and these cases could represent ideal candidates for Herceptin targeted therapy.
HER2/neu overexpression is significantly associated with tumor grade. Therefore it could be applied as a reliable immunmarker, with patients overexpressing HER2/neu being potential candidates for new adjuvant monoclonal antibody-based targeted immunotherapy.
The study design was valid and the data was sufficient to make a conclusion on the frequency of HER2/neu proteins overexpression in adenocarcinomas of the GIT system.
P- Reviewer: Parra-Palau JL S- Editor: Zhai HH L- Editor: Rutherford A E- Editor: Liu XM
| 1. | Schlessinger J. All signaling is local? Mol Cell. 2002;10:218-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3110] [Cited by in RCA: 3090] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 2. | Lara PN, Laptalo L, Longmate J, Lau DH, Gandour-Edwards R, Gumerlock PH, Doroshow JH, Gandara DR. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer. 2004;5:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Lara PN, Chee KG, Longmate J, Ruel C, Meyers FJ, Gray CR, Edwards RG, Gumerlock PH, Twardowski P, Doroshow JH. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California Cancer Consortium Screening and Phase II Trial. Cancer. 2004;100:2125-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Lara PN, Meyers FJ, Gray CR, Edwards RG, Gumerlock PH, Kauderer C, Tichauer G, Twardowski P, Doroshow JH, Gandara DR. HER-2/neu is overexpressed infrequently in patients with prostate carcinoma. Results from the California Cancer Consortium Screening Trial. Cancer. 2002;94:2584-2589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Coogan CL, Estrada CR, Kapur S, Bloom KJ. HER-2/neu protein overexpression and gene amplification in human transitional cell carcinoma of the bladder. Urology. 2004;63:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, Hesketh P, Rathore R, Wolff R, Tantravahi U. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Tsai JY, Aviv H, Benevenia J, Chang VT, Patterson F, Aisner S, Hameed M. HER-2/neu and p53 in osteosarcoma: an immunohistochemical and fluorescence in situ hybridization analysis. Cancer Invest. 2004;22:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Järvinen TA, Liu ET. HER-2/neu and topoisomerase IIalpha--simultaneous drug targets in cancer. Comb Chem High Throughput Screen. 2003;6:455-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570-6578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17:1-12. [PubMed] |
| 11. | Noble F, Kelly JJ, Bailey IS, Byrne JP, Underwood TJ. A prospective comparison of totally minimally invasive versus open Ivor Lewis esophagectomy. Dis Esophagus. 2013;26:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Qu JJ, Shi YR, Hao FY. [Clinical study of the predictors to neoadjuvant chemotherapy in patients with advanced gastric cancer]. Zhonghua Weichang Waike Zazhi. 2013;16:276-280. [PubMed] |
| 13. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5317] [Article Influence: 354.5] [Reference Citation Analysis (3)] |
| 14. | Talsma K, van Hagen P, Grotenhuis BA, Steyerberg EW, Tilanus HW, van Lanschot JJ, Wijnhoven BP. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol. 2012;19:2142-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Rosai J. Rosai and Ackerman's Surgical Pathology. 10th ed. St Louis, MO: St Louis Mosby 2011; 615-885. |
| 16. | Schuell B, Gruenberger T, Scheithauer W, Zielinski Ch, Wrba F. HER 2/neu protein expression in colorectal cancer. BMC Cancer. 2006;6:123. [PubMed] |
| 17. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 868] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 18. | Peiró G, Mayr D, Hillemanns P, Löhrs U, Diebold J. Analysis of HER-2/neu amplification in endometrial carcinoma by chromogenic in situ hybridization. Correlation with fluorescence in situ hybridization, HER-2/neu, p53 and Ki-67 protein expression, and outcome. Mod Pathol. 2004;17:227-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Nathanson DR, Culliford AT, Shia J, Chen B, D’Alessio M, Zeng ZS, Nash GM, Gerald W, Barany F, Paty PB. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer. 2003;105:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Leung SP, Griffith OL, Masoudi H, Gown A, Jones S, Phang T, Wiseman SM. Clinical utility of type 1 growth factor receptor expression in colon cancer. Am J Surg. 2008;195:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Xie SD, Xu CY, Shen JG, Jiang ZN, Wang LB. HER 2/neu protein expression in gastric cancer is associated with poor survival. Mol Med Rep. 2009;2:943-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai X, Yu BH, Du YQ. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World J Gastroenterol. 2011;17:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Li Q, Chen Y, Kong P, Ming W, Ma Y, Yang Z, Du Y, Zhang D. [Correlation between the expression of HER-2/neu, nm23 protein and local invasion and lymph node metastasis of human LSCC]. Linchuang Erbiyanhouke Zazhi. 2006;20:259-261. [PubMed] |
| 26. | Sato-Kuwabara Y, Neves JI, Fregnani JH, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using Fluorescence in situ Hybridization (FISH) and immunohistochemistry. BMC Cancer. 2009;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | McKay JA, Loane JF, Ross VG, Ameyaw MM, Murray GI, Cassidy J, McLeod HL. c-erbB-2 is not a major factor in the development of colorectal cancer. Br J Cancer. 2002;86:568-573. [PubMed] |
| 28. | Kapitanović S, Radosević S, Kapitanović M, Andelinović S, Ferencić Z, Tavassoli M, Primorać D, Sonicki Z, Spaventi S, Pavelic K. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology. 1997;112:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |