Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5839
Revised: January 30, 2014
Accepted: March 6, 2014
Published online: May 21, 2014
Processing time: 166 Days and 19.3 Hours
AIM: To evaluate the significance of γ-catenin in clinical pathology, cellular function and signaling mechanism in esophageal squamous cell carcinoma (ESCC).
METHODS: The mRNA expression of γ-catenin was detected by real-time quantitative reverse transcription-polymerase chain reaction in 95 tissue specimens and evaluated for association with the clinicopathologic characteristics and survival time of patients with ESCC. siRNAs against human γ-catenin were used to inhibit γ-catenin expression. Hanging drop aggregation assay and dispase-based dissociation assay were performed to detect the effect of γ-catenin on ESCC cell-cell adhesion. Transwell assay was performed to determine cell migration. Luciferase-based transcriptional reporter assay (TOPflash) was used to measure β-catenin-dependent transcription in cells with reduced γ-catenin expression. The expression and subcellular localizations of β-catenin and E-cadherin were examined using Western blot and immunofluorescence analysis.
RESULTS: γ-catenin mRNA expression was significantly associated with tumor histological grade (P = 0.017) in ESCC. Kaplan-Meier survival analysis showed that γ-catenin expression levels had an impact on the survival curve, with low γ-catenin indicating worse survival (P = 0.003). The multivariate Cox regression analysis demonstrated that γ-catenin was an independent prognostic factor for survival. Experimentally, silencing γ-catenin caused defects in cell-cell adhesion and a concomitant increase in cell migration in both KYSE150 and TE3 ESCC cells. Analysis of Wnt signaling revealed no activation event associated with γ-catenin expression. Total β-catenin and Triton X-100-insoluble β-catenin were significantly reduced in the γ-catenin-specific siRNA-transfected KYSE150 and TE3 cells, whereas Triton X-100-soluble β-catenin was not altered. Moreover, knocking down γ-catenin expression resulted in a significant decrease of E-cadherin and Triton X-100-insoluble desmocollin-2, along with reduced β-catenin and E-cadherin membrane localization in ESCC cells.
CONCLUSION: γ-catenin is a tumor suppressor in ESCC and may serve as a prognostic marker. Dysregulated expression of γ-catenin may play important roles in ESCC progression.
Core tip: We, like others, have shown in previous work that reduced classic and desmosomal cadherin expression correlated with increased metastasis both in laboratory models and in clinical esophageal squamous cell carcinoma (ESCC) samples; however, a direct functional role of γ-catenin in this process has not been shown. In this study, we report that γ-catenin plays a critical role in ESCC progression. Our work demonstrates that γ-catenin is a tumor metastasis suppressor in ESCC and its expression may serve as a prognostic marker. Loss of γ-catenin leads to significant changes in esophageal cancer cell phenotypes.
- Citation: Fang WK, Liao LD, Gu W, Chen B, Wu ZY, Wu JY, Shen J, Xu LY, Li EM. Down-regulated γ-catenin expression is associated with tumor aggressiveness in esophageal cancer. World J Gastroenterol 2014; 20(19): 5839-5848
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5839
Esophageal squamous cell carcinoma (ESCC) is the most common malignancy in China, and has high mortality[1]. It is difficult to diagnose ESCC during early stages of disease development, and advanced ESCC is frequently associated with local invasion and lymph node metastasis[2]. Therefore, a better understanding of tumor dissemination and growth is paramount, and identification of genes crucial for tumor metastasis is urgently needed for the development of ESCC diagnostics and therapeutics.
Adhering junctions, such as adherens junctions and desmosomes, are vital for the unity of cells in esophageal epithelial sheets[3]. Generally, adhering junctions are comprised of a transmembrane component and a variety of cytoplasmic adapter proteins that in turn link cytoskeletal structures to sites of cell-cell contact. Alterations in expression of these cytoplasmic adapter proteins have been linked to tumor progression and/or suppression[4]. One such protein is a close relative of β-catenin called γ-catenin (also known as junction plakoglobin), which can bind to both classic and desmosomal cadherins, but is found primarily in desmosomes and is critically important in the maintenance of normal epithelial tissue architecture[5,6]. Reduction of γ-catenin expression has been reported in numerous human carcinomas such as oral, lung, colorectal, prostate, ovarian, and bladder cancers[7-12]. In esophageal cancer, loss or reduced γ-catenin expression has been correlated with poor survival[13].
Despite the growing number of correlative studies, the possible role or mechanism by which depletion of γ-catenin may contribute to neoplastic progression is not fully understood. Previous study shows that γ-catenin can function as an inhibitor of β-catenin/Tcf-dependent gene transcription and highlights γ-catenin as a potentially novel tumor suppressor protein in a subset of human lung cancers[14]. However, another study reveals no activated Wnt signaling event associated with γ-catenin expression in the bladder model[15]. In oral squamous cell carcinoma, γ-catenin has tumor metastasis suppressor function by regulating the metastasis suppressor activity of Nm23[16]. In keratinocytes, γ-catenin deficiency results in extracellular matrix (ECM)-dependent disruption of mature focal adhesions and actin organization, through distinct ECM-Src and RhoGTPase-dependent pathways [17]. Recently, we have shown that desmocollin-2 (DSC2), the most widely distributed desmosomal cadherin family member, plays a causal role in esophageal cellular invasion and metastasis. The loss of DSC2 initiates tumor cell metastasis by affecting the subcellular localization of γ-catenin, activating the β-catenin pathway, and eventually inducing an epithelial-mesenchymal transition-like process[18,19]. However, from these experiments we can not assess the contribution of γ-catenin to esophageal cancer suppression. Whether these observed changes in γ-catenin play a causal role in ESCC progression has not been determined.
In this study, using a real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) technique, we identified differential expression of γ-catenin in ESCC. The significance of γ-catenin in clinical pathology, cellular function and the signaling mechanism by which γ-catenin exerts its tumor suppressor activity in ESCC cells was evaluated. Results of these studies may provide a foundation for further study of potential clinical applications in diagnosis, prognosis or therapeutics.
This study was approved by the ethical committee of the Central Hospital of Shantou City and the Medical College of Shantou University, and written informed consent was obtained from all surgical patients to use resected samples for research.
ESCC tissue specimens and paired adjacent normal epithelial tissues were obtained from 95 patients (median age, 55 years, range 40-88 years) who underwent surgery in the Department of Pathology of Shantou Central Hospital from 2007 to 2008. The specimens were immediately frozen in liquid nitrogen following surgery and stored at -70 °C until RNA isolation. All of the tumors were confirmed as ESCC by the Clinical Pathology Department of the Hospital, and the cases were classified according to the 7th edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer and were included in this study only if a follow-up was obtained. Patients’ data are summarized in Table 1.
| Parameter | Five year survival | P value |
| Age (yr) | ||
| < 55 | 38 (63.2) | 0.225 |
| ≥ 55 | 57 (52.6) | |
| Gender | ||
| Female | 24 (54.2) | 1.000 |
| Male | 71 (57.7) | |
| Tumor size | ||
| ≤ 3 cm | 30 (56.7) | 0.078 |
| 3-5 cm | 49 (62.5) | |
| > 5 cm | 16 (37.5) | |
| Differentiation grade | ||
| G1 | 23 (73.9) | 0.060 |
| G2 | 55 (58.2) | |
| G3 | 17 (38.5) | |
| Invasive depth | ||
| T1 + T2 | 24 (70.8) | 0.169 |
| T3 + T4 | 71 (52.1) | |
| Regional lymph node metastasis | ||
| N0 | 57 (64.9) | 0.034 |
| N1 | 38 (44.7) | |
| pTNM stage | ||
| IA +IB + IIA + IIB | 61 (68.9) | 0.007 |
| IIIA + IIIB + IIIC + IV | 29 (41.4) | |
Total RNA was extracted from frozen stored tissues with TRIzol reagent (Invitrogen, United States) in accordance with the manufacturer’s instructions. Reverse transcription was performed in a total volume of 20 μL using 1 μg of total RNA by using the Reverse Transcription System (Promega, United States). Real-time quantitative PCR was carried out on the Rotor-Gene 6000 system (Corbett Life Science, Sydney, Australia). SYBR® Premix Ex Taq™ (TaKaRa) was used according to the manufacturer’s instructions. γ-catenin PCR primers were designed based on human γ-catenin mRNA sequence (GenBank accession number NM_002230.1). The primer sequences are as follows: forward 5’-CCC ATC AAT GAG CCC TAT GGA G-3’ and reverse 5’-GGG CAC ATC GCT GGA GTA CA-3’. As an internal control, a fragment of human β-actin was amplified using the following primers: forward 5’-CAA CTG GGA CGA CAT GGA GAA A-3’ and reverse 5’-GAT AGC AAC GTA CAT GGC TGG G-3’. PCR conditions were an initial denaturation step of 10 s at 95 °C, followed by 40 cycles consisting of 5 s at 95 °C, 20 s at 60 °C and 15 sec at 72 °C. Quantification was performed using the 2-ΔΔCT method. The absolute levels of γ-catenin mRNA were normalized to that of β-actin mRNA. The status of differentially expressed γ-catenin gene in the ESCC tissue was defined as “positive expression” if 2-ΔΔCT was > 0.5-fold or “negative expression” if 2-ΔΔCT was ≤ 0.5-fold, when compared to that detected in the adjacent normal tissue.
The human esophageal squamous carcinoma cell lines KYSE150 and TE3[20] were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal calf serum. For siRNA transfection, approximately 5 × 104 cells per well were inoculated into 6-well plates, cultured for 24 h and then transfected with the relevant siRNA (50 nmol/L) using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, United States). siRNAs were synthesized by Shanghai GenePharma Co., Ltd and contained two siRNAs against human γ-catenin (siRNA-1: 5’-GCU GAU CAU CCU GGC CAA U-3’ and siRNA-2: 5’-AGU CGG CCA UUG UGC AUC U-3’). A control siRNA oligonucleotide, which does not match any known human coding cDNA, was used as a control.
Transwell migration assay was conducted as previously described[19]. Cells were placed into the upper chamber of an insert in serum-free medium (BD Biosciences, NJ, United States). After 24 h of incubation, cells remaining in the upper chamber were carefully removed. Cells adhering to the lower membrane were fixed and stained with Giemsa reagent, imaged, and counted using an inverted microscope (Olympus, Tokyo, Japan). The mean value was calculated from data obtained from three separate chambers.
Hanging drop cultures of aggregated cells were generated from 1 × 103 cells. Cells were allowed to aggregate overnight on the underside of a culture dish as previously described[21]. Resulting cell clusters were subjected to 30 rounds of pipetting through a 200 μL Gilson pipette, and the degree of dissociation was quantified by counting the particles after trituration.
Cell cultures were seeded in triplicate onto 6-well plates. Twenty-four hours after reaching confluency, cultures were washed twice in phosphate buffered saline (PBS) and then incubated in 1 mL of dispase (2.4 U/mL; Sigma) for more than 30 min as previously described[21]. Released monolayers were carefully washed twice with PBS and transferred to 15 mL conical tubes. Enough PBS was added to a final volume of 2 mL. Tubes were secured to a rocker and subjected to 50 inversion cycles. Fragments were counted using an inverted microscope (Olympus, Tokyo, Japan).
Cells were co-transfected with either TOPflash or FOPflash reporter plasmids along with the siRNA-1/-2 or control siRNA. The TOPflash reporter plasmid contained β-catenin/TCF binding motifs, whereas the FOPflash reporter plasmid contained mutant β-catenin/Tcf-binding sites and was used as a control. The level of β-catenin-dependent transcription was determined by a TOPflash luciferase activity assay[19]. Luciferase reporter activities were normalized to the activity of the Renilla internal control. Data represent the results of triplicate dishes from two independent experiments.
Western blot was performed as described previously[19]. Total cell lysates were prepared in RIPA buffer (Paragon Biotech, China). For analysis of the Triton X-100-insoluble pool, cells were lysed in 1% Triton X-100 buffer (1% Triton X-100, 145 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.4, 5 mmol/L EDTA, 2 mmol/L EGTA, and 1 mmol/L PMSF) followed by centrifugation (16000 g, 10 min). The Triton X-100-insoluble pellet was solubilized in Laemmli sample buffer, resolved by SDS/PAGE (12% gels) and immunoblotted. Experiments were repeated two to three times with similar results.
The staining procedure was performed as previously described[19]. After fixation in 4% paraformaldehyde solution for 15 min, cells were incubated with donkey serum blocking buffer for 30 min and a primary antibody overnight at 4 °C, followed by donkey anti-mouse IgG (DyLight 488) and/or donkey anti-rabbit IgG (DyLight 594) (Jackson, Germany) for 30 min at 37 °C. Samples were counterstained with DAPI (Sigma, St. Louis, MO, United States) for 10 min. Finally, the cells were examined under a confocal microscope (OLYMPUS, FV-1000).
Associations of γ-catenin with clinicopathological characteristics including age, gender, tumor size, differentiation grade, invasive depth, lymph node metastasis, and TNM classification were assessed by the χ2 test. Kaplan-Meier curves were constructed for overall survival analysis by a log-rank test. All statistical analyses were performed with SPSS 13.0 software (version 13.0; SPSS, Inc., Chicago, IL). Each P-value is two-tailed and significance level is 0.05.
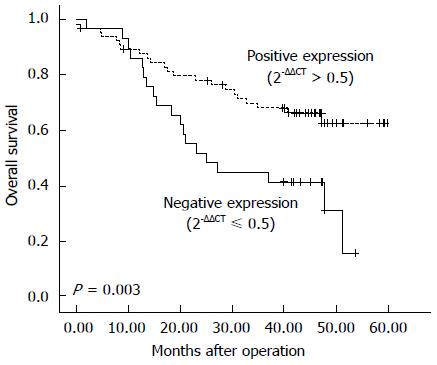
To assess the clinical implications of γ-catenin mRNA expression in ESCC, we analyzed expression of this gene by real-time quantitative PCR. With a median follow-up of 39.6 mo for the 95 patients analyzed in this study, the mean survival was 41.4 mo (range 37.0-45.8 mo), and that the 5-year survival rate was 48.9%. The absolute levels of γ-catenin mRNA were normalized to that of β-actin mRNA. The status of differentially expressed γ-catenin gene in the ESCC tissue was defined as “positive expression” if 2-ΔΔCT was > 0.5-fold or “negative expression” if 2-ΔΔCT was ≤ 0.5-fold, when compared to that detected in the adjacent normal tissue. By this criterion, 29 (31%) patients had a negative γ-catenin expression status and 66 (69%) patients had a positive γ-catenin expression status. Table 2 shows associations between the clinicopathologic characteristics of patients with ESCC and γ-catenin expression status. A significant correlation was observed between γ-catenin expression status and histologic grade of tumors (P = 0.017). Positive γ-catenin expression cases (2-ΔΔCT > 0.5) were found in 78% of grade I tumors, 76% of grade II, and 38% of grade III. ESCC patients with histologic grade III tumors were more likely to have low levels of γ-catenin expression (2-ΔΔCT≤ 0.5). There were no significant correlations between γ-catenin expression levels and other clinical parameters, such as pTNM classification, in patients with ESCC.
| Parameter | γ-catenin status | P value | |
| 2-ΔΔCT ≤ 0.5 | 2-ΔΔCT > 0.5 | ||
| Age (yr) | |||
| ≤ 55 | 18 (36) | 32 (64) | 0.216 |
| > 55 | 11 (24) | 34 (76) | |
| Gender | |||
| Female | 11 (46) | 13 (54) | 0.079 |
| Male | 18 (25) | 53 (75) | |
| Tumor size | |||
| ≤ 3 cm | 11 (37) | 19 (63) | 0.522 |
| 3-5 cm | 12 (25) | 36 (75) | |
| > 5 cm | 5 (31) | 11 (69) | |
| Differentiation grade | |||
| G1 | 5 (22) | 18 (78) | 0.017 |
| G2 | 13 (24) | 42 (76) | |
| G3 | 8 (62) | 5 (38) | |
| Invasive depth | |||
| T1 + T2 | 8 (33) | 16 (67) | 0.734 |
| T3 + T4 | 21 (30) | 50 (70) | |
| Regional lymph node metastasis | |||
| N0 | 16 (28) | 41 (72) | 0.528 |
| N1 | 13 (34) | 25 (66) | |
| pTNM stage | |||
| IA +IB + IIA + IIB | 16 (26) | 45 (74) | 0.640 |
| IIIA + IIIB + IIIC + IV | 9 (31) | 20 (69) | |
The expression level of γ-catenin was next evaluated for association with survival time using the Kaplan-Meier method. The results showed that patient survival time was positively correlated with γ-catenin expression. ESCC patients with tumors demonstrating negative expression of γ-catenin (2-ΔΔCT≤ 0.5) exhibited poor prognosis. Among 95 ESCC patients, in 29 cases of negative γ-catenin expression (2-ΔΔCT≤ 0.5), the median survival time was 25.0 mo and the 5-year survival rate was 15.5%, whereas in 66 cases of positive γ-catenin expression (2-ΔΔCT > 0.5), the median survival time was 42.1 mo and the 5-year survival rate was 62.6% (P = 0.003, Figure 1). The use of the Cox regression model in multivariate analysis showed that γ-catenin expression status was an independent prognostic predictor (P = 0.004) (Table 3).
| Cox regression analysis | Percentage of survival (95%CI) | P value | |
| Univariate | Time to survival (mo) | ||
| 2-ΔΔCT ≤ 0.5 | 31.309 ± 3.343 | 34.5 (24.757-37.860) | 0.007 |
| 2-ΔΔCT > 0.5 | 45.463 ± 2.609 | 66.7 (40.349-50.576) | |
| Multivariate | Relative risk | ||
| Tumor size | 1.256 | 0.751-2.101 | 0.385 |
| Differentiation grade | 1.651 | 0.914-2.981 | 0.097 |
| Regional lymph node metastasis | 1.846 | 0.963-3.539 | 0.065 |
| γ-catenin | 0.370 | 0.189-0.723 | 0.004 |
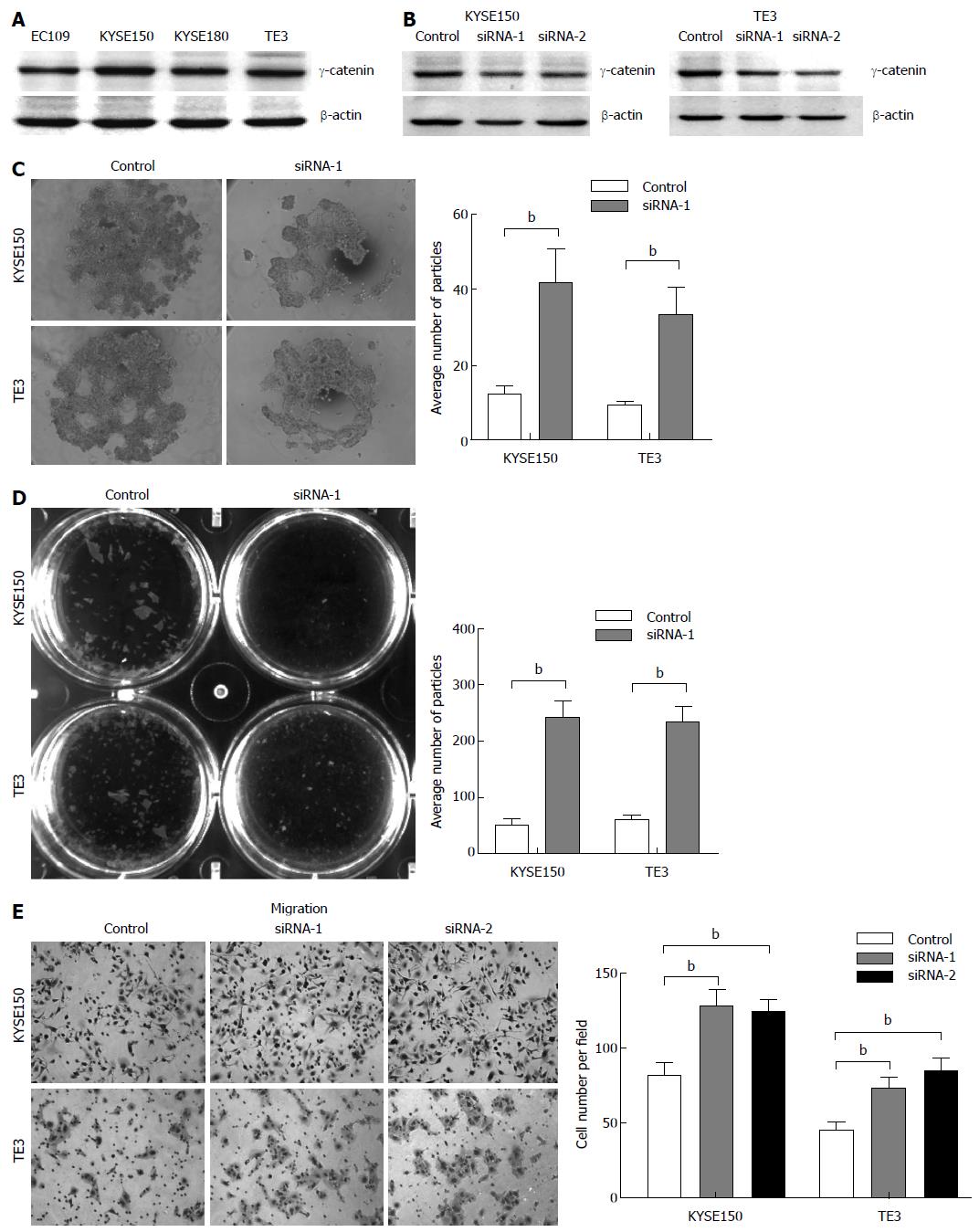
We next explored the expression of γ-catenin in ESCC cell lines. As shown in Figure 2A, γ-catenin expression was detected in all cell lines evaluated, with KYSE150 and TE3 cells contained higher γ-catenin expression levels. These cell lines were selected as the model for the subsequent function studies.
Two double-stranded siRNAs (siRNA-1 and -2) targeting the human γ-catenin gene were synthesized. These sequences are specific to γ-catenin mRNA and there is no match to other genes in the NCBI nucleotide database, in particular to other catenin family members, by BLAST searching. Western blot analysis revealed that γ-catenin expression decreased markedly in treated cells compared with the control. Transfection with siRNAs resulted in a 38%-54% reduction in γ-catenin expression in KYSE150 cells (Figure 2B, left panel) and a 25% to 44% reduction in expression in TE3 cells (Figure 2B, right panel), when compared to that transfected with the scramble sequence.
In view of the fact that the malignant transformation of epithelial cells involves alteration of cell-cell adhesion that enables these cells to adopt a phenotype of migration leading to tumor invasion and metastasis[22], we next investigated whether reduced γ-catenin expression levels affected cell-cell adhesion in ESCC cells. We first employed a previously described hanging drop aggregation assay[21]. Cells allowed to aggregate for 24 h in a hanging drop on the underside of a culture dish were subjected to trituration through a 200 μL-Gilson pipette tip in an attempt to disrupt intercellular adhesion. The degree of dissociation was quantified by counting the particles that dissociated from the original cluster. KYSE150 and TE3 cells transfected with siRNA against γ-catenin were more susceptible to dissociation than control cells (Figure 2C), which exhibited on average an approximately threefold increase in particle number when compared with the control (P < 0.01).
To further confirm the effect of γ-catenin on ESCC cell-cell adhesion, we performed a Dispase-based dissociation assay. This assay was previously used to quantify the adhesion strength of a variety of epithelial cells[21,23]. Confluent monolayers of siRNA transfected KYSE150 and TE3 cells were harvested from tissue culture dishes by incubation with dispase. Monolayers were then transferred to 15 mL conical tubes. After inverting the tubes 50 times on a rocker, monolayer fragments were counted (Figure 2D). γ-catenin-specific siRNA-transfected monolayers dissociated into numerous smaller fragments, whereas control cell monolayers exhibited only minimal dissociation. Quantification of the number of fragments showed a four-fold increase in fragmentation (P < 0.01), suggesting that knocking down γ-catenin led to a significant decrease in cell-cell adhesion.
As weakening of cell-cell adhesions has been reported to increase cell motility[22], we next investigated whether γ-catenin status in cells correlated with their relative motility. Towards this end, we performed a transwell migration assay to compare cell motility. The assay revealed that γ-catenin knockdown cells were significantly more migratory than control siRNA-transfected cells over a 24-hperiod. The transfection of KYSE150 and TE3 cells with a γ-catenin-specific siRNA increased cell migration up to 25% compared with the RNAi control transfection (P < 0.01; Figure 2E). These data imply that reduced expression of γ-catenin promotes cell motility.
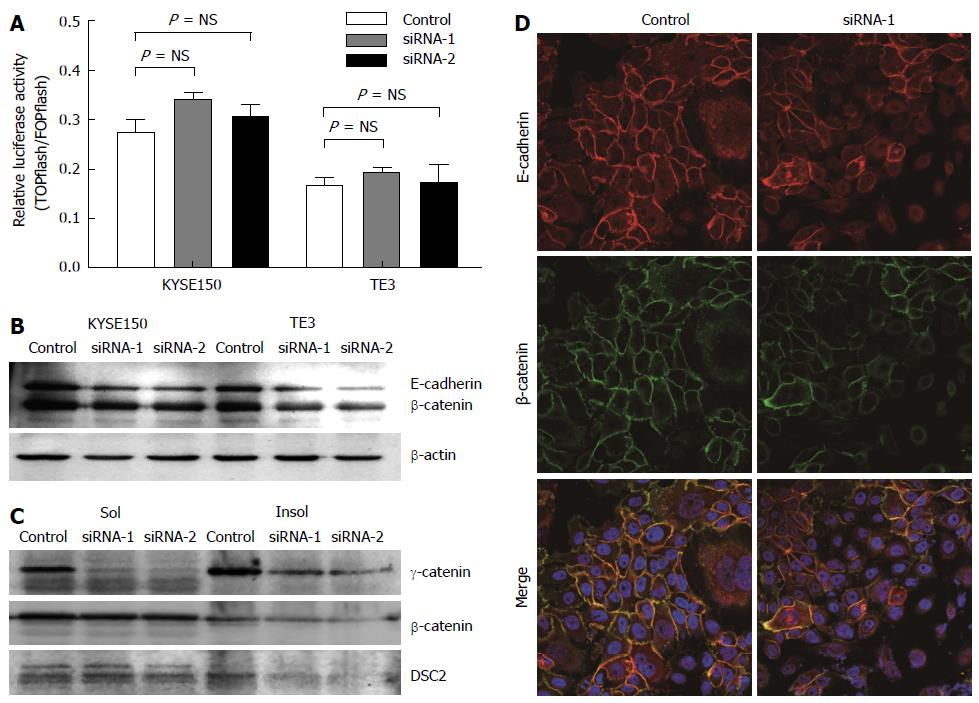
Although the role of β-catenin in Wnt signaling has been well established, the role of γ-catenin in this pathway is less clear[24]. To investigate whether γ-catenin influences β-catenin signaling in esophageal carcinoma cells, we first used a luciferase-based transcriptional reporter assay (TOPflash) to measure β-catenin-dependent transcription in cells with reduced γ-catenin expression. As illustrated in Figure 3A, KYSE150 and TE3 cells transfected with γ-catenin-specific siRNA showed no significant Wnt activation (P > 0.05), when compared with the RNAi control transfected cells. We next examined β-catenin protein expression levels by Western blot. Total β-catenin and Triton X-100-insoluble β-catenin were significantly reduced in the γ-catenin-specific siRNA-transfected KYSE150 and TE3 cells, whereas Triton X-100-soluble β-catenin was not altered (Figure 3B and C). These results suggest that γ-catenin does not mediate its effects through the Wnt pathway in this model.
As γ-catenin is a member of the Armadillo family of proteins and binds directly to both classic and desmosomal cadherins[5]. We therefore investigated the effects of γ-catenin knockdown on expression of E-cadherin and DSC2. As shown in Figure 3, reducing expression of γ-catenin resulted in a significant decrease of E-cadherin and Triton X-100-insoluble DSC2 in ESCC cells. To confirm this result, the subcellular localizations of β-catenin and E-cadherin were examined using immunofluorescence. Consistent with the Western blot results, knocking down γ-catenin expression caused reduced β-catenin and E-cadherin membrane localization (Figure 3D).
Accumulating evidence suggests that adhesive interactions are critical in the process of metastatic tumor migration and that adhesion molecules can act as both positive and negative modulators of the metastatic process[25]. The cadherin-catenin complex promotes homotypic tumor cell adhesion, maintaining intercellular contacts that confine cells to the primary tumor site. Previous studies have shown a correlation between reduced classic and desmosomal cadherin expression and increased metastasis both in laboratory models and in clinical ESCC samples[18,19,26]; however, a direct functional role of γ-catenin in this process has not been shown. In this study, we report that γ-catenin plays a critical role in ESCC progression. Our work demonstrates that γ-catenin is a tumor metastasis suppressor in ESCC and its expression may serve as a prognostic marker. Loss of γ-catenin leads to significant changes in esophageal cancer cell phenotypes.
Evidence from clinical studies suggests that reduced expression of γ-catenin in human cancers is associated with increased tumor progression and adverse clinical outcome[7-13]. To confirm whether these observed changes in γ-catenin play a causal role in ESCC progression, we first use a real-time quantitative RT-PCR method to analyze the prognostic importance of γ-catenin expression in ESCC. Reduced γ-catenin mRNA expression is associated with an unfavorable prognosis of ESCC patients. Patients with positive γ-catenin- expressing tumors have a better prognosis than those with reduced expression of γ-catenin. Moreover, γ-catenin expression level is an independent prognostic predictor. Our findings are consistent with earlier immunohistochemistry studies revealing that decreased γ-catenin expression was significantly associated with poorer prognosis[13], which implies more aggressive malignant behaviors of esophageal carcinoma cells.
Several studies have discovered that γ-catenin was involved in cell invasion and metastasis of human oral squamous cell cancer, lung cancer, and bladder cancer[14-16]. In our clinical investigation, expression of γ-catenin does not correlate with the degree of lymph node metastasis. However, Lin et al[13] have reported that reduced protein expression of γ-catenin is associated with lymph node metastasis in human ESCC. It seems that this confusing result conducted from the statistical analysis might be due to the insufficient ESCC specimens, or the discordance between protein and mRNA expression levels of γ-catenin. In in vitro experiments, we show that γ-catenin expression enhances cell-cell adhesion and suppresses the motility of ESCC cells, suggesting that γ-catenin might play as a metastasis suppressor in ESCC. As a junctional protein, γ-catenin interacts with both desmocollin and E-cadherin in both the soluble and cytoskeleton associated pools of cellular proteins[5]. Recently, it has been suggest that γ-catenin may play a central role in desmosome organization and is required for effective intermediate filament anchorage to desmosomes[27]. Consistent with these observations, the data presented in this work suggest that a decreased level of γ-catenin in the cell leads to a decrease in E-cadherin and desmocollin protein expression, which is accompanied by a decrease in cell-cell adhesion and an increase in cell migration. These results are consistent with data from Simpson et al[28] that show that increasing γ-catenin levels rescue cadherin expression, desmosome organization, and functional adhesion, leading us to conclude that knocking down γ-catenin expression with siRNA destabilizes other cadherins. Although we did not determine whether this represents transcriptional downregulation or increased turnover of protein possibly complexed with γ-catenin, the reduction of cell adhesion protein suggests that the inhibition of cell migration is partly dependent upon γ-catenin interactions with the classic and desmosomal cadherin proteins.
The signaling mechanism(s) by which γ-catenin exerts its tumour suppressor activity in ESCC cells remains unclear. γ-catenin localizes to the desmosomal plaque and seems to be required for desmosome organization as discussed earlier. Alternatively, desmosomes have been postulated to serve as signaling centers[29], and it is possible that an alteration in desmosome composition results in an alteration in signal transduction resulting in increased transformation. In addition, γ-catenin may directly modify intracellular signaling, thus altering cell behavior, such as cell migration under certain circumstances[17,30]. In numerous human cancers including ESCC, the Wnt/β-catenin signaling pathway is constitutively activated[31]. The previous study on human lung cancer cells shows that re-expression of γ-catenin reduces TCF activity[14]. However, it is interesting that we report here that γ-catenin does not affect β-catenin/TCF-dependent gene transcription. Our observations support the work of Rieger-Christ et al[15], who revealed no activated Wnt signaling event associated with γ-catenin expression in the bladder model. Hence, in esophageal carcinoma cells, γ-catenin might be exerting a tumor suppressor role via alternative signaling pathways from those established in other carcinoma cell models, which will require extensive study to determine.
In summary, this study provides both clinical and mechanistic evidence supporting the critical role of γ-catenin in ESCC progression. Our data demonstrate that γ-catenin is a tumor suppressor in ESCC and its expression may serve as a prognostic marker. Results of this study may provide a foundation for further study of potential clinical applications in diagnosis, prognosis or therapeutics.
γ-catenin is a member of the armadillo family of proteins, which can bind to both classic and desmosomal cadherins, but is found primarily in desmosomes and is critically important in the maintenance of normal epithelial tissue architecture. Reduction of γ-catenin expression has been reported in numerous human carcinomas.
Accumulating evidence suggests that adhesive interactions are critical in the process of metastatic tumor migration and that adhesion molecules can act as both positive and negative modulators of the metastatic process. Previous studies have shown a correlation between reduced desmosomal cadherin expression and increased metastasis both in laboratory models and in clinical esophageal squamous cell carcinoma (ESCC) samples. However, a direct functional role of γ-catenin in ESCC progression has not been determined.
The significance of γ-catenin in clinical pathology, cellular function and the signaling mechanism by which γ-catenin exerts its tumor suppressor activity in ESCC cells was evaluated. The work demonstrates that γ-catenin is a tumor metastasis suppressor in ESCC and its expression may serve as a prognostic marker. Loss of γ-catenin leads to significant changes in esophageal cancer cell phenotypes.
This study may provide a foundation for further study of potential clinical applications of γ-catenin in ESCC diagnosis, prognosis or therapeutics.
This is a good descriptive study in which the authors analyzed the relationship between γ-catenin mRNA expression and disease prognosis in patients with ESCC, and the cellular function and signaling mechanism by which γ-catenin exerts its tumor suppressor activity in ESCC cells. They conclude that γ-catenin is a tumor metastasis suppressor in ESCC and its expression may serve as a prognostic marker. Dysregulated expression of γ-catenin may play important roles in ESCC progression. The results are interesting and provide insights into esophageal cancer.
P- Reviewers: Deans C, Lu JC, Rajeshwari K S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2645] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 2. | Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 306] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | McCrea PD, Gu D. The catenin family at a glance. J Cell Sci. 2010;123:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Lewis JE, Wahl JK, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 210] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Zhurinsky J, Shtutman M, Ben-Ze’ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113:3127-3139. [PubMed] |
| 7. | Närkiö-Mäkelä M, Pukkila M, Lagerstedt E, Virtaniemi J, Pirinen R, Johansson R, Kosunen A, Lappalainen K, Hämäläinen K, Kosma VM. Reduced gamma-catenin expression and poor survival in oral squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Nagel JM, Kriegl L, Horst D, Engel J, Gautam S, Mantzoros CS, Kirchner T, Göke B, Kolligs FT. γ-Catenin is an independent prognostic marker in early stage colorectal cancer. Int J Colorectal Dis. 2010;25:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Pantel K, Passlick B, Vogt J, Stosiek P, Angstwurm M, Seen-Hibler R, Häussinger K, Thetter O, Izbicki JR, Riethmüller G. Reduced expression of plakoglobin indicates an unfavorable prognosis in subsets of patients with non-small-cell lung cancer. J Clin Oncol. 1998;16:1407-1413. [PubMed] |
| 10. | van Oort IM, Tomita K, van Bokhoven A, Bussemakers MJ, Kiemeney LA, Karthaus HF, Witjes JA, Schalken JA. The prognostic value of E-cadherin and the cadherin-associated molecules alpha-, beta-, gamma-catenin and p120ctn in prostate cancer specific survival: a long-term follow-up study. Prostate. 2007;67:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Imai T, Horiuchi A, Shiozawa T, Osada R, Kikuchi N, Ohira S, Oka K, Konishi I. Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol. 2004;35:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Syrigos KN, Harrington K, Waxman J, Krausz T, Pignatelli M. Altered gamma-catenin expression correlates with poor survival in patients with bladder cancer. J Urol. 1998;160:1889-1893. [PubMed] |
| 13. | Lin YC, Wu MY, Li DR, Wu XY, Zheng RM. Prognostic and clinicopathological features of E-cadherin, alpha-catenin, beta-catenin, gamma-catenin and cyclin D1 expression in human esophageal squamous cell carcinoma. World J Gastroenterol. 2004;10:3235-3239. [PubMed] |
| 14. | Winn RA, Bremnes RM, Bemis L, Franklin WA, Miller YE, Cool C, Heasley LE. gamma-Catenin expression is reduced or absent in a subset of human lung cancers and re-expression inhibits transformed cell growth. Oncogene. 2002;21:7497-7506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Rieger-Christ KM, Ng L, Hanley RS, Durrani O, Ma H, Yee AS, Libertino JA, Summerhayes IC. Restoration of plakoglobin expression in bladder carcinoma cell lines suppresses cell migration and tumorigenic potential. Br J Cancer. 2005;92:2153-2159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Aktary Z, Chapman K, Lam L, Lo A, Ji C, Graham K, Cook L, Li L, Mackey JR, Pasdar M. Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene. 2010;29:2118-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Todorović V, Desai BV, Patterson MJ, Amargo EV, Dubash AD, Yin T, Jones JC, Green KJ. Plakoglobin regulates cell motility through Rho- and fibronectin-dependent Src signaling. J Cell Sci. 2010;123:3576-3586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Fang WK, Gu W, Li EM, Wu ZY, Shen ZY, Shen JH, Wu JY, Pan F, Lv Z, Xu XE. Reduced membranous and ectopic cytoplasmic expression of DSC2 in esophageal squamous cell carcinoma: an independent prognostic factor. Hum Pathol. 2010;41:1456-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Fang WK, Liao LD, Li LY, Xie YM, Xu XE, Zhao WJ, Wu JY, Zhu MX, Wu ZY, Du ZP. Down-regulated desmocollin-2 promotes cell aggressiveness through redistributing adherens junctions and activating beta-catenin signalling in oesophageal squamous cell carcinoma. J Pathol. 2013;231:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277-284. [PubMed] |
| 21. | Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, Hudson TY, Mongiu AK, Leigh IM, Kelsell DP. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J Cell Biol. 2002;159:1005-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3628] [Cited by in RCA: 3665] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 23. | Yin T, Getsios S, Caldelari R, Godsel LM, Kowalczyk AP, Müller EJ, Green KJ. Mechanisms of plakoglobin-dependent adhesion: desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J Biol Chem. 2005;280:40355-40363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Zhurinsky J, Shtutman M, Ben-Ze’ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol Cell Biol. 2000;20:4238-4252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920-6929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 602] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 26. | Takayama N, Arima S, Haraoka S, Kotho T, Futami K, Iwashita A. Relationship between the expression of adhesion molecules in primary esophageal squamous cell carcinoma and metastatic lymph nodes. Anticancer Res. 2003;23:4435-4442. [PubMed] |
| 27. | Acehan D, Petzold C, Gumper I, Sabatini DD, Müller EJ, Cowin P, Stokes DL. Plakoglobin is required for effective intermediate filament anchorage to desmosomes. J Invest Dermatol. 2008;128:2665-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Simpson CL, Kojima S, Cooper-Whitehair V, Getsios S, Green KJ. Plakoglobin rescues adhesive defects induced by ectodomain truncation of the desmosomal cadherin desmoglein 1: implications for exfoliative toxin-mediated skin blistering. Am J Pathol. 2010;177:2921-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Dusek RL, Attardi LD. Desmosomes: new perpetrators in tumour suppression. Nat Rev Cancer. 2011;11:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Franzen CA, Todorović V, Desai BV, Mirzoeva S, Yang XJ, Green KJ, Pelling JC. The desmosomal armadillo protein plakoglobin regulates prostate cancer cell adhesion and motility through vitronectin-dependent Src signaling. PLoS One. 2012;7:e42132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Ninomiya I, Endo Y, Fushida S, Sasagawa T, Miyashita T, Fujimura T, Nishimura G, Tani T, Hashimoto T, Yagi M. Alteration of beta-catenin expression in esophageal squamous-cell carcinoma. Int J Cancer. 2000;85:757-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |