Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5540
Revised: December 13, 2013
Accepted: January 19, 2014
Published online: May 14, 2014
Processing time: 260 Days and 11.1 Hours
AIM: To investigate the effectiveness of endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) in treating superficial esophageal cancer (SEC).
METHODS: Studies investigating the safety and efficacy of ESD and EMR for SEC were searched from the databases of Pubmed, Web of Science, EMBASE and the Cochrane Library. Primary end points included the en bloc resection rate and the curative resection rate. Secondary end points included operative time, rates of perforation, postoperative esophageal stricture, bleeding and local recurrence. The random-effect model and the fixed-effect model were used for statistical analysis.
RESULTS: Eight studies were identified and included in the meta-analysis. As shown by the pooled analysis, ESD had significantly higher en bloc and curative resection rates than EMR. Local recurrence rate in the ESD group was remarkably lower than that in the EMR group. However, operative time and perforation rate for ESD were significantly higher than those for EMR. As for the rate of postoperative esophageal stricture and procedure-related bleeding, no significant difference was found between the two techniques.
CONCLUSION: ESD seems superior to EMR in the treatment of SEC as evidenced by significantly higher en bloc and curative resection rates and by obviously lower local recurrence rate.
Core tip: This meta-analysis was performed on the basis of previously published reports aiming to compare endoscopic submucosal dissection (ESD) with endoscopic mucosal resection (EMR) in treating superficial esophageal cancer. Eight studies involving 1081 patients were analyzed. Of those, 448 lesions were treated by ESD and 744 were treated by EMR. Compared with EMR, ESD had significantly higher overall en bloc and curative resection rates, and lower local recurrence rate. There was no significant difference in the complication rate between the two methods.
-
Citation: Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection
vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014; 20(18): 5540-5547 - URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5540
Nowadays superficial esophageal cancer (SEC) is increasingly detected with the innovation of endoscopy techniques. Before the advent of endoscopic therapy, radical esophagectomy served as a standard therapeutic strategy for SEC, which was however reported to be related with high mortality and impaired quality of life (QOL)[1,2]. Later, the technique of endoscopic mucosal resection (EMR) initiated a new era of endoscopic treatment for SEC[3]. To date, EMR has been widely applied in clinical practice due to less invasion, lower cost, more patient tolerance, and better postoperative QOL. Nevertheless, when applying EMR techniques to large lesion, it is always difficult to evaluate the margin of specimen by histopathology or to determine the risk of lymph node metastasis. In addition, EMR was reported to have high local recurrence rates after piecemeal resection[4].
In 1990s, endoscopic submucosal dissection (ESD) was developed to remove large lesions in the alimentary tract, thus providing en bloc specimens for adequate pathological evaluation of lateral and deep margins[5]. Using improved needle-knife, the technique of ESD makes it possible to dissect the tumors from the submucosal layer. However, some studies showed that ESD could have a higher rate of complications, such as bleeding, perforation and postoperative esophageal stricture resulting from complicated procedures[6]. To date, several studies[7-11] have compared the technique of ESD with EMR for the treatment of SEC in terms of safety and efficacy, while the results of these studies were confounding when pooled together. To our knowledge, there was still a lack of a systematic review that specifically compares the safety and efficacy of ESD and EMR in the treatment of SEC. Aiming to provide a clinical basis for endoscopic treatment of SEC, this meta-analysis included several retrospective studies that evaluated the efficacy and safety of ESD and EMR in patients with SEC.
To identify all relevant studies that compared ESD with EMR from 1995 to October 31, 2012, a systematical literature search was performed through the databases of Pubmed, Web of Science, EMBASE, and the Cochrane Library with the language restricted to English. The following search terms were used: “ESD”, “EMR”, “endoscopic submucosal dissection”, “endoscopic mucosal resection”, “esophageal” and “esophagus”. The references listed in the retrieved articles were screened manually.
The studies were included in our meta-analysis according to the following criteria: (1) enrolling patients diagnosed with SEC by histology test; (2) comparing ESD and EMR for the treatment of SEC; (3) reporting the endpoints regarding therapeutic effect and complications; and (4) having the sample size of more than ten. In case of duplicated reports, the one with more cases was selected. Any comment, review, or guideline articles without original data were excluded. Besides, studies comparing ESD and EMR for lesions resulting from Barret’s esophagus were also excluded, as such lesions were mostly located in the gastroesophageal junction and the neoplasms were mostly adenoma.
In this meta-analysis, primary end points included the rates of both en bloc and curative resections; secondary end points included surgical duration and rates of bleeding, perforation, postoperative esophageal stricture and local recurrence. In this study “en bloc” was defined as resection of the lesion in one piece without piecemeal, and “curative resection” was defined as resection without undifferentiated-type cell nests or lymphvascular invasion that could be detected by histopathology in both lateral and vertical margins. Surgical duration was defined as the time from marking to resection of the lesions. Bleeding was defined as the blood loss during surgical procedures. Perforation was diagnosed endoscopically when mediastinal connective tissue was observed during surgical procedures, or by the presence of free air on an imaginological examination. Postoperative stricture was defined as a stricture requiring endoscopic dilation. Local recurrence was defined as the same histology type of the neoplasm diagnosed by histology at the resection site during the follow-up of the patient.
Two investigators extracted detailed data independently and reached consensus by discussing possibly different opinions. The data were extracted from each literature including name of the first author, year of the publication, country of the study, the number of patients and lesions, and duration of the follow-up.
The quality of each study was assessed according to the following six items: number of patients (more or fewer than ten patients); follow-up (more or less than 6 mo); comparable; comparison of operative procedures, ESD vs EMR; consecutive; and whether there was a clear protocol for the evaluation of surgical outcome. A quality score was calculated for each study, with a maximum of six points indicating the best quality[12].
RevMan 5.0 software (Cochrane Collaboration) was used in this study. The forest plot can provide the summary data entered for each study including the weight for each study, the overall effect estimate, and the statistical significance of the analysis, while a summary receiver operating characteristic curve analysis can be used to select the optimal threshold under a variety of clinical circumstances, thereby showing an optimum cut-off for sensitivity and specificity. Since the primary purpose of current study was to compare the effectiveness of two different therapeutic methods for superficial esophageal cancer, the forest plot was applied for the statistical analysis. The weighted mean difference (WMD) was recommended for continuous data of surgical duration, and the ORs with 95%CIs were recommended for dichotomous data, such as en bloc resection, curative resection, local recurrence, perforation, and stricture. In the meta-analyses, we estimated heterogeneity with Chi-square and I2. P values < 0.1 or I2 more than 50% were considered significantly heterogeneous[13]. Pooled data were calculated using a fixed-effects model when there was no heterogeneity; or a random-effects model would be applied instead. Software Stata 11 (Stata Corporation, College Station, TX, United States) was used to detect publication bias, and then the symmetry of the funnel plot was confirmed by Egger’s test with a P value less than 0.05[14].
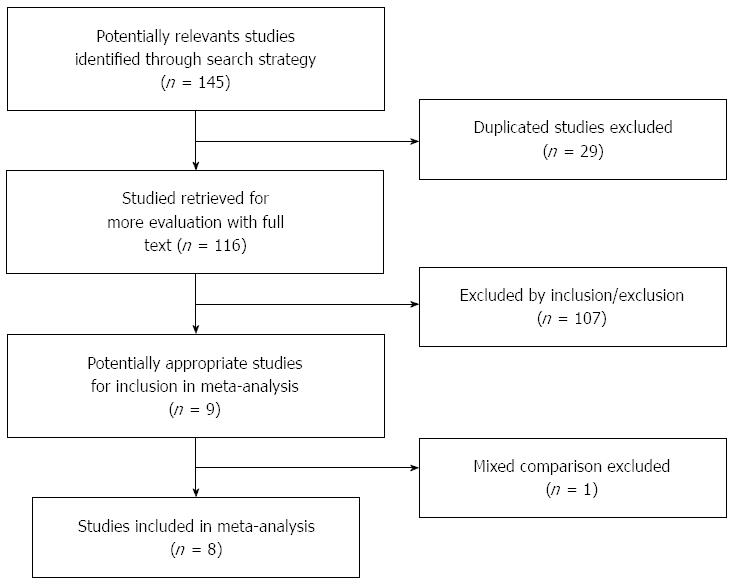
A total of 145 studies were initially yielded using the above-mentioned search strategy. According to inclusion/exclusion criteria, eight reports were finally included in our meta-analysis[7-11,15-17]. The procedure of study selection was shown in Figure 1. Overall, a total of 1080 patients with SEC were enrolled in the selected eight studies, which contained 448 lesions in the ESD group and 744 lesions in the EMR group. The main characteristics and quality assessment of the finally included reports are shown in Table 1.
| Ref. | Country | Full text/Abstract | Patient n (ESD/EMR) | Lesionn(ESD/EMR) | Quality score | Follow-up (mo) |
| Ishihara et al[11] 2008 | Japan | Full Text | 148 (29/119) | 171 (31/140) | 5 | Not recorded |
| Jung et al[15] 2008 | South Korea | Abstract | 62 (34/28) | 69 (37/32) | 4 | Not recorded |
| Kubota et al[16] 2010 | Japan | Abstract | 165 (129/36) | 167 (36/131) | 5 | ESD 29.8 |
| EMR 64.0 | ||||||
| Takahashi et al[10] 2010 | Japan | Full Text | 300 (116/184) | 300 (116/184) | 6 | 65 (8-174) |
| Teoh et al[9] 2010 | China | Full Text | 28 (18/10) | 35 (22/13) | 5 | 22.20 ± 17.31 |
| Urabe et al[7] 2011 | Japan | Full Text | 122 (59/63) | 162 (79/83) | 6 | ESD 18.9 |
| EMR 30.7 | ||||||
| Yamashita et al[8] 2011 | Japan | Full Text | 112 | 127 (71/56) | 6 | 39 (8-123) |
| Konishi et al[17] 2012 | Japan | Abstract | 143 (93/50) | 161 (56/105) | 5 | ESD 15 (1-48) |
| EMR 70 (4-146) |
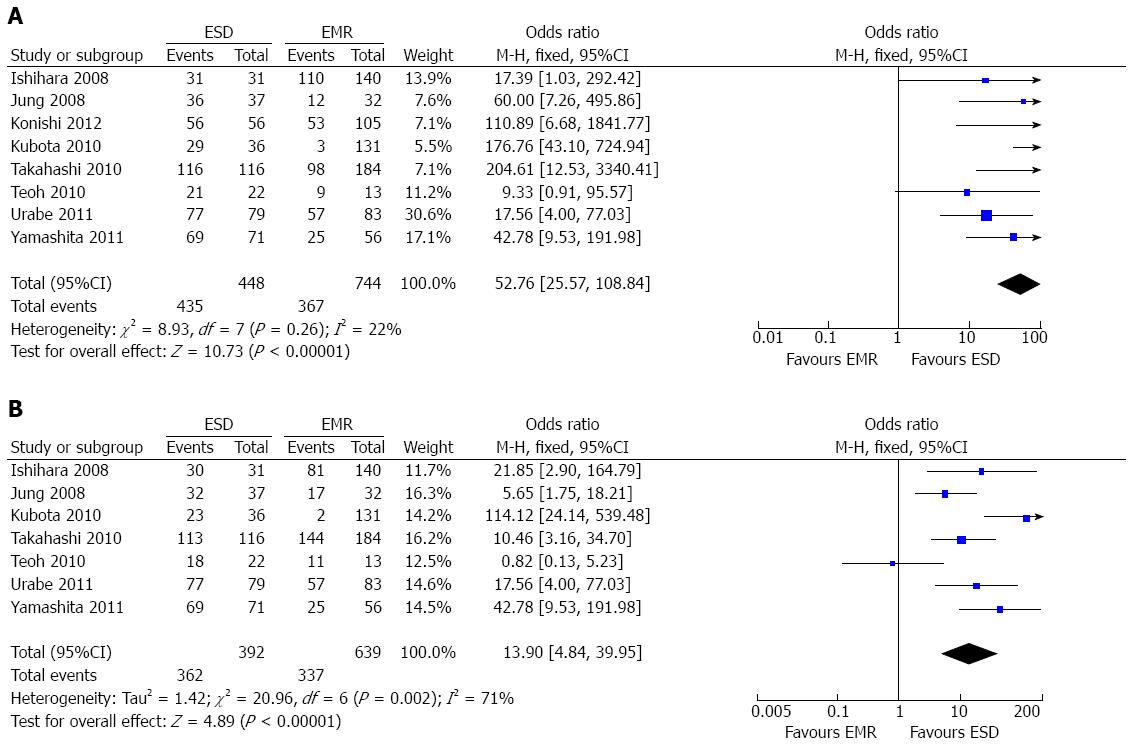
The en bloc resection rate was reported in eight papers[7-11,15-17]. Since there was no heterogeneity among these studies (P = 0.26; I2 = 22%), a fixed-effect model was applied, and the overall analysis showed a significantly higher en bloc resection rate (97.1%; 435/448) in the ESD group than in the EMR group (49.3%; 367/744) irrespective of the diameter of the lesion (OR = 52.76; 95%CI: 25.57-108.84; P < 0.001) (Figure 2A). Four studies[7,8,11,16] subgrouped the patients according to tumor size with the cut-off point set at 20 mm. In both subgroups, the en bloc resection rates were significantly higher in the ESD group than in the EMR group (OR = 14.99; 95%CI: 3.30-68.03; P = 0.0005, OR = 115.88; 95%CI: 23.35-575.12; P < 0.00001, respectively).
Seven studies[7-11,15,16] provided the data of curative resection rate for SEC. There was significant heterogeneity among these studies (P = 0.02; I2 = 71%), therefore a random-effect model was applied to perform the analysis, which showed a higher curative resection rate in the ESD group (92.3%; 362/392) than in the EMR group (52.7%; 337/639) (OR = 13.9; 95%CI: 4.84-39.95; P < 0.001) (Figure 2B). On the basis of the result of the sensitivity analysis, one study[9] that might bias the result was excluded. The result of remaining data still showed a higher curative resection rate in the ESD group (OR = 20.17; 95%CI: 8.30-49.05; P < 0.00001), and the heterogeneity could be partially explained (P = 0.05; I2 = 56%).
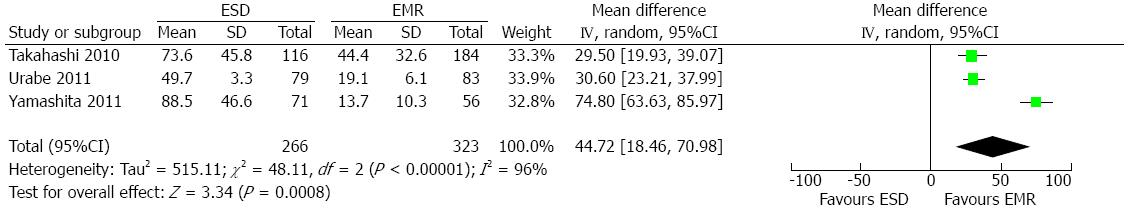
Five involved studies[7,8,10,11,17] reported the comparison of surgical duration between the ESD group and EMR group. A random-effect model was applied since there was heterogeneity among the studies (P < 0.00001; I2 = 96%). The result demonstrated that significantly longer surgical duration was needed in the ESD group than in the EMR group (WMD = 44.72; 95%CI: 18.46-70.98; P = 0.0008) (Figure 3).
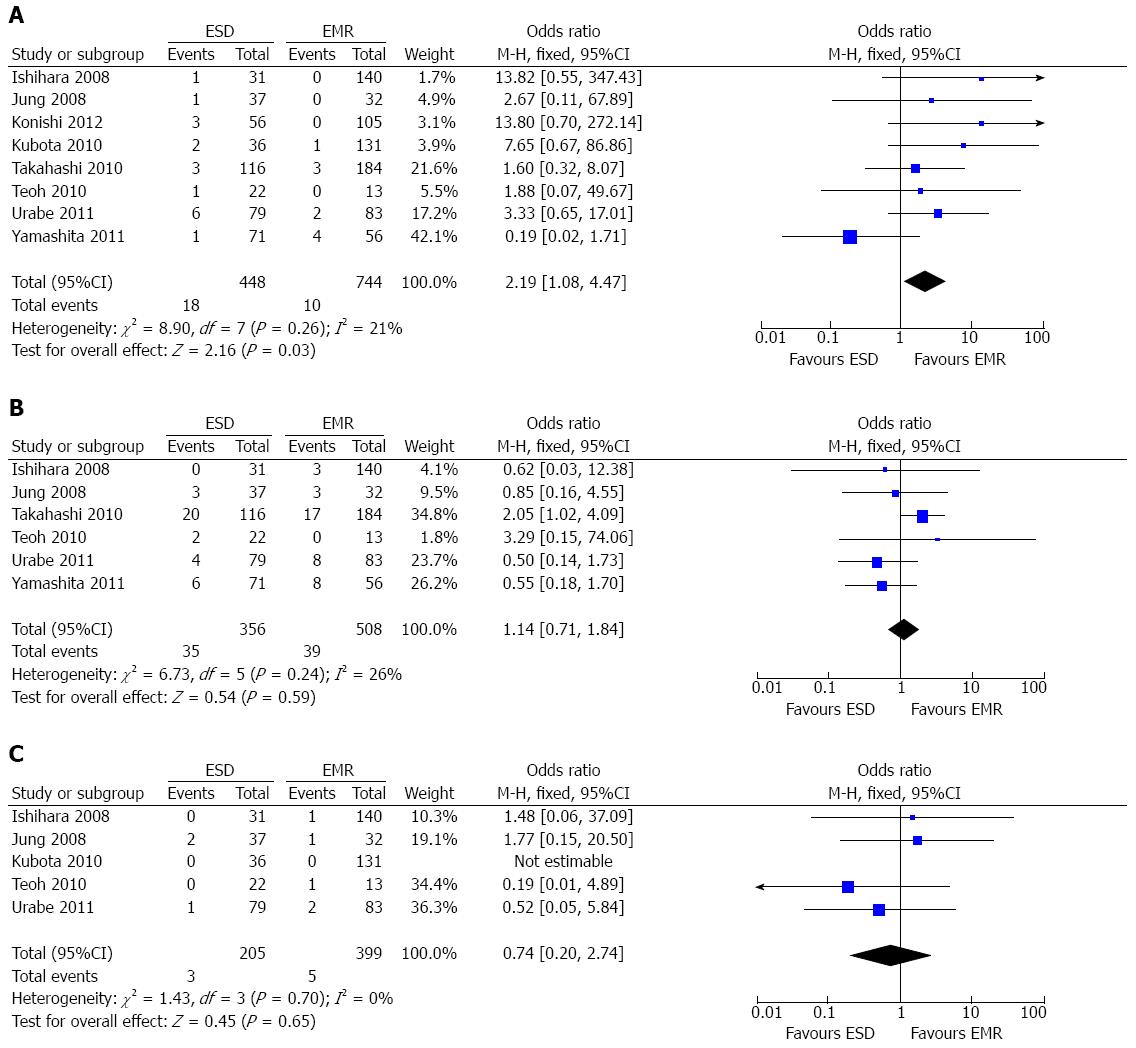
Perforation rate: All the included studies[7-11,15-17] reported the data of procedure-related perforation. A fixed-effect model was applied as there was no heterogeneity among the studies (P = 0.26; I2 = 21%). The pooled analysis demonstrated that the perforation rate for ESD was higher than that for EMR (OR = 2.19; 95%CI: 1.08-4.47; P = 0.03) (Figure 4A).
Postoperative esophageal stricture: Six studies[7-11,15] reported the data of postoperative esophageal stricture. A fixed-effect model was applied as there was no heterogeneity (P = 0.24; I2 = 26%). The overall result demonstrated that there was no significant difference between the ESD group and the EMR group concerning the esophageal stricture rate (OR = 1.14; 95%CI: 0.71-1.84; P = 0.59) (Figure 4B).
Bleeding rate: Five studies[7,9,11,15,16] reported operation-related bleeding. There was no heterogeneity (P = 0.70; I2 = 0%) among the studies, thereby a fixed-effect model was applied. No statistical difference was noted in terms of bleeding rates between the two groups (OR = 0.74; 95%CI: 0.20-2.74; P = 0.65) (Figure 4C).
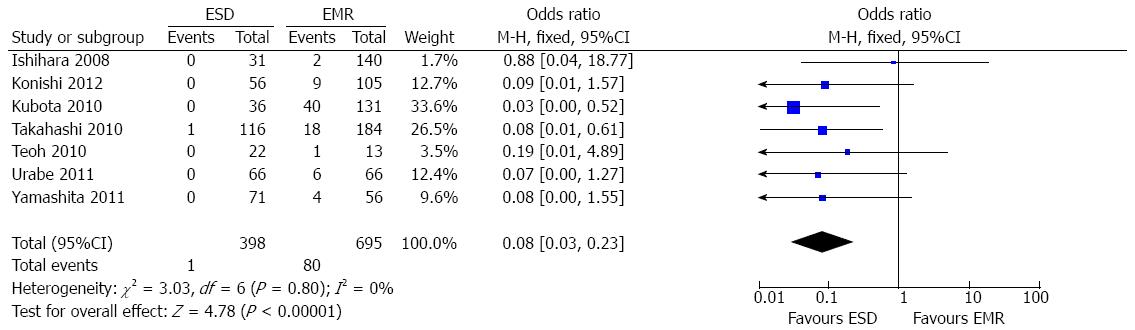
Recurrence rate: Seven studies[7-11,15,17] reported the data of local recurrence. The pooled analysis showed a significantly lower recurrence rate in the ESD group (0.3%; 1/398) than in the EMR group (11.5%; 80/695) (OR = 0.08; 95%CI: 0.03-0.23; P < 0. 001). As there was no heterogeneity among the studies (P = 0.80; I2 = 0%), a fixed-effect model was applied (Figure 5). However, subgroup analysis showed that the recurrence rate for ESD was not higher than that for EMR when lesion size was smaller than 20 mm (OR = 0.34; 95%CI: 0.06-2.08; P = 0.25), and there was no heterogeneity (P = 0.77; I2 = 0%). The result was not influenced when lesion size was larger than 20 mm (OR = 0.05; 95%CI: 0.01-0.28; P = 0.0006), and no heterogeneity was found among studies (P = 0.82; I2 = 0%).
Publication bias: No publication bias was detected by funnel plot and the Egger test when the data of en bloc resection and perforation were used as the outcome (P = 0.792 and 0.413, respectively).
Compared with traditional surgery, endoscopic therapy was widely accepted due to less invasion and better QOL of patients after removal of mucosal lesions[18], which could ideally facilitate en bloc and curative resection and reduce the risk of local recurrence[11]. The current study confirmed a distinct advantage of ESD over EMR in terms of clinical effectiveness. The en bloc resection rate in the ESD group was significantly higher than that in the EMR group, which was consistent with the previous meta-analysis studies[19,20] on the efficacy and safety of ESD and EMR in early gastric cancer and rectal carcinoid tumours. Considering that ESD was more applied to the lesion larger than 20 mm, we did subgroup analyses that demonstrated the same superiority of ESD in the en bloc resection for SEC than EMR regardless of the lesion size[11].
Compared with piecemeal resection, en bloc resection could be more effective to achieve an entire pathologic specimen and provide precise histopathologic assessment, making it possible to raise the curative resection rate for SEC[21]. In our study, the pooled result showed that the curative resection rate was higher in the ESD group than in the EMR group. Considering that endoscopist teams in Japan and South Korea could be more experienced, we did a sensitivity analysis after excluding one study from Hongkong which has quite different result from that of other studies. The sensitivity analysis still showed a superior curative resection rate in the ESD group than in the EMR group. Along with the high curative rate for SEC in the ESD group, the pooled analysis showed the local recurrence rate in the ESD group was much lower than that in the EMR group. The subsequent subgroup analysis demonstrated that this advantage was only observed when lesion size was larger than 20 mm.
ESD is composed of a series of complicated procedures[22], and some authors reported that some procedure-related complications can remarkably prolong the surgical duration. In this study, the pooled analysis showed that surgical duration in the ESD group was significantly longer than that in the EMR group. Nevertheless, with the accumulation of surgical experience of endoscopists, the procedure time for ESD may be reduced[23].
Although showing great advantages in terms of en bloc resection rates, curative resection rates, and local recurrence rates, ESD was found to have a higher rate of perforation in our study. Perforation is a common and serious complication of endoscopic treatment, which might be associated with lesion size and the depth of invasion[24]. Another serious complication related to the procedure of ESD is postoperative esophageal stricture, which might lead to severe dysphagia and decrease the QOL of the patient[25]. However, the pooled data of our study showed that the rate of stricture in the ESD group was comparable with that in the EMR group. From data of studies reporting the rate of esophageal stricture, we found that stricture mostly occurred in patients with large lesions, reflecting the fact that lesion resection in such patients can inevitably result in cicatricial stenosis since the mucosal defects always exceeded three-fourths of the circumference[10].
Several limitations existed in our study. Similar to earlier meta-analysis on endoscopic methods, the current study included a limited number of studies that were currently available. Inevitably, there was heterogeneity in the quality of the studies, such as the definitions of complication were not unified across the studies. Finally, the results of our analysis was dominated by studies from Asian population, therefore the conclusions may not be applicable to patients in other populations.
ESD showed considerable advantages regarding the en bloc resection rate, curative resection rate, and local recurrence rate when compared with EMR for SEC. Although there was a higher rate of perforation for ESD, the rates of esophageal stricture and bleeding for ESD in the treatment of SEC was similar to those for EMR. High quality and randomize-control trials from more countries with larger samples are warranted to validate these results. Compared with EMR, ESD had significantly higher en bloc and curative resection rates in the treatment of SEC, while its local recurrence rate was lower. A multicentre randomized controlled trial is warranted in order to acquire stronger evidence.
Nowadays superficial esophageal cancer (SEC) is increasingly detected with the innovation of endoscopy techniques. Both endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) can overcome the shortcoming of radical esophagectomy, and thus extensively used in the treatment of SEC.
In earlier literature concerning the treatment of SEC, EMR was demonstrated to have advantages in terms of less invasion, lower cost, more patient tolerance, and better postoperative quality of life of patient, which was however reported to have high local recurrence rates after piecemeal resection. By contrast, ESD was found to yield lower local recurrence rate, which nevertheless could have higher rates of procedure-related bleeding, perforation and postoperative esophageal stricture. To date, several studies have compared the technique of ESD with EMR for the treatment of SEC in terms of safety and efficacy, while the results of these studies were confounding when pooled together.
This meta-analysis demonstrated that ESD is superior to EMR in the treatment of SEC as evidenced by significantly higher en bloc and curative resection rates and by obviously lower local recurrence rate.
This meta-analysis provided a clinical basis for endoscopic treatment of SEC.
Superficial esophageal cancer means the cancer confined to mucosal or submucosal layer of the esophagus, without lymph node metastasis. EMR and ESD are endoscopic therapy methods, which can remove the lesions of the alimentary tract without surgical procedures and have been extensively used in clinical practice.
This paper was performed to compare ESD and EMR in terms of safety and effectiveness when treating SEC, and it was concluded that ESD could be superior to EMR in the treatment of SEC as evidenced by significantly higher en bloc and curative resection rates and by obviously lower local recurrence rate. The study is concise and informative.
P- Reviewers: Shi HY, Snyder N S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Nigro JJ, Hagen JA, DeMeester TR, DeMeester SR, Theisen J, Peters JH, Kiyabu M. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg. 1999;230:433-438; discussion 438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 277] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Saito Y, Takisawa H, Suzuki H, Takizawa K, Yokoi C, Nonaka S, Matsuda T, Nakanishi Y, Kato K. Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy. Gastrointest Endosc. 2008;67:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Hirasaki S, Tanimizu M, Moriwaki T, Hyodo I, Shinji T, Koide N, Shiratori Y. Efficacy of clinical pathway for the management of mucosal gastric carcinoma treated with endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med. 2004;43:1120-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Sumiyama K, Kaise M, Nakayoshi T, Kato M, Mashiko T, Uchiyama Y, Goda K, Hino S, Nakamura Y, Matsuda K. Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early stage gastric cancer. Gastrointest Endosc. 2004;60:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Urabe Y, Hiyama T, Tanaka S, Yoshihara M, Arihiro K, Chayama K. Advantages of endoscopic submucosal dissection versus endoscopic oblique aspiration mucosectomy for superficial esophageal tumors. J Gastroenterol Hepatol. 2011;26:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Yamashita T, Zeniya A, Ishii H, Tsuji T, Tsuda S, Nakane K, Komatsu M. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc. 2011;25:2541-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Teoh AY, Chiu PW, Yu Ngo DK, Wong SK, Lau JY, Ng EK. Outcomes of endoscopic submucosal dissection versus endoscopic mucosal resection in management of superficial squamous esophageal neoplasms outside Japan. J Clin Gastroenterol. 2010;44:e190-e194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-264, 264.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-ix, 1-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1693] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 13. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30429] [Article Influence: 780.2] [Reference Citation Analysis (0)] |
| 14. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40577] [Article Influence: 1449.2] [Reference Citation Analysis (2)] |
| 15. | Jung H, Choi K, Song H, Kim D, Lee G, Kim J. Endoscopic resection for superficial esophageal cancer: comparison between EMR and ESD methods. J Gastroenterol Hepatol. 2008;23 Suppl 5:A88. |
| 16. | Kubota Y, Kojima T, Fukuda D, Saraya T, Tsunoda C, Ikeda E, Satake H, Nagahisa E, Yoda Y, Mochizuki S. Comparison of Endoscopic Mucosal Resection (EMR) and Endoscopic Submucosal Dissection (ESD) for Large Squamous Cell Carcinoma of the Esophagus. Gastrointest Endosc. 2010;72:AB177. [DOI] [Full Text] |
| 17. | Konishi J, Kobayashi N, Hirahara Y, Sekiguchi R. The Usefulness of Endoscopic Submucosal Dissection for Superficial Esophageal Cancers Comparing With Endoscopic Mucosal Resection. Gastrointest Endosc. 2010;75:AB469. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Conio M, Ponchon T, Blanchi S, Filiberti R. Endoscopic mucosal resection. Am J Gastroenterol. 2006;101:653-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Zhong DD, Shao LM, Cai JT. Endoscopic mucosal resection vs endoscopic submucosal dissection for rectal carcinoid tumours: a systematic review and meta-analysis. Colorectal Dis. 2013;15:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Conio M, Gostout CJ. Histopathologic findings predicting lymph node metastasis and prognosis of patients with superficial esophageal carcinoma. Analysis of 240 surgically resected tumors. Gastrointest Endosc. 2001;54:668-669. [PubMed] |
| 22. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 23. | Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |