Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5881
Revised: September 21, 2013
Accepted: October 19, 2013
Published online: May 21, 2014
Processing time: 351 Days and 16.9 Hours
AIM: To evaluate the incidence of late biliary complications in non-resectable alveolar echinococcosis (AE) under long-term chemotherapy with benzimidazoles.
METHODS: Retrospective analysis of AE patients with biliary complications occurring more than three years after the diagnosis of AE. We compared characteristics of patients with and without biliary complications, analyzed potential risk factor for biliary complications and performed survival analyses.
RESULTS: Ninety four of 148 patients with AE in Zurich had non-resectable AE requiring long-term benzimidazole chemotherapy, of which 26 (28%) patients developed late biliary complications. These patients had a median age of 55.5 (35.5-65) years at diagnosis of AE and developed biliary complications after 15 (8.25-19) years of chemotherapy. The most common biliary complications during long-term chemotherapy were late-onset cholangitis (n = 14), sclerosing cholangitis-like lesions (n = 8), hepatolithiasis (n = 5), affection of the common bile duct (n = 7) and secondary biliary cirrhosis (n = 7). Thirteen of the 26 patients had undergone surgery (including 12 resections) before chemotherapy. Previous surgery was a risk factor for late biliary complications in linear regression analysis (P = 0.012).
CONCLUSION: Late biliary complications can be observed in nearly one third of patients with non-resectable AE, with previous surgery being a potential risk factor. After the occurrence of late biliary complications, the median survival is only 3 years, suggesting that late biliary complications indicate a poor prognostic outcome.
- Citation: Frei P, Misselwitz B, Prakash MK, Schoepfer AM, Prinz Vavricka BM, Müllhaupt B, Fried M, Lehmann K, Ammann RW, Vavricka SR. Late biliary complications in human alveolar echinococcosis are associated with high mortality. World J Gastroenterol 2014; 20(19): 5881-5888
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5881.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5881
Human alveolar echinococcosis (AE) is caused by the larval stage of the fox tapeworm, Echinococcus multilocularis. The parasites’ dominant definitive host is the red fox, which can be infected in up to 60%[1]. Echinococcus multilocularis is widely distributed throughout the Northern Hemisphere, with a disease-endemic area stretching from North America through central Europe (eastern France, southern Germany, Switzerland and western Austria) to central and east Asia[2,3]. AE remains a rare disease in Western Europe, but not in highly endemic regions such as Sibiria and China, where a human prevalence rate of 3% or higher has been described[4].
In early untreated cohorts, the fatality rate exceeded 90% within 10 years[5]. However, survival rates have improved tremendously within the last three decades due to the increasing success of several treatment options, including surgery as the preferred first line therapy[6], long-term chemotherapy with benzimidazoles (available since 1975[7]), interventional procedures, and in rare cases liver transplantation[8]. As a result, the life expectancy for a middle aged patient with AE has increased by approximately 20 years and is now only slightly reduced (by about 3 years) compared to healthy controls[7]. However, if complete surgical resection of the parasite is not possible, long-term (life-long) chemotherapy with benzimidazoles remains the standard of care[6], and AE continues to be a disease with significant morbidity and mortality.
In unresected AE, biliary complications can influence the course of the disease significantly. The intrahepatic bile ducts or the extrahepatic biliary tree can either be directly infiltrated and destroyed by the growing parasite or compressed due to surrounding growth. Biliary (and other) complications include cholangitis, liver abscess, septic shock, portal hypertension and biliary cirrhosis[9]. Detailed knowledge of biliary complications is crucial for the management of patients with non-resectable AE.
Currently, our knowledge of adverse effects in AE is very limited. There are case series describing biliary complications of cystic hydatid disease (caused by Echinococcus granulosus). In these publications, bile duct obstruction with liver atrophy in the cyst-bearing lobes[10], rupture of hepatic hydatid cysts in the biliary tree with secondary cholangitis[11] or biliary obstruction by daughter cysts with further complications such as portal hypertension, ascites, abscesses and the development of bronchobiliary fistulas have been described[12,13]. However, these data are not directly applicable to alveolar hydatid disease (AE; caused by Echinococcus multilocularis) which is much more frequent in central Europe. We aimed to evaluate late biliary complications in a cohort of patients with non-resectable AE.
From the prospective database of the Swiss National Center for Echinococcosis, we retrospectively analysed all available data of AE patients followed at the University Hospital of Zurich with a diagnosis of AE before 2003. The detailed study protocol has been published earlier[14]. The study was performed according to the declaration of Helsinki and the protocol was approved by the hospital ethics committee. All patients provided written informed consent to participate in the study. Patients were examined at yearly intervals, including a physical examination, routine laboratory tests, and additional immunodiagnostic tests, a chest radiograph, ultrasonography, and CT scan of the abdomen according to the study protocol. Further magnetic resonance imaging of the upper abdomen was performed in unclear cases.
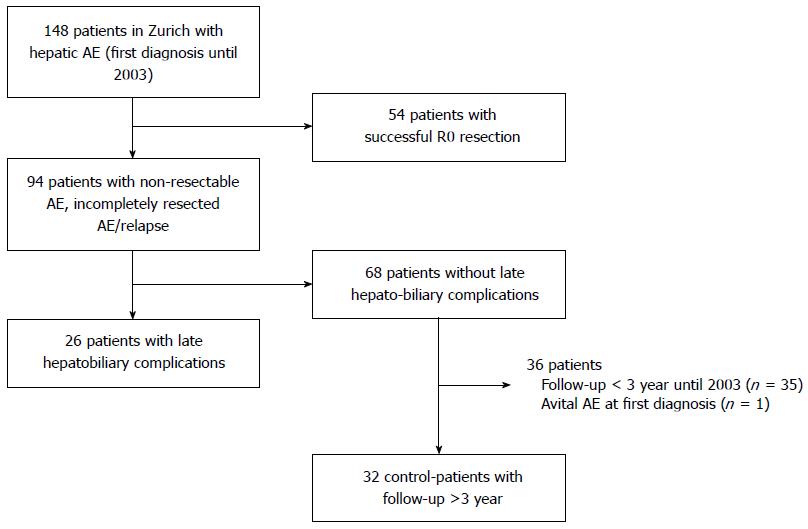
The patient selection process as described above is illustrated in Figure 1. All patients in Zurich with a diagnosis of AE between 1967 and 2003 were included (n = 148). Fifty-four patients with successful R0 resection were excluded. A total of 94 patients had non-resectable or recurrent AE and received continuous long-term chemotherapy with benzimidazole carbamates (either mebendazole or albendazole). Of the 94 non-resectable AE patients, 26 patients developed late biliary complications as diagnosed by imaging [endoscopic retrograde cholangiopancreaticography (ERCP), computed tomography scan (CT), magnetic resonance cholangiopancreaticography (MRCP)] and blood tests. Liver biopsies were not routinely performed in these patients to find biliary cirrhosis.
To assess the prognostic value of late hepatobiliary complications, we compared the 26 patients with control patients without any biliary complications. The control group consisted of 32 patients with a first diagnosis of non-resectable AE between 1979 and 2003 and a follow up of at least 3 years.
Late biliary complications were defined as biliary disease occurring at least 3 years after initial diagnosis of AE. The following were assessed: (1) Sclerosing cholangitis-like lesions, defined as abnormal ERCP or MRCP findings with features resembling those of primary sclerosing cholangitis such as multilocular annular strictures within the intrahepatic and/or extrahepatic bile ducts with alternating normal or slightly dilated segments; (2) Stenosis of the common bile duct, defined as isolated strictures in the proximal or distal common bile duct as well as strictures in the hilar region or extensions to the extrahepatic left and right duct; (3) One or several episodes of late cholangitis; (4) Hepatolithiasis and choledocholithiasis; (5) Secondary biliary cirrhosis; (6) Postoperative biliary stenosis after hepaticojejunostomy; and (7) Biliary fistulas.
Descriptive statistics were calculated using Microsoft Office Excel 2007. Results of numerical data are presented as medians (quartiles). Group comparisons were performed using the Fisher’s exact test or the Mann-Whitney-U test of the statistics program GraphPad Prism 5. Furthermore, Pearson’s correlation analysis and linear regression analysis were done using SPSS. For the linear regression analysis the potentially confounding factors: gender, age at diagnosis of AE, years of follow-up, years from diagnosis of AE until endpoint (either late biliary complications or end of follow-up), years of albendazole, mebendazole and total benzimidazole treatment as well as previous surgery were considered.
Late biliary complications developed in 26 patients with non-resectable AE undergoing chemotherapy after a median of 15 (interquartile range, IQR: 8.25-19) years. The 11 male and 15 female patients had a median age of 55.5 (35.5-65) years at diagnosis of AE. Further patient characteristics are provided in Table 1, which summarizes patients with and without late biliary complications. AE was diagnosed between 1967 and 1997. All patients received long-term chemotherapy with benzimidazoles, mostly mebendazole (25/26), rarely albendazole (5/26). Switching from one benzimidazole to the other was necessary in 4/26 patients. Benzimidazoles were given for a median of 13 (IQR: 8.1-20) years.
| Patients with late biliary complications (n = 26) | Control group without late biliary complications (n = 32) | P1value | |
| Male | 42.30% | 37.50% | NS |
| Year of AE diagnosis | 1967-1997 | 1979-2003 | |
| Age at AE diagnosis, (yr) | 55.5 (35.5-65) | 60.0 (44.2-64.6) | NS |
| Previous liver surgery | 13 (50) | 5 (15.6) | 0.009 |
| Years between AE diagnosis and first biliary complication, (yr) | 15.0 (8.3-19.0 ) | NA | |
| Age at first biliary complication, (yr) | 64.5 (54.0-75.8) | NA | |
| Deaths during follow-up until 2006 | 15 (57.7) | 5 (15.6) | NA2 |
| Liver related | 5 | 1 | |
| Non-liver related | 10 | 4 | |
| Age at death, (yr) | 80.0 (67.5-81 ) | 78.5 (65.0-85.1) | NS |
| Benzimidazole treatment (total) , (yr) | 13.0 (8.1-20.0) | 7.2 (4.2-16.1) | 0.0404 |
| Follow-up (total) , (yr) | 23.0 (13.0-25.0) | 8.6 (5.1-18.8) | < 0.0001 |
| Follow-up of survivors until study endpoint (end of 2006 or drop-out), (yr) | 11 | 27 | |
| From AE diagnosis | - 24.0 (23.0-29.0) | 7.1 (5.0-14.7) | 0.0001 |
| From biliary complication | - 9.0 (7.3-11.8) | NA | |
| Follow-up until death, (yr) | 15 | 5 | NS |
| From AE diagnosis | 15.0 (11.0-24.5) | 21.2 (9.7-25.1 ) | |
| From biliary complication | 2.0 (1.5-5.0) | NA |
Late biliary complications were grouped according to the presenting clinical syndrome or disease and the underlying pathogenic mechanisms. Table 2 summarizes the clinical syndromes of the 26 patient with late biliary complications and the respective long-term outcome. Symptoms occurred 15 years after diagnosis of AE at a median age of 64.5 (IQR: 54-75 ) years.
| Biliary complication | No. of patients | Outcome Number deaths during follow-up | Time to death of non-survivors, (yr) | Length of follow-up after BC, (yr) |
| Sclerosing cholangitis-like lesions (subtype2) | 8 (31) | 7/8 (87.5) | 3 (1.5-5) | 3.75 (1.75-6) |
| Stenosis of the common bile duct (subtype 4) | 7 (27) | 4/7 (57) | 4 (1.75-6.75) | 6 (4-10) |
| Late cholangitis (subtype 1) | 14 (54) | 5/14 (35) | 2 (2.5-6) | 7 (4-10.25) |
| Hepatolithiasis (subtype 3) | 5 (19) | 3/5 (60) | 2 (2-3) | 4 (2-6) |
| Secondary biliary cirrhosis(subtype 5) | 7 (27) | 4/7 (57) | 3 (2-4.5) | 6 (3-7) |
| Postoperative biliary stenosis after hepaticojejunostomy (subtype 7) | 1 (4) | No deaths | 11 | |
| Biliary fistula (subtype 8) | 2 (8) | No deaths | 8 |
Biliary complications included late-onset cholangitis (n = 14), sclerosing cholangitis-like lesions (n = 8), hepaticolithiasis (n = 5), secondary biliary cirrhosis (n = 7), stenosis of the common bile duct (n = 2), and postoperative stenosis after hepaticojejunostomy (n = 1). In addition, biliary fistulas occurred in 2 patients, including one biliary cutaneous and one bronchobiliary fistula. Altogether, 44 biliary complications in our 26 patients were noted. No obvious associations between different biliary complications could be detected. Eleven patients (42%) required interventions for late biliary complications, a total of 23 procedures were performed. 10 patients (38.5%) needed ERCP for treatment of biliary complications. In 2 patients (7.7%), PTCD was performed. Five patients (19.2%) needed surgery, including 3 hepaticojejunostomies and 2 resections. In 1 patient (3.8%), percutaneous enterostomy was performed.
To identify potential risk factors for late biliary complications, we performed Pearsons’ correlation and linear regression analysis of several possible risk factors (Table 3). Age at diagnosis of AE, age at diagnosis of biliary complications and time from diagnosis of AE until endpoint were not associated with an increased risk. However, longer duration of benzimidazole treatment was associated with an increased risk of developing biliary complications, also after adjustment for additional factors in the linear regression analysis. Both years of albendazole and mebendazole treatment correlated with the risk for the development of biliary complication even though directionality was different with albendazole apparently protective and mebendazole increasing the risk. At this point, this different directionality is based on very small subgroups, difficult to explain and other unknown confounding factors could be responsible.
| Pearson correlation | Linear regression analysis | ||||
| Variable number | Variable | Pearson correlation coefficients | P value for Pearson correlation | Unadjusted regression coefficients | P value for regression coefficients |
| 1 | Gender | 0.049 | NS | 0.141 | NS |
| 2 | Age at diagnosis of AE | -0.146 | NS | Redundant | - |
| 3 | Mebendazole treatment | 0.403 | 0.001 | Redundant | - |
| 4 | Albendazole treatment | -0.351 | 0.003 | -0.037 | 0.016 |
| 5 | Benzimidazole treatment | 0.274 | 0.019 | 0.030 | 0.013 |
| 6 | Whether or not surgery was performed | 0.370 | 0.02 | 0.354 | 0.031 |
| 7 | Age at diagnosis of biliary complication | -0.069 | NS | 0.001 | NS |
| 8 | Years from diagnosis of AE until endpoint (either BC or end of follow up) | 0.198 | NS | -0.024 | NS |
Importantly, previous surgery was a risk factor for biliary complications both in the correlation and regression analysis (P = 0.02 and 0.031, respectively). While 13 of the 26 patients with biliary complications had previously undergone surgery, only 5 out of 32 patients without biliary complications had a history of surgery. However, within the biliary complication group, biliary complications were observed both in patients with (n = 13) and without previous surgery (n = 13). Surgery was performed shortly after the diagnosis of AE in 10 patients or within the first 5 year after diagnosis in 3 patients (after 1, 2 and 5 year). Thus, previous surgery seems to be a risk factor for late biliary complications, but these are nevertheless not simply always consequence of earlier surgical procedures (Table 4).
| Previous surgery (n = 13) | No previous surgery (n = 13) | |
| Late cholangitis | 8 | 6 |
| Sclerosing-cholangitis like lesions | 2 | 6 |
| Hepatolithiasis | 3 | 2 |
| Affection of big bile ducts | 3 | 4 |
| Secondary biliary cirrhosis | 4 | 3 |
| Postoperative biliary stenosis (after hepaticojejunostomy) | 1 | 0 |
| Biliary fistula | 2 | 0 |
A total of 15/26 patients with late biliary complications died until the endpoint of 2006. Five of the 15 deaths were AE induced liver failures. The ten other deaths were caused by infectious complications (n = 3), lethal gastrointestinal bleeding (n = 2), advanced age (n = 2) and cardiovascular diseases (n = 3). Overall, a substantial number of deaths (8/15, 53%) were probably or likely linked to the AE infection (5 liver failures, 1 variceal bleed after secondary biliary cirrhosis and 2 septic shocks in patients with biliary cirrhosis). 7/17 deaths (47%) were not clearly liver-related or due to biliary complications.
Death occurred on average 3.0 (2-5) years after the diagnosis of biliary complications.
The short time interval between the occurrence of late biliary complications and death underlines the prognostic value of such complications. However, survival after first diagnosis of AE was not different in the biliary complications group compared to the control group. Of note, only 4 out of 7 patients with secondary biliary cirrhosis died (1 due to progressive liver disease after terminating treatment, 1 due to gastrointestinal bleeding, 2 because of septic shock). Five of 14 patients with late cholangitis died during follow-up.
To assess the prognostic relevance of biliary complications, we compared the 26 patients with late biliary complications to 32 control patients without biliary complications (Table 1). The control group included 27 patients with inoperable AE, 4 patients who had received non-curative surgery and 1 patient with a relapse after curatively intended AE surgery but no postoperative benzimidazole treatment due to non-compliance.
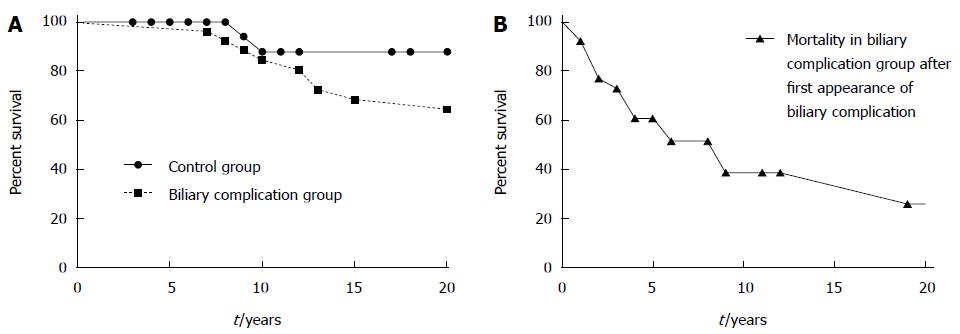
In the group with late biliary complications, substantially more patients died within the follow-up period until 2006 (57.5% vs 15.6%). However, the mean and median follow up time in patients with late biliary complications was substantially longer than in control patients, leading to a possible bias. Nevertheless, the range of follow-up in these patient groups was comparable. Moreover, as shown in Figure 2, the diagnosis of a biliary complication seemed to mark a turning point in the natural course of the disease. When we compared the survival of patients immediately after the occurance of biliary complications to the overall survival of patients with and without biliary complications after AE diagnosis a much shorter survival became apparent (Figure 2B). When survival after biliary complications was compared to survival of the control group, this difference reached statistical significance. In contrast, a comparison of the groups with and without biliary complications after diagnosis of AE showed no differences in survival. This is probably due to the much longer mean and median follow-up time in the biliary complication group (Table 1), leading to a possible bias. In summary, our data suggest that the occurrence of biliary complications indicates a “turning point” in the natural course of non-resectable AE and implies a poor prognosis after this timepoint.
AE is a severe infectious disease with relevant morbidity despite substantial increases in life expectancy over the last three decades[5,7]. Diagnostic and therapeutic improvements include better imaging techniques, improvements in surgical and perioperative strategies and the possibility of indefinite treatment with benzimidazoles in patients with non-resectable AE. Our data from a large national AE cohort demonstrate that biliary complications are a frequent problem in these patients in the course of the disease. Despite prolonged life expectancy, our data show that late biliary complications may predict a poor prognostic outcome. Mortality after the occurrence of biliary complications is substantially increased, with a median and mean survival of only 3 and 3.6 years, respectively.
Literature on biliary complications in non-resectable AE is scarce. There are many case series on biliary complications in “hydatid disease” (“cystic hydatid disease”), due to cystic echinococcosis, which differs from AE (“alveolar hydatid disease”) and shows a divergent biological behaviour. Thus, our study fills a gap in the knowledge of the clinical course of AE.
We tried to analyze potential risk factors for late biliary complications in non-resectable AE. In our analysis, length of benzimidazole treatment and previous liver surgery were associated with late biliary complications. The association of benzimidazole treatment and late biliary complications cannot be equated with a causal relationship. However, linear regression analysis showed that surgical interventions are a risk factor for biliary complications, although late biliary complications ocurred both in patients with and without previous surgery. According to the literature, liver surgery can be associated with perioperative complications, and biliary complications after hepatic resections are known to be associated with a high risk of liver failure and operative mortality[15]. Indeed, other case series have shown that there is a learning curve in liver resections (for other indications), accounting for up to 10% of all postoperative biliary complications[16]. In a Turkish study, focussing on hydatid liver surgery (treatment of cystic hydatid disease), biliary leakage occurred in 26% of 54 patients[17]. It should be noted that the frequency of non-curative “debulking” resections in our cohort was high, but most of these operations were performed several decades ago. Today it has become clear that such surgery should be avoided[18], in line with case series reporting postoperative recurrences in 3 of 9 patients[19]. Although length of albendazole treatment was associated with biliary complications in linear regression analysis, this seems difficult to explain. Of note, the albendazole subgroup was very small, including only 5 (19%) patients, of which 4 had previously been treated with mebendazole.
Despite the known beneficial effect of benzimidazoles on longterm survival[5,7], benzimidazoles seem to be unable to completely prevent biliary complications in non-resectable AE. Despite the regression or stabilization of lesions, 28% (26/94) of patients in our study with non-resectable AE developed late cholestatic complications such as cholestatic jaundice or cholangitis 3 to 26 year after the initial diagnosis of AE. In regression analysis, follow-up time was not associated with biliary complications. This suggests that our data are credible despite different mean and median follow-up times in the biliary complications and control group (mean follow up 11 years vs 20.3 years, median follow up 8.6 years vs 32 years). However, the range of follow-up was not substantially different. This makes it unlikely that differences in follow-up time are distorting the data.
The reasons for late cholestatic complications on benzimidazole treatment are probably multifactorial and not just a consequence of previous surgery, larval proliferation and biliary infiltration. Hypothetical explanations include malcompliance with benzimidazole treatment, which was not addressed in our study. However, in clinical practice benzimidazole levels are measured regularly (usually 4 h after drug intake) making malcompliance an unlikely cause for most patients. Furthermore, inactive AE cysts may trigger a non-productive immunological reaction, resulting in progressive fibrosis. In another scenario, the altered bile duct anatomy after AE infiltration might promote secondary infections or duodenal reflux causing secondary inflammation and fibrosis. This would explain why benzimidazoles seem to be able to inhibit AE growth, but not always the occurrence of AE complications such as biliary obstructions[19]. In any case, we are unable to determine whether benzimidazoles are only partially effective for the prevention of biliary complications or were started too late in the course of the disease.
Our study has several strengths and limitations. A major strength is the considerable number of affected patients who were evaluated in this nationwide prospective cohort and who followed a rigorous study protocol since 1980. A first limitation is that some of the studied complications occurred many years before the current data analysis, not all details about specific procedures performed and their immediate complications could be retrieved. We therefore focused on the first occurrence of biliary complications and death as major endpoints, for which a robust analysis could be performed despite these limitations. Second, follow-up times in patients with and without late biliary complications were substantially different. However, the range of follow-up was comparable between the two groups, as discussed above. Third, our analysis has to remain descriptive in many aspects and cannot provide clear risk factors for late biliary complications. There are no “parasitic factors” which could be identified as risk factors for late biliary complications. One could speculate that the PNM stage might be crucial, but we did not have access to concise PNM data. Thus, previous surgical interventions stay as major risk factor for late biliary complications.
How should the knowledge of biliary complications and their impact on long term survival change our management of non-resectable AE? It seems crucial that primary care physicians and gastroenterologists caring for such patients pay attention to the relevant risk of late biliary complications under long-term benzimidazole treatment, which is no guarantee for symptom-free long-term survival. After the detection of biliary complications, patients might benefit from advanced treatment options and tertiary care centers should be consulted. Centers will consider the benefits and risks of interventional procedures including ERCP and PTCD in the treatment of biliary complications. Furthermore, early liver transplantation in incurable symptomatic biliary AE has been suggested[20], which has so far only rarely been done due to insufficient available data. Nevertheless, an individual discussion about the risks and potential benefits of liver transplantation with otherwise eligible patients can be considered.
In summary, we have been able to demonstrate in a large cohort of patients with non-resectable AE that biliary complications are a frequent problem despite long-term chemotherapy with benzimidazoles. Late biliary complications occurred in nearly one third of patients, with previous surgery as a main risk factor. The survival in patients after occurrence of late biliary complications was short, with a median and mean survival of only 3 and 3.6 years. These data suggest that the occurrence of biliary complications can cause substantial morbidity and is associated with increased mortality.
We thank our study nurse, Karin Riederer, for her meticulous help in conducting this study.
P- Reviewers: Herszenyi L, Kawa S S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
| 1. | Gottstein B. [Epidemiology and systematics of cystic and alveolar hydatid disease]. Chirurg. 2000;71:1-8. [PubMed] |
| 2. | Calderini P, Magi M, Gabrielli S, Brozzi A, Kumlien S, Grifoni G, Iori A, Cancrini G. Investigation on the occurrence of Echinococcus multilocularis in Central Italy. BMC Vet Res. 2009;5:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Romig T. Echinococcus multilocularis in Europe--state of the art. Vet Res Commun. 2009;33 Suppl 1:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Craig PS, Rogan MT, Campos-Ponce M. Echinococcosis: disease, detection and transmission. Parasitology. 2003;127 Suppl:S5-20. [PubMed] |
| 5. | Ammann RW, Hoffmann AF, Eckert J. [Swiss study of chemotherapy of alveolar echinococcosis--review of a 20-year clinical research project]. Schweiz Med Wochenschr. 1999;129:323-332. [PubMed] |
| 6. | Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, Pavone P, Cappellani A, Cacopardo B. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 241] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 7. | Torgerson PR, Schweiger A, Deplazes P, Pohar M, Reichen J, Ammann RW, Tarr PE, Halkik N, Müllhaupt B. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol. 2008;49:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Moray G, Shahbazov R, Sevmis S, Karakayali H, Torgay A, Arslan G, Savas N, Yilmaz U, Haberal M. Liver transplantation in management of alveolar echinococcosis: two case reports. Transplant Proc. 2009;41:2936-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Fleiner-Hoffmann AF, Pfammatter T, Leu AJ, Ammann RW, Hoffmann U. Alveolar echinococcosis of the liver: sequelae of chronic inferior vena cava obstructions in the hepatic segment. Arch Intern Med. 1998;158:2503-2508. [PubMed] |
| 10. | Prousalidis J, Tzardinoglou E, Kosmidis C, Katsohis K, Aletras O. Surgical management of calcified hydatid cysts of the liver. HPB Surg. 1999;11:253-259. [PubMed] |
| 11. | Atli M, Kama NA, Yuksek YN, Doganay M, Gozalan U, Kologlu M, Daglar G. Intrabiliary rupture of a hepatic hydatid cyst: associated clinical factors and proper management. Arch Surg. 2001;136:1249-1255. [PubMed] |
| 12. | Kammerer WS, Schantz PM. Echinococcal disease. Infect Dis Clin North Am. 1993;7:605-618. [PubMed] |
| 13. | Pawlowski Z, Eckert J, Vuitton D, Ammann R, Kern P, Craig P. Echinococcosis in humans: clinical aspects, diagnosis and treatment. Paris: World Organization for Animal Health and World Health Organization 2001; . |
| 14. | Woodtli W, Bircher J, Witassek F, Eckert J, Wüthrich B, Ammann RW. Effect of plasma mebendazole concentrations in the treatment of human echinococcosis. Am J Trop Med Hyg. 1985;34:754-760. [PubMed] |
| 15. | Lo CM, Fan ST, Liu CL, Lai EC, Wong J. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133:156-161. [PubMed] |
| 16. | Lam CM, Lo CM, Liu CL, Fan ST. Biliary complications during liver resection. World J Surg. 2001;25:1273-1276. [PubMed] |
| 17. | Kayaalp C, Bzeizi K, Demirbag AE, Akoglu M. Biliary complications after hydatid liver surgery: incidence and risk factors. J Gastrointest Surg. 2002;6:706-712. [PubMed] |
| 18. | Kadry Z, Renner EC, Bachmann LM, Attigah N, Renner EL, Ammann RW, Clavien PA. Evaluation of treatment and long-term follow-up in patients with hepatic alveolar echinococcosis. Br J Surg. 2005;92:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Wilson JF, Rausch RL, Wilson FR. Alveolar hydatid disease. Review of the surgical experience in 42 cases of active disease among Alaskan Eskimos. Ann Surg. 1995;221:315-323. [PubMed] |
| 20. | Koch S, Bresson-Hadni S, Miguet JP, Crumbach JP, Gillet M, Mantion GA, Heyd B, Vuitton DA, Minello A, Kurtz S. Experience of liver transplantation for incurable alveolar echinococcosis: a 45-case European collaborative report. Transplantation. 2003;75:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (1)] |