Published online May 21, 2014. doi: 10.3748/wjg.v20.i19.5801
Revised: March 12, 2014
Accepted: April 8, 2014
Published online: May 21, 2014
Processing time: 97 Days and 2.5 Hours
Acute pancreatitis is an inflammatory disorder of the pancreas that may cause life-threatening complications. Etiologies of pancreatitis vary, with gallstones accounting for the majority of all cases, followed by alcohol. Other causes of pancreatitis include trauma, ischemia, mechanical obstruction, infections, autoimmune, hereditary, and drugs. The main events occurring in the pancreatic acinar cell that initiate and propagate acute pancreatitis include inhibition of secretion, intracellular activation of proteases, and generation of inflammatory mediators. Small cytokines known as chemokines are released from damaged pancreatic cells and attract inflammatory cells, whose systemic action ultimately determined the severity of the disease. Indeed, severe forms of pancreatitis may result in systemic inflammatory response syndrome and multiorgan dysfunction syndrome, characterized by a progressive physiologic failure of several interdependent organ systems. Stress occurs when homeostasis is threatened, and stressors can include physical or mental forces, or combinations of both. Depending on the timing and duration, stress can result in beneficial or harmful consequences. While it is well established that a previous acute-short-term stress decreases the severity of experimentally-induced pancreatitis, the worsening effects of chronic stress on the exocrine pancreas have received relatively little attention. This review will focus on the influence of both prior acute-short-term and chronic stress in acute pancreatitis.
Core tip: Depending on the timing and duration, stress can result in beneficial or harmful consequences. Regarding the exocrine pancreas, a previous acute-short-term stress decreases the severity of experimentally-induced pancreatitis. This protection is conferred by distinct heat shock proteins (HSP) including HSP27, HSP60 and HSP70. Conversely, chronic stress increases the susceptibility of the exocrine pancreas, aggravating pancreatitis episodes. These worsening effects are mainly mediated by tumor necrosis factor alpha.
- Citation: Binker MG, Cosen-Binker LI. Acute pancreatitis: The stress factor. World J Gastroenterol 2014; 20(19): 5801-5807
- URL: https://www.wjgnet.com/1007-9327/full/v20/i19/5801.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i19.5801
Acute pancreatitis is an inflammatory disorder of the pancreas with an overall mortality of approximately 5%[1]. Etiologies of pancreatitis vary, with gallstones accounting for the majority of all cases, followed by alcohol. Other causes of pancreatitis include trauma, ischemia, mechanical obstruction, infections, autoimmune, hereditary, and drugs[2].
The main events occurring in the pancreatic acinar cell that initiate and propagate acute pancreatitis include inhibition of secretion, intracellular activation of proteases, and generation of inflammatory mediators[3]. These cellular events can be correlated with the acinar morphological changes (retention of enzyme content, formation of large vacuoles containing both digestive enzymes and lysosomal hydrolases, and necrosis), which are observed in the well-established in vivo experimental model of supraphysiological cerulein-induced pancreatitis[4], as well as in human acute pancreatitis[5]. Chemokines released from damaged pancreatic cells attract inflammatory cells, whose systemic action ultimately determined the severity of the disease. Indeed, severe forms of pancreatitis may result in systemic inflammatory response syndrome and multiorgan dysfunction syndrome, characterized by a progressive physiologic failure of several interdependent organ systems[6].
Stress can be defined as “threatened homeostasis”, and stressors can include physical or mental forces, or combinations of both. The reaction of an individual to a given stressor involves the stimulation of pathways within the brain leading to activation of the hypothalamic-pituitary-adrenal axis and the central sympathetic outflow[7]. This can result in visceral hypersensitivity through the release of different substances, such as substance P and calcitonin gene-related peptide from afferent nerve fibers[8].
The main source of pancreatic innervation comes from both vagus nerves and the celiac ganglion complex. The cephalic segment is innervated by the right celiac complex and the hepatic and mesenteric plexus coming from the right vagus. The splenic segment is innervated by the left celiac nerve and the splanchnic nervous network. Except for the gastro-duodenal branches network, most of the nerves enter the gland by its periphery and concentrate in the cephalic segment, which exhibits an important number of ganglion cells. These characteristics of the macroscopic innervation decrease in a significant and progressive fashion towards the splenic segment[9,10].
While it is well established that a previous acute-short-term stress decreases the severity of experimentally-induced pancreatitis[11-17], the worsening effects of chronic stress on the exocrine pancreas have received relatively little attention[18-20]. This review will focus on the influence of both prior-acute-short-term and chronic stress in acute pancreatitis.
Preceding acute-short-term stress is a well-known inductor of cellular protection against numerous pathological conditions, including renal ischaemia, heart ischaemia, brain ischaemia, enterocolitis and pancreatitis[11-17,21-25]. Exposure of organisms to an initial sublethal stress leads to the synthesis of heat shock proteins (HSP) and confers protection against further stress[26]. HSP comprise a highly conserved family of proteins with molecular sizes ranging from 10 to 110 kDa. These molecular chaperones are involved in synthesis, folding, transport and degradation of proteins, and can be induced by stressful conditions such as infection, inflammation, hypoxia, starvation, heat shock, water immersion, and oxidative stress[27-29].
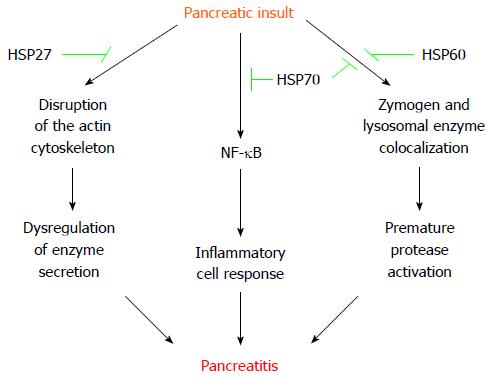
The eventual protection conferred by acute stress-induced HSP in pancreatitis, seems to be stressor- and disease-inducer-dependent[30,31]. Water immersion and heat shock induce pancreatic HSP60 and HSP70, respectively, and protect rats from cerulein-induced acute pancreatitis by inhibiting autophagy, which prevents the subcellular redistribution of cathepsin B and the activation of trypsinogen[14,32,33]. Additionally, hyperthermia- or chemical-stimulated HSP70 also decrease the production of inflammatory mediators by downregulation of NF-κB[34,35]. Remarkably, transgenic mice knock-out for HSP70 (HSP70.1-/-) develop spontaneous activation of pancreatic trypsinogen[36]. However, transgenic knock-in mice over-expressing HSP72 do not exhibit protection for development of cerulein-induced acute pancreatitis, but HSP72 over-expression accelerates tissue injury recovery by lessening NF-κB signaling[37]. Heat shock also induces pancreatic protection against cerulein hyperstimulation by upregulating HSP27[38]. Indeed, over-expression of HSP27 preserves the actin cytoskeleton of pancreatic acinar cells and protect against cerulein-induced pancreatitis in a specific phosphorylation-dependent manner[39]. HSP27 exerts a similar protective effect in coronary arteries[40]. Vessels (endothelial and/or smooth muscle cells) from patients with ischemic heart disease exhibit decreased levels of HSP27 (in particular phospho-HSP27), which correlates with destabilization of the actin cytoskeleton[40]. Regardless of the underlying mechanism, disorganization of the actin cytoskeleton is associated with dysregulation of pancreatic enzyme secretion[41]. Interestingly, HSP27 seems to coordinate activity with other HSP members to provide the full extent of resistance to injury[42]. For instance, , depletion of HSP70 in renal cells does not impede association of HSP27 with actin, but prevents maximal cytoprotective effect against energy depletion[42].
Other pancreatitis-induced models exhibit some differences with the previously mentioned, secretagogue hyperstimulation. Thus, hyperthermia protects against subsequent L-arginine-induced acute pancreatitis in rats by increasing pancreatic expression of HSP70 and HPS27, and phosphorylation of HSP27, but without changing HSP60 levels[15,43]. As observed in the cerulein model, transgenic mice over-expressing HSP72 do not exhibit protection for L-arginine-induced acute pancreatitis[37]. However, HSP72 over-expression does not accelerate tissue injury recovery in L-arginine treated animals[37]. Although both hot and cold water immersion induce pancreatic HSP72 and HSP60, respectively, only cold water immersion slightly protect rats from sodium tauracholate-induced acute pancreatitis, pointing the transcendence of the subcellular redistribution of cathepsin B in this necrohemorrhagic pancreatitis model[13].
Nevertheless, prior-acute-short-term stress protects against pancreatitis by distinct HSP, which seem to exert its beneficial effects through different pathways (Figure 1).
Chronic stress has been proved to increase the susceptibility of different rat organs, such as the small intestine, colon and brain, to inflammatory diseases[8,20,44-46], as well as to aggravate atherosclerotic lesions in mice[47].
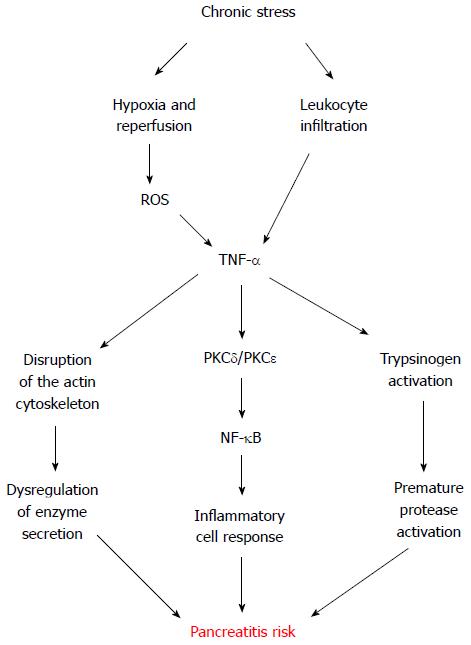
Even though oxidative stress and inflammation each occur in the pancreas during the early stage of supramaximal cerulein-induced acute pancreatitis model, neither oxidative stress nor an inflammatory insult alone cause the characteristic changes of acute pancreatitis[48]. However, chronic stress leaves the exocrine pancreas susceptible to pancreatitis by submaximal cerulein stimulation[20]. Pancreatic tissue from rats chronically exposed to restraint exhibit measurable levels of the pro-inflammatory cytokine tumor necrosis factor α (TNF-α) as well as a low but detectable leukocyte infiltrate and myeloperoxidase activity[20], suggesting leukocytes as a feasible source of TNF-α induced by chronic stress. Interestingly, in vitro incubation of mice pancreatic acini with phorbol-12-myristate-13-acetate-activated neutrophils or macrophages directly induce intracellular trypsinogen activation and cell death, being protease activation and necrosis mediated by leukocyte-secreted TNF-α in a cathepsin-B and calcium-dependent manner[49].
TNF-α has an important role in various biological functions, including cell proliferation, cell differentiation, survival, apoptosis and necrosis[50], and in stress-related inflammatory disorders[45-47,51]. For a long time, it has been known that TNF-α participates in the inflammatory cascade which propagates pancreatitis[52]. Nevertheless, its relevance in the genesis of this debilitating disease only recently captured the attention of research investigation[20,49].
Secretion of TNF-α by several stress stimuli has been demonstrated in vitro in many cell types, including pancreatic acinar cells[53-60], and in vivo in different tissues[47,51,61-63]. Our lab has shown that in vitro hypoxia-reoxygenation conditions also induce TNF-α secretion by acinar cells[20]. These conditions are concomitant with ischemia-reperfusion processes, which can be the result of microcirculatory disturbances generated by stress[64]. Indeed, local pancreatic blood flow is reduced by stress[65]. Hence, alternate vasoconstriction and vasodilatation leading to tissue ischemia and reperfusion could reflect another putative local origin of chronic stress-derived TNF-α found in the pancreatic tissue. This is supported by the increased levels of the transcription factor hypoxia inducible factor 1 alpha (HIF-1α) observed in experimentally stressed rats[20]. HIF-1α is induced by hypoxic conditions and is involved in different inflammatory processes, such as dermatitis, rheumatoid arthritis[66], and also pancreatitis[67].
Different reports evaluated the response of pancreatic acinar cells to exogenous TNF-α, showing disruption of the typical filamentous actin distribution[20,68]. A similar redistribution of actin from apical to basolateral membranes was observed in pancreatic acini supra-stimulated with CCK[69]. While TNF-α alone does not stimulate amylase secretion in human pancreas[70] or in isolated rat pancreatic acini[20,68], it certainly inhibits submaximal CCK-stimulated amylase secretion[20]. Although necessary, the inhibition of pancreatic enzyme secretion alone is not sufficient to induce pancreatitis[3]. Nonetheless, TNF-α also activates pancreatic acinar nuclear factor-κB (NF-κB), a key transcriptional regulator of the expression of inflammatory molecules[20,68,71,72]. Consistently, rat pancreatic acinar cells treated with high doses of exogenous TNF-α, exhibit a notable increase in the production of cytokines interleukin (IL)-1β, IL-4, IL-6, IL-10, as well as TNF-α[73].
TNF-α has been shown to regulate the activity of distinct protein kinase C (PKC) isoforms in diverse cell types, including the pancreatic acinar cell[72,74,75]. PKC family comprises at least 12 members differing in tissue distribution and activation requirements. There are three subclasses: classical PKC isozymes (-α, -β1, -β2, and -γ), which require calcium and are activated by diacylglycerol and phorbol ester; the novel PKC isozymes (-δ, -ε, -η, and -θ), which are activated by diacylglycerol and phorbol ester independently of calcium; and the atypical PKC isozymes (-λ, -ι, and -ζ), which are calcium independent and not responsive to phorbol ester. Rat pancreatic acini express the α, δ, ε, and ζ PKC isozymes[76]. Changes in PKC activity are associated with inflammation in a variety of tissues, including skin, kidney, intestine, and pancreas[77-80]. Specifically, PKC-δ and PKC-ε regulate the signal transduction pathways implicated in the pathophysiological activation of NF-κB and trypsinogen in rat pancreatic acini[72,81]. TNF-α activates both PKC-δ and PKC-ε in rat pancreatic acini[72], which convert physiological CCK concentrations into phytopathogenic concentrations[20]. Different studies have consistently shown that modulation of PKC activity sensitizes acinar cells to physiological secretagogue treatments, resulting in harmful levels of NF-κB and trypsin activity[81,82]. In agreement, TNF-α plus submaximal CCK pathologically activates NF-κB and trypsinogen in rat pancreatic acini, and induced both apoptosis and necrosis[20]. However, pancreatic acini response from rats seems to differ from that observed in mice, since TNF-α by itself only induces trypsinogen activation and necrosis in mice, with an extent comparable to supramaximal cerulein stimulation[20,49]. This could be a concentration-dependent effect or relative to differences between species, which is well-documented for experimentally-induced pancreatitis in rodents[83-86], but further studies are required to address this disparity in pancreatic acinar response to exogenous TNF-α.
Summarizing this topic, chronic stress appears as a risk factor to develop pancreatitis by sensitizing the exocrine pancreas through TNF-α, which seems to exert its detrimental effects through different pathways (Figure 2).
Depending on the timing and duration, stress can result in beneficial or harmful consequences for the exocrine pancreas. Prior acute-short-term stress could be useful for high-risk procedures such as endoscopic retrograde cholangiopancreatography. Conversely, the management of chronic stress appears critical for patients with risk of pancreatitis. Nonetheless, the mechanisms underlying protection by previous-acute-short-term stress as well as burden by chronic stress, have to be further explored.
P- Reviewers: Bauer P, Cosen-Binker L S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1150] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 2. | Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 408] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Klöppel G, Dreyer T, Willemer S, Kern HF, Adler G. Human acute pancreatitis: its pathogenesis in the light of immunocytochemical and ultrastructural findings in acinar cells. Virchows Arch A Pathol Anat Histopathol. 1986;409:791-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | McFadden DW. Organ failure and multiple organ system failure in pancreatitis. Pancreas. 1991;6 Suppl 1:S37-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117:854-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 284] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Chen JH, Wei SZ, Chen J, Wang Q, Liu HL, Gao XH, Li GC, Yu WZ, Chen M, Luo HS. Sensory denervation reduces visceral hypersensitivity in adult rats exposed to chronic unpredictable stress: evidences of neurogenic inflammation. Dig Dis Sci. 2009;54:1884-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Tiscornia OM, Martinez JL, Sarles H. Some aspects of human and canine macroscopic pancreas innervation. Am J Gastroenterol. 1976;66:353-361. [PubMed] |
| 10. | Salvioli B, Bovara M, Barbara G, De Ponti F, Stanghellini V, Tonini M, Guerrini S, Cremon C, Degli Esposti M, Koumandou M. Neurology and neuropathology of the pancreatic innervation. JOP. 2002;3:26-33. [PubMed] |
| 11. | Grisé K, Kim F, McFadden D. Hyperthermia induces heat-shock protein expression, reduces pancreatic injury, and improves survival in necrotizing pancreatitis. Pancreas. 2000;21:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Rakonczay Z, Takács T, Mándi Y, Iványi B, Varga S, Pápai G, Boros I, Lonovics J. Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: possible role of HSP72. Int J Hyperthermia. 2001;17:520-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Rakonczay Z, Takács T, Iványi B, Mándi Y, Pápai G, Boros I, Varga IS, Jost K, Lonovics J. The effects of hypo- and hyperthermic pretreatment on sodium taurocholate-induced acute pancreatitis in rats. Pancreas. 2002;24:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Bhagat L, Singh VP, Song AM, van Acker GJ, Agrawal S, Steer ML, Saluja AK. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Tashiro M, Ernst SA, Edwards J, Williams JA. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Takács T, Rakonczay Z, Varga IS, Iványi B, Mándi Y, Boros I, Lonovics J. Comparative effects of water immersion pretreatment on three different acute pancreatitis models in rats. Biochem Cell Biol. 2002;80:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Cosen-Binker LI, Binker MG, Negri G, Tiscornia O. Influence of stress in acute pancreatitis and correlation with stress-induced gastric ulcer. Pancreatology. 2004;4:470-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Harada E, Kanno T. Progressive enhancement in the secretory functions of the digestive system of the rat in the course of cold acclimation. J Physiol. 1976;260:629-645. [PubMed] |
| 19. | Harada E. Lowering of pancreatic amylase activity induced by cold exposure, fasting and adrenalectomy in rats. Comp Biochem Physiol A Comp Physiol. 1991;98:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Binker MG, Binker-Cosen AA, Richards D, Gaisano HY, de Cosen RH, Cosen-Binker LI. Chronic stress sensitizes rats to pancreatitis induced by cerulein: role of TNF-α. World J Gastroenterol. 2010;16:5565-5581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Hencz L, Kertesz V. [Dynamic urination disorders caused by anomalous renal vessels]. Magy Seb. 1953;6:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Sommerschild HT, Kirkebøen KA. Preconditioning - endogenous defence mechanisms of the heart. Acta Anaesthesiol Scand. 2002;46:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Akhlaghi M, Bandy B. Preconditioning and acute effects of flavonoids in protecting cardiomyocytes from oxidative cell death. Oxid Med Cell Longev. 2012;2012:782321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Thompson JW, Dave KR, Young JI, Perez-Pinzon MA. Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics. 2013;10:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Kim EK, Lee KY, Lee HJ, Lee JA, Choi CW, Kim HS, Kim BI, Choi JH. Heat shock pretreatment reduces intestinal injury in a neonatal rat model of early necrotizing enterocolitis. Neonatology. 2013;103:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1421] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 27. | Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063-1081. [PubMed] |
| 28. | Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 450] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 29. | Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 30. | Rakonczay Z, Takács T, Boros I, Lonovics J. Heat shock proteins and the pancreas. J Cell Physiol. 2003;195:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Saluja A, Dudeja V. Heat shock proteins in pancreatic diseases. J Gastroenterol Hepatol. 2008;23 Suppl 1:S42-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Lee HS, Bhagat L, Frossard JL, Hietaranta A, Singh VP, Steer ML, Saluja AK. Water immersion stress induces heat shock protein 60 expression and protects against pancreatitis in rats. Gastroenterology. 2000;119:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Kim JN, Lee HS, Ryu SH, Kim YS, Moon JS, Kim CD, Chang IY, Yoon SP. Heat shock proteins and autophagy in rats with cerulein-induced acute pancreatitis. Gut Liver. 2011;5:513-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1157-G1162. [PubMed] |
| 35. | Bhagat L, Singh VP, Dawra RK, Saluja AK. Sodium arsenite induces heat shock protein 70 expression and protects against secretagogue-induced trypsinogen and NF-kappaB activation. J Cell Physiol. 2008;215:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Hwang JH, Ryu JK, Yoon YB, Lee KH, Park YS, Kim JW, Kim N, Lee DH, Jeong JB, Seo JS. Spontaneous activation of pancreas trypsinogen in heat shock protein 70.1 knock-out mice. Pancreas. 2005;31:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Lunova M, Zizer E, Kucukoglu O, Schwarz C, Dillmann WH, Wagner M, Strnad P. Hsp72 overexpression accelerates the recovery from caerulein-induced pancreatitis. PLoS One. 2012;7:e39972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Fetaud-Lapierre V, Pastor CM, Farina A, Hochstrasser DF, Frossard JL, Lescuyer P. Proteomic analysis of heat shock-induced protection in acute pancreatitis. J Proteome Res. 2010;9:5929-5942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Kubisch C, Dimagno MJ, Tietz AB, Welsh MJ, Ernst SA, Brandt-Nedelev B, Diebold J, Wagner AC, Göke B, Williams JA. Overexpression of heat shock protein Hsp27 protects against cerulein-induced pancreatitis. Gastroenterology. 2004;127:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Robinson AA, Dunn MJ, McCormack A, dos Remedios C, Rose ML. Protective effect of phosphorylated Hsp27 in coronary arteries through actin stabilization. J Mol Cell Cardiol. 2010;49:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Torgerson RR, McNiven MA. The actin-myosin cytoskeleton mediates reversible agonist-induced membrane blebbing. J Cell Sci. 1998;111:2911-2922. [PubMed] |
| 42. | Sreedharan R, Riordan M, Thullin G, Van Why S, Siegel NJ, Kashgarian M. The maximal cytoprotective function of the heat shock protein 27 is dependent on heat shock protein 70. Biochim Biophys Acta. 2011;1813:129-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Tashiro M, Schäfer C, Yao H, Ernst SA, Williams JA. Arginine induced acute pancreatitis alters the actin cytoskeleton and increases heat shock protein expression in rat pancreatic acinar cells. Gut. 2001;49:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Colón AL, Madrigal JL, Menchén LA, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Stress increases susceptibility to oxidative/nitrosative mucosal damage in an experimental model of colitis in rats. Dig Dis Sci. 2004;49:1713-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | de Pablos RM, Villarán RF, Argüelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Munhoz CD, García-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res. 2008;41:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 47. | Gu H, Tang C, Peng K, Sun H, Yang Y. Effects of chronic mild stress on the development of atherosclerosis and expression of toll-like receptor 4 signaling pathway in adolescent apolipoprotein E knockout mice. J Biomed Biotechnol. 2009;2009:613879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Fu K, Sarras MP, De Lisle RC, Andrews GK. Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am J Physiol. 1997;273:G696-G705. [PubMed] |
| 49. | Sendler M, Dummer A, Weiss FU, Krüger B, Wartmann T, Scharffetter-Kochanek K, van Rooijen N, Malla SR, Aghdassi A, Halangk W. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut. 2013;62:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 50. | Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Mazzon E, Cuzzocrea S. Role of TNF-alpha in ileum tight junction alteration in mouse model of restraint stress. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1268-G1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 53. | Dong W, Simeonova PP, Gallucci R, Matheson J, Flood L, Wang S, Hubbs A, Luster MI. Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol Appl Pharmacol. 1998;151:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Wang JY, Wang JY. Hypoxia/Reoxygenation induces nitric oxide and TNF-alpha release from cultured microglia but not astrocytes of the rat. Chin J Physiol. 2007;50:127-134. [PubMed] |
| 56. | Wang BW, Hung HF, Chang H, Kuan P, Shyu KG. Mechanical stretch enhances the expression of resistin gene in cultured cardiomyocytes via tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2007;293:H2305-H2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Kim HG, Kim JY, Gim MG, Lee JM, Chung DK. Mechanical stress induces tumor necrosis factor-{alpha} production through Ca2+ release-dependent TLR2 signaling. Am J Physiol Cell Physiol. 2008;295:C432-C439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Liu J, Xia Q, Zhang Q, Li H, Zhang J, Li A, Xiu R. Peroxisome proliferator-activated receptor-gamma ligands 15-deoxy-delta(12,14)-prostaglandin J2 and pioglitazone inhibit hydroxyl peroxide-induced TNF-alpha and lipopolysaccharide-induced CXC chemokine expression in neonatal rat cardiac myocytes. Shock. 2009;32:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Kim H, Seo JY, Roh KH, Lim JW, Kim KH. Suppression of NF-kappaB activation and cytokine production by N-acetylcysteine in pancreatic acinar cells. Free Radic Biol Med. 2000;29:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Seo JY, Kim H, Seo JT, Kim KH. Oxidative stress induced cytokine production in isolated rat pancreatic acinar cells: effects of small-molecule antioxidants. Pharmacology. 2002;64:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783-1788. [PubMed] |
| 62. | Munhoz C, Madrigal JL, García-Bueno B, Pradillo JM, Moro MA, Lizasoain I, Lorenzo P, Scavone C, Leza JC. TNF-alpha accounts for short-term persistence of oxidative status in rat brain after two weeks of repeated stress. Eur J Neurosci. 2004;20:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, Klapp BF, Arck PC, Peters EM. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Kopylova GN, Smirnova EA, Sanzhieva LTs, Umarova BA, Lelekova TV, Samonina GE. Glyprolines and semax prevent stress-induced microcirculatory disturbances in the mesentery. Bull Exp Biol Med. 2003;136:441-443. [PubMed] |
| 65. | Furukawa M, Kimura T, Sumii T, Yamaguchi H, Nawata H. Role of local pancreatic blood flow in development of hemorrhagic pancreatitis induced by stress in rats. Pancreas. 1993;8:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1528] [Cited by in RCA: 1623] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 67. | Gomez G, Englander EW, Wang G, Greeley GH. Increased expression of hypoxia-inducible factor-1alpha, p48, and the Notch signaling cascade during acute pancreatitis in mice. Pancreas. 2004;28:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Satoh A, Gukovskaya AS, Edderkaoui M, Daghighian MS, Reeve JR, Shimosegawa T, Pandol SJ. Tumor necrosis factor-alpha mediates pancreatitis responses in acinar cells via protein kinase C and proline-rich tyrosine kinase 2. Gastroenterology. 2005;129:639-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Schäfer C, Ross SE, Bragado MJ, Groblewski GE, Ernst SA, Williams JA. A role for the p38 mitogen-activated protein kinase/Hsp 27 pathway in cholecystokinin-induced changes in the actin cytoskeleton in rat pancreatic acini. J Biol Chem. 1998;273:24173-24180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Denham W, Yang J, Fink G, Denham D, Carter G, Bowers V, Norman J. TNF but not IL-1 decreases pancreatic acinar cell survival without affecting exocrine function: a study in the perfused human pancreas. J Surg Res. 1998;74:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 303] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Shimosegawa T, Pandol SJ. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582-G591. [PubMed] |
| 73. | Robinson K, Vona-Davis L, Riggs D, Jackson B, McFadden D. Peptide YY attenuates STAT1 and STAT3 activation induced by TNF-alpha in acinar cell line AR42J. J Am Coll Surg. 2006;202:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Kilpatrick LE, Sun S, Mackie D, Baik F, Li H, Korchak HM. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of delta-PKC and ERK1/2. J Leukoc Biol. 2006;80:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Chang Q, Tepperman BL. Effect of selective PKC isoform activation and inhibition on TNF-alpha-induced injury and apoptosis in human intestinal epithelial cells. Br J Pharmacol. 2003;140:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Pollo DA, Baldassare JJ, Honda T, Henderson PA, Talkad VD, Gardner JD. Effects of cholecystokinin (CCK) and other secretagogues on isoforms of protein kinase C (PKC) in pancreatic acini. Biochim Biophys Acta. 1994;1224:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Breitkreutz D, Braiman-Wiksman L, Daum N, Denning MF, Tennenbaum T. Protein kinase C family: on the crossroads of cell signaling in skin and tumor epithelium. J Cancer Res Clin Oncol. 2007;133:793-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109 Suppl 2:S424-S437. [PubMed] |
| 79. | Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Shi C, Zhao X, Wang X, Zhao L, Andersson R. Potential effects of PKC or protease inhibitors on acute pancreatitis-induced tissue injury in rats. Vascul Pharmacol. 2007;46:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1344-G1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Satoh A, Gukovskaya AS, Reeve JR, Shimosegawa T, Pandol SJ. Ethanol sensitizes NF-kappaB activation in pancreatic acinar cells through effects on protein kinase C-epsilon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G432-G438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295-C1304. [PubMed] |
| 84. | Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189-G196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 85. | Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2006;281:3370-3381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 86. | Hartwig W, Schimmel E, Hackert T, Fortunato F, Bergmann F, Baczako A, Strobel O, Büchler MW, Werner J. A novel animal model of severe pancreatitis in mice and its differences to the rat. Surgery. 2008;144:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |