Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5533
Revised: February 8, 2014
Accepted: March 19, 2014
Published online: May 14, 2014
Processing time: 186 Days and 18.9 Hours
AIM: To determine the correlation between invasiveness, migration and prognosis in esophageal squamous cell carcinoma (ESCC) and expression of the B-cell-specific Moloney leukemia virus insert site 1 (Bmi-1) and plasminogen activator inhibitor-1 (PAI-1).
METHODS: Eighty previously untreated patients who underwent surgical excision of ESCC were included. The expression of Bmi-1 and PAI-1 was examined immunohistochemically in formalin-fixed paraffin-embedded primary tissue specimens. The relationships between the expression of Bmi-1 and PAI-1, the clinicopathologic features of ESCC, and the survival rate of ESCC patients were also discussed. The correlation between Bmi-1 and PAI-1 protein expression in ESCC was analyzed. The relationship between Bmi-1 and PAI-1 expression and ESCC prognosis was evaluated using a Cox regression model and Kaplan-Meier survival curve analysis.
RESULTS: The rates of positive Bmi-1 and PAI-1 expression in ESCC were higher than those in normal esophageal tissue (P < 0.05). The expression of Bmi-1 and PAI-1 was correlated with depth of invasion and lymph node metastasis (P < 0.05), but not with patient age, tumor size or nationality (P > 0.05). The expression of Bmi-1 was positively correlated with that of PAI-1 (P < 0.05). The 10-year overall survival rate for all patients was 20% (16/80). Univariate Kaplan-Meier survival analysis showed that patients with high expression of esophageal PAI-1 and Bmi-1 had lower survival, however, the difference was not statistically significant. Cox multivariate analysis showed that PAI-1 and Bmi-1 were not independent factors for survival rate, while the depth of tumor invasion and metastasis were independent factors affecting patient survival.
CONCLUSION: The expression of Bmi-1 and PAI-1 plays a role in ESCC progression, and may be used as a prognostic marker in ESCC.
Core tip: In this study, B-cell-specific Moloney leukemia virus insert site 1 (Bmi-1) and plasminogen activator inhibitor-1 (PAI-1) expression, their correlation with clinicopathological features, and their prognostic significance in esophageal squamous cell carcinoma (ESCC) were examined. The results showed that the Bmi-1 protein promoted malignant transformation, increased invasion and metastasis of cells. PAI-1 was not an independent prognostic factor. Although these new markers are important, the molecular mechanism of ESCC metastasis is far from being fully understood and represents a new prerequisite for developing better treatment strategies.
- Citation: Zhang Y, Zhang YL, Chen HM, Pu HW, Ma WJ, Li XM, Ma H, Chen X. Expression of Bmi-1 and PAI-1 in esophageal squamous cell carcinoma. World J Gastroenterol 2014; 20(18): 5533-5539
- URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5533.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5533
Esophageal squamous cell carcinoma (ESCC) is a common malignant digestive system tumor worldwide. There are approximately 240000 new cases of esophageal cancer in China each year. According to statistics, the 5-year survival rate for ESCC patients ranges from 3.5% to 13.9%. Mortality due to esophageal cancer is 13.05/100000 in the Xinjiang Uygur Autonomous Region of China, while in Kazakh it is 68.88/100000. ESCC is a serious threat to human health[1]. The Bmi-1 gene was discovered in 1999. It produced a synergistic effect with the c-myc gene in mice with lymphoma and this synergy promoted the formation of tumor cells[2]. Since then, other researchers have detected excessive Bmi-1 gene expression in a variety of human cancers, such as head and neck cancer[3] and breast cancer[4], and Bmi-1 gene expression was also found to be associated with the development of tumors[5,6]. In the present study, the expression levels of B-cell-specific Moloney leukemia virus insert site 1 (Bmi-1) and plasminogen activator inhibitor-1 (PAI-1) in 80 cases of ESCC were determined to explore their influence on the prognosis of patients with ESCC.
The study was conducted at the First Affiliated Hospital of Xinjiang Medical University, and 80 ESCC tissue specimens were collected from patients between 1997 and 2004. All specimens were obtained following surgical resection and were proved by pathologists. Of the 80 patients enrolled, 40 were of Kazakh nationality and 40 were of Han nationality, and 57 were male and 23 were female with an average age of 60 years. None of the patients had received preoperative radiotherapy or chemotherapy. According to the World Health Organization (WHO) standard pathology classification, 46 cases showed moderate to high differentiation and 34 cases showed low differentiation. Fifty-three patients had no lymph node metastasis and 27 had lymph node metastasis. Normal esophageal tissue was obtained from 20 individuals who served as controls. Specimens were fixed in 40 g/L formaldehyde, embedded in conventional paraffin, and slices 4 mm thick were cut. Mouse monoclonal antibody for Bmi-1 was obtained from ABCAM, United States, and the SP immunohistochemical kit, antibody diluent, and the DAB chromogenic reagent kit were purchased from Beijing Fir Company, China.
Immunohistochemical EnVision two footwork method was used to determine the protein expression of Bmi-1 and PAI-1 in each group. The procedure was as follows: paraffin sections were dewaxed step by step, hydrated in water, incubated with 30 mL/L H2O2 for 20 min at room temperature (inactivated endogenous peroxidase), and rinsed with PBS. The slices were placed in citrate buffer salt at pH 6.0 and heated for 20 min in a microwave (temperature controlled at 95 °C-100 °C) for antigen retrieval. The slices were then cooled to room temperature and rinsed with PBS. The antibody was then added and placed in the refrigerator at 4 °C overnight. The slices were washed using the same method, DAB chromogenic reagent was added and the slices were placed in a wet box. Termination of the chromogenic reaction was achieved with tap water. Slices were modified by prolonging the hematoxylin staining time and adding a step of differentiation in hydrochloric acid-alcohol differentiation. Slices was then dehydrated and mounted.
Bmi-1 expression in the endonuclear area was seen as tan or brown granular particles, and was also visible in the cytoplasm. PAI-1 expression was seen as tan or brown granular particles within the cytoplasm. Expression in normal esophageal mucosa cells was used as a positive control. Slices of normal esophagus were randomly examined in five fields of view at 400 × magnification, and a negative result was regarded if there were < 5% of positive cells and a positive result if there were > 5% of positive cells.
Statistical analyses were performed using SPSS 17.0 software for χ2 test, Kaplan-Meier, survival analysis, and the Cox regression model. P < 0.05 was considered statistically significant.
The rates of expression of Bmi-1 in esophageal cancer tissue and normal mucosa were 78.7% and 5.0%, respectively. The rates of expression of PAI-1 in esophageal cancer tissue and normal mucosa were 75% and 45%, respectively. The differences in PAI-1 and Bmi-1 expression were statistically significant between normal esophageal mucosa and esophageal cancer tissue (P < 0.05 for both) (Tables 1 and 2).
| Pathological characteristic | Total cases | Bmi-1 | χ2 | Pvalue | ||
| Positive | Negative | Positive rate (%) | ||||
| Cancerous tissue | 80 | 63 | 17 | 78.7 | 37.77 | 0.01 |
| Normal tissue | 20 | 1 | 19 | 5.0 | ||
| Pathological characteristic | Total cases | PAI-1 | χ2 | Pvalue | ||
| Positive | Negative | Positive rate (%) | ||||
| Cancerous tissue | 80 | 61 | 19 | 75.0 | 28.69 | 0.01 |
| Normal tissue | 20 | 2 | 18 | 10.0 | ||
Bmi-1 protein expression in patients with lymph node metastasis was lower than that in patients without lymph node metastasis, and the difference was statistically significant (P < 0.05). Bmi-1 expression gradually increased with increased depth of infiltration in the muscularis and serosa layer (P < 0.05). There was no significant difference in expression due to different tumor sizes (P > 0.05). The difference in Bmi-1 expression was not statistically significant between the Han and Kazakh subjects (P > 0.05) (Table 3).
| Characteristic | Total cases | Positive cases | Positive rate (%) | χ2 | Pvalue |
| Differentiation degree | |||||
| Moderate to high | 46 | 38 | 82.6 | 0.963 | 0.411 |
| Poor | 34 | 25 | 73.5 | ||
| Lymphatic metastasis | |||||
| Yes | 27 | 17 | 63.0 | 6.07 | 0.024 |
| No | 53 | 46 | 86.6 | ||
| Sex | |||||
| Male | 57 | 47 | 73.5 | 1.627 | 0.234 |
| Female | 23 | 16 | 82.6 | ||
| Age (yr) | |||||
| < 60 | 34 | 25 | 87.5 | 0.963 | 0.414 |
| ≥ 60 | 46 | 38 | 70.0 | ||
| Nationality | |||||
| Han | 40 | 35 | 78.8 | 3.66 | 0.099 |
| Kazakh | 40 | 28 | 78.7 | ||
| Tumor size (cm) | |||||
| ≤ 3 | 33 | 26 | 92.3 | 0 | 0.613 |
| > 3 | 47 | 37 | 72.2 | ||
| Depth of infiltration | |||||
| Muscular layer | 26 | 24 | 92.3 | 4.231 | 0.0451 |
| Tunica externa | 54 | 39 | 72.2 | ||
| Pathological type | |||||
| Ulcerative | 37 | 27 | 73.0 | 0.885 | 0.829 |
| Fungating | 11 | 10 | 90.9 | ||
| Medullary | 26 | 21 | 80.7 | ||
| Constrictive | 6 | 5 | 83.3 | ||
PAI-1 protein expression in patients with lymph node metastasis was lower than that in patients without lymph node metastasis, and the difference was statistically significant (P < 0.01). PAI-1 expression gradually increased with increased depth of infiltration in the muscularis and serosa layer (P < 0.05). There was no significant difference in expression due to different tumor sizes (P > 0.05) (Table 4).
| Characteristic | Total cases | Positive cases | Positive rate (%) | χ2 | P value |
| Differentiation degree | |||||
| Moderate to high | 46 | 39 | 84.8 | 4.351 | 0.061 |
| Poor | 34 | 22 | 64.7 | ||
| Lymphatic metastasis | |||||
| Yes | 27 | 16 | 59.3 | 6.497 | 0.024 |
| No | 53 | 45 | 84.9 | ||
| Sex | |||||
| Male | 57 | 44 | 77.2 | 0.097 | 0.777 |
| Female | 23 | 17 | 73.9 | ||
| Age (yr) | |||||
| < 60 | 34 | 29 | 85.3 | 2.671 | 0.119 |
| ≥ 60 | 46 | 32 | 69.6 | ||
| Nationality | |||||
| Han | 40 | 30 | 75.0 | 0.069 | 1.000 |
| Kazakh | 40 | 31 | 77.5 | ||
| Tumor size (cm) | |||||
| ≤ 3 | 33 | 25 | 75.8 | 0.008 | 1.000 |
| > 3 | 47 | 36 | 76.6 | ||
| Depth of infiltration | |||||
| Muscular layer | 26 | 14 | 53.8 | 10.676 | 0.022 |
| Tunica externa | 54 | 47 | 87.0 | ||
| Pathological type | |||||
| Ulcerative | 37 | 27 | 73.0 | 0.580 | 0.901 |
| Fungating | 11 | 9 | 81.8 | ||
| Medullary | 26 | 20 | 76.9 | ||
| Constrictive | 6 | 5 | 76.0 | ||
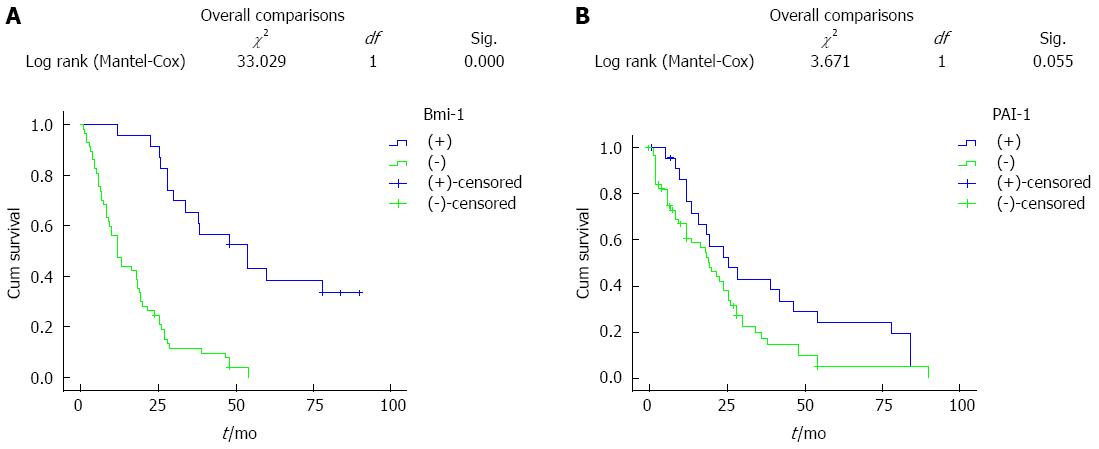
This study included 80 patients with ESCC, 16 patients survived, the median survival time was 60 mo, and the 10-year survival rate was 20% (16/80). Bmi-1 expression in the univariate Kaplan-Meier survival analysis showed that both the average survival time and median survival time in patients with positive expression of Bmi-1 were less than those in patients with negative expression. The difference, however, was not statistically significant (Figure 1A). PAI-1 expression in the univariate Kaplan-Meier survival analysis showed that both the average survival time and median survival time in patients with positive expression of PAI-1 were less than those in patients with negative expression. The difference, however, was not statistically significant (Figure 1B).
It is well known that ESCC, a common malignant disease, is prone to invade adjacent regions and to metastasize to lymph nodes and distant organs. To develop novel treatments and cures, it is imperative to address the factors underlying tumorigenesis, invasion and metastasis. In this study, we identified and functionally characterized Bmi-1 as an important player in ESCC progression. We determined the expression of Bmi-1, demonstrated the association between Bmi-1 expression and clinicopathologic parameters and evaluated the role of Bmi-1 in ESCC prognosis. Differential expression of Bmi-1 was detected in ESCC patients with and without lymph node metastasis, and in primary cancer tissues and matched adjacent non-cancerous tissues. Bmi-1 expression was significantly up-regulated in ESCC tissues compared with adjacent non-cancerous tissues.
It has been reported that high Bmi-1 gene expression is closely related to the occurrence, development, invasion, infiltration, metastasis and prognosis of colorectal[5] and breast cancers[6]. A number of studies have also shown that the Bmi-1 gene is important in the cell-cycle regulation system. Tumor cell invasion is regulated by various factors which can stimulate or promote cell motility, destruction of the matrix, angiogenesis and other biological events at the molecular level. In laryngeal neoplasms[7,8], Bmi-1 over-expression can promote transformation of epithelial-ectomesenchymal tissue, while lower Bmi-1 expression can inhibit transformation of epithelium-ectomesenchymal tissue and metastasis of tumor cells. Abd El hafez et al[9] reported the immunohistochemical expression of Bmi-1 protein in epithelial ovarian cancer tissue specimens and its relevance to clinicopathologic prognostic variables and patient survival. High Bmi-1 expression was strongly associated with advanced stages and carcinomas with serous histology. Bmi-1 expression displayed a significant inverse association with patient overall and mean survival. It has been reported that Bmi-1 plays a very important role in the invasion and metastasis of neoplasms, especially as a proto-oncogene. He et al[10] showed that the expression level of Bmi-1 mRNA was significantly positively correlated with histological grade, clinical grade and lymph node metastasis in ESCC using polymerase chain reaction and immunocytochemistry, however, protein levels of Bmi-1 were not associated with the above-mentioned factors.
We found that positive expression of Bmi-1 increased with the depth of cancer invasion; Bmi-1 expression in ESCC patients with lymph node metastasis was higher than that in patients without lymph node metastasis, and the difference was statistically significant (P < 0.05). This showed that Bmi-1 protein promoted malignant transformation of cancer cells and increased invasion and metastasis of cells. However, Bmi-1 protein was not related to the degree of esophageal tissue differentiation, age, sex, nationality, or pathological type. Survival analyses (Kaplan-Meier) indicated that patients with high expression of Bmi-1 had a lower 10-year survival rate than patients with negative expression. The difference, however, was not statistically significant (P > 0.05). The analysis showed that Bmi-1 was not an independent prognostic factor, which was confirmed by further multivariate Cox regression analysis. Metastatic relapse remains a major challenge in the management of ESCC. Many factors are involved in tumor progression, including changes in cell adhesion, cell communication, increased migration or motility and invasiveness. In this study, Bmi-1 was shown to contribute to each of these events. The result was in accordance with a previous observation that Bmi-1 alone did not influence prognosis.
Qian et al[11] recently demonstrated that plasma PAI-l concentration was associated with pathologic stage in pancreatic cancer patients. PAI-l concentration was greater in stage IV than in stage III, and PAI-l accelerated degradation of the extracellular matrix. PAI-l concentration was found to be high during progression in late stage pancreatic cancer. Jiang et al[12] found that the expression of PAI-l protein in pancreatic cancer tissue was significantly higher than that in normal pancreatic tissue using immunohistochemistry, which was closely related to clinical TNM stage and lymph node metastasis in pancreatic cancer. However, the findings were not affected by gender, age, location, size and histopathological grade. Tumor cells produce cytokines and other substances to promote tumor angiogenesis, stroma infiltration, microenvironmental changes, and tumor cell detachment from mucous membrane, resulting in tumor invasion and metastasis along lymphatic and blood vessels and the transfer of tumor cells to surrounding tissue and distant organs.
In this study, PAI-l protein expression was shown to be related to lymph node metastasis and depth of infiltration in ESCC, however, there was no obvious relationship with age, nationality, degree of differentiation, pathological classification, or sex. From the histology results, it was shown that PAI-l protein expression plays an important role in the development of tumors, and is closely related to tumor invasion and metastasis. However, patient nationality had no effect on PAI-1 expression.
To the best of our knowledge, there are few studies on the relationship between Bmi-1 expression and the prognosis of tumors. It was found that up-regulated expression of Bmi-1 was the main factor in the poor prognosis of nasopharyngeal carcinoma[13]. High expression of Bmi-1 protein was related to the degree of tumor differentiation[14,15] and clinical stage in ovarian cancer tissue[16,17]. Therefore, over-expression of Bmi-1 protein may be involved in ovarian cancer occurrence and development. Zhan et al[18] found that Bmi-1 expression in non-small cell lung cancer is higher than that in normal lung tissue, which suggests that Bmi-1 may be related to the development and prognosis of lung cancer. Chen et al[19] pointed out that Bmi-1 expression in gastric cancer tissue was higher than that in adjacent tissues, Bmi-1 expression was correlated with tumor size and lymph node metastasis, and patients with positive expression had a lower survival rate than patients with negative expression. Despite more evidence to show that poor prognosis is related to Bmi-1 expression, other studies have not shown the same results. It was found that only 25% of breast cancer patients in Africa had Bmi-1 protein expression. Another relevant study demonstrated that the mRNA expression level of Bmi-1 significantly increased in patients without lymph node metastasis in the early stages of the disease, and Bmi-1 up-regulation was associated with the occurrence of nasopharyngeal carcinoma and a low survival rate. It was recently proved that increased Bmi-1 protein expression was associated with survival in patients treated with etoposide or platinum-containing agents. Bmi-1 gene expression was closely associated with poor prognosis and treatment failure in patients with malignant tumors, such as acute myelogenous leukemia, chronic myelogenous leukemia, myelodysplastic syndrome, and lymphoma. Farivar et al[20] found that increased expression of Bmi-1 in pediatric brain tumors may be important in the acquisition of an aggressive phenotype. Bmi-1 can be used as a strong and independent molecular marker of prognosis in pediatric brain tumors. Abd El hafez et al[9] proved that patients with epithelial ovarian cancer and expression of Bmi-1 had a significantly lower 5-year survival rate than patients with negative Bmi-1 expression. Bmi-1 expression is an independent prognostic factor in gastric cancer, and up-regulation of Bmi-1 is a sign of poor prognosis in patients with gastric cancer.
The results of this study showed that the 5-year survival rate in patients with positive Bmi-1 expression was lower compared to patients with negative Bmi-1 expression, according to the results of the Kaplan Meier survival analysis, however, the difference was not statistically significant (P > 0.05). These results may be due to a small number of samples, and further investigations are needed using a larger patient cohort to extend our findings.
Recent reports indicate the possible role of PAI-1 in different carcinomas. Allott et al[21] reported that infiltration and invasion were greater in patients with esophageal tumors and positive PAI-1 expression, resulting in decreased 10-year overall survival. Fang et al[22] suggested that PAI-1 exerts a protective effect against apoptosis in tumor cells by a mechanism that, in part, involves Fas/Fas-L-mediated apoptosis and plasmin activation, and that both tumor- and host-derived PAI-1 play a role in tumor progression. A number of studies have also shown that PAI-1 mediated effects are not restricted to tumor cells of bladder origin[23-26]. Collectively, these data show that targeting PAI-1 may be beneficial and support the notion that novel drugs such as tiplaxtinin[27] could be investigated as anticancer agents.
In the present study, the 5-year survival rate was lower in patients with positive PAI-1 expression compared to patients with negative PAI-1 expression, according to survival analyses (Kaplan-Meier). The difference, however, was not statistically significant (P > 0.05).
In conclusion, the findings of the present study demonstrated that PAI-1 is not an independent prognosis factor and PAI-1 is related to the depth of invasion and lymph node metastasis following identification of prognostic factors using multivariate Cox regression analysis. Patients with ECSS who had higher PAI-1 expression showed progression, suggesting that PAI-1 is a novel molecular marker that predicts progression and prognosis. These data also support PAI-1 as a therapeutic target in cancer and preclinical studies should be performed to test PAI-1 inhibitors in cancer therapy. Further studies will be needed to determine whether PAI-1 is a novel therapeutic target for ESCC.
Worldwide, the majority of esophageal cancers are squamous cell carcinomas. Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors. Over the past few years, the incidence of ESCC has increased, and its mortality rate ranked the sixth in cancer-related deaths.
Over the past few years, several studies have confirmed that B-cell-specific Moloney leukemia virus insert site 1 (Bmi-1) is over-expressed in all tumor cells and tissues in lung cancer, colorectal cancer, breast cancer, nasopharyngeal cancer, oral carcinoma, cutaneous carcinoma, and gastric cancer. It has been reported that high Bmi-1 gene expression is closely related to the occurrence, development, invasion, infiltration, metastasis and prognosis of colorectal and breast cancers. A number of studies have also shown that the Bmi-1 gene is important in the cell-cycle regulation system. It has been reported that positive plasminogen activator inhibitor-1 (PAI-1) protein expression in pancreatic cancer tissue is significantly higher than that in normal pancreatic tissue using immunohistochemistry, which is closely related to clinical TNM stage and lymph node metastasis of pancreatic cancer. Previous studies demonstrated that Bmi-1 and PAI-1 are over-expressed in tumor cells and cancer tissues. More recent reports, however, suggest that PAI-1 may regulate tumor cell apoptosis and has a protumorigenic function. Whether Bmi-1 and PAI-1 influence cell senescence and metastasis of human ESCC is unknown.
Currently, there are few studies on the relationship between the expression of Bmi-1 and prognosis of ESCC. The role of Bmi-1 and PAI-l expression in ESCC remains unclear. This study determined the correlation between invasiveness, migration and prognosis and both Bmi-1 and PAI-1 expression in ESCC. It is suggested that the expression of Bmi-1 and PAI-1 play a role in ESCC progression. The expression of Bmi-1 and PAI-1 may be used as a prognostic marker in ESCC. The findings of the present study demonstrated that PAI-1 and Bmi-1 are not independent factors for survival, while the depth of tumor invasion and metastasis are independent factors affecting survival.
These new markers and methods are important, and provide a platform for the development of better treatment strategies. This research provides additional support for the notion that Bmi-1 and PAI-1 inhibitors should be developed as new agents for ESCC.
Bmi-1, a member of the polycomb group, functions as a transcriptional repressor and has high expression in many tumors, indicating a poor prognosis. Several lines of evidence suggest that Bmi-1 blocks cell senescence and proliferation, and the Bmi-1 gene is also associated with tumor invasion and metastasis. PAI-1, a member of the serine protease inhibitors superfamily, which is expressed by endothelial cells, epithelial cells, monocytes and macrophages, is the principal inhibitor of tissue-type plasminogen activator and urokinase-type plasminogen activator and thus inhibits fibrinolysis.
In this manuscript, the authors examined the expression of Bmi-1 and PAI-1. The results may represent a molecular mechanism of esophageal cancer. It is a well written paper with important clinical implications. Overall, this topic is very interesting.
P- Reviewers: Budzynski J, Romano C S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Pu XH, Pu HW, Chen X, Shen QL. Expression and its significance of RASSF1A and FGF3 in esophageal carcinoma. Yixue Yanjiu Zazhi. 2010;27:11-14. [DOI] [Full Text] |
| 2. | Guo WJ, Datta S, Band V, Dimri GP. Mel-18, a polycomb group protein, regulates cell proliferation and senescence via transcriptional repression of Bmi-1 and c-Myc oncoproteins. Mol Biol Cell. 2007;18:536-546. [PubMed] |
| 3. | Dalley AJ, Pitty LP, Major AG, Abdulmajeed AA, Farah CS. Expression of ABCG2 and Bmi-1 in oral potentially malignant lesions and oral squamous cell carcinoma. Cancer Med. 2014;3:273-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, Song LB, Zeng MS. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer. 2011;10:10. [PubMed] |
| 5. | Li DW, Tang HM, Fan JW, Yan DW, Zhou CZ, Li SX, Wang XL, Peng ZH. Expression level of Bmi-1 oncoprotein is associated with progression and prognosis in colon cancer. J Cancer Res Clin Oncol. 2010;136:997-1006. [PubMed] |
| 6. | Guo P, Gao A, Zhang G, Han H, Zhou Q. Decoding the knots of initiation of oncogenic epithelial-mesenchymal transition in tumor progression. Curr Cancer Drug Targets. 2013;13:996-1011. [PubMed] |
| 7. | Yan W, Zhang W, Sun L, Liu Y, You G, Wang Y, Kang C, You Y, Jiang T. Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res. 2011;1411:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Choy B, Bandla S, Xia Y, Tan D, Pennathur A, Luketich JD, Godfrey TE, Peters JH, Sun J, Zhou Z. Clinicopathologic characteristics of high expression of Bmi-1 in esophageal adenocarcinoma and squamous cell carcinoma. BMC Gastroenterol. 2012;12:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Abd El hafez A, El-Hadaad HA. Immunohistochemical expression and prognostic relevance of Bmi-1, a stem cell factor, in epithelial ovarian cancer. Ann Diagn Pathol. 2014;18:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | He XT, Cao XF, Ji L, Zhu B, Lv J, Wang DD, Lu PH, Cui HG. Association between Bmi1 and clinicopathological status of esophageal squamous cell carcinoma. World J Gastroenterol. 2009;15:2389-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Qian P, Lu ZX, Yang CX. Determination of the Plasma PAI-1 and Its Clinical Significance in Pancreatic Cancer Patients. Fangshe Mianyixue Zazhi. 2012;25:559-560. [DOI] [Full Text] |
| 12. | Jiang L, Sun CL, Li H, Yu H. Expression of Smad7,uPA and PAI-1 in pancreatic cancer and its clinical significance. Jiangsu Daxue Xuebao. 2010;20:527-531. [DOI] [Full Text] |
| 13. | Wu J, Hu D, Yang G, Zhou J, Yang C, Gao Y, Zhu Z. Down-regulation of BMI-1 cooperates with artemisinin on growth inhibition of nasopharyngeal carcinoma cells. J Cell Biochem. 2011;112:1938-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Yu J, Lu Y, Cui D, Li E, Zhu Y, Zhao Y, Zhao F, Xia S. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol Rep. 2014;31:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Yu X, Jiang X, Li H, Guo L, Jiang W, Lu SH. miR-203 inhibits the proliferation and self-renewal of esophageal cancer stem-like cells by suppressing stem renewal factor Bmi-1. Stem Cells Dev. 2014;23:576-585. [PubMed] |
| 16. | Gavrilescu MM, Todosi AM, Aniţei MG, Filip B, Scripcariu V. Expression of bmi-1 protein in cervical, breast and ovarian cancer. Rev Med Chir Soc Med Nat Iasi. 2012;116:1112-1117. [PubMed] |
| 17. | Xin T, Zhang FB, Sui GJ, Jin XM. Bmi-1 siRNA inhibited ovarian cancer cell line growth and decreased telomerase activity. Br J Biomed Sci. 2012;69:62-66. [PubMed] |
| 18. | Zhan HB, Cai XG, Wu FM. Expression and clinical significance of Bmi-1 protein and hTERT protein in non-small cell lung cancer. Linchuang Zhongliuxue Zazhi. 2009;14:4-10. [DOI] [Full Text] |
| 19. | Chen Y, Lian G, Zhang Q, Zeng L, Qian C, Chen S, Huang K. Overexpression of Bmi-1 induces the malignant transformation of gastric epithelial cells in vitro. Oncol Res. 2013;21:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Farivar S, Zati Keikha R, Shiari R, Jadali F. Expression of bmi-1 in pediatric brain tumors as a new independent prognostic marker of patient survival. Biomed Res Int. 2013;2013:192548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Allott EH, Morine MJ, Lysaght J, McGarrigle SA, Donohoe CL, Reynolds JV, Roche HM, Pidgeon GP. Elevated Tumor Expression of PAI-1 and SNAI2 in Obese Esophageal Adenocarcinoma Patients and Impact on Prognosis. Clin Transl Gastroenterol. 2012;3:e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Fang H, Placencio VR, DeClerck YA. Protumorigenic activity of plasminogen activator inhibitor-1 through an antiapoptotic function. J Natl Cancer Inst. 2012;104:1470-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Sogutlu Sari E, Yazici A, Eser B, Erol MK, Kilic A, Ermis SS, Koytak A, Akşit H, Yakut T. The prevalence of 4G/5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in central serous chorioretinopathy and its association with plasma PAI-1 levels. Cutan Ocul Toxicol. 2014;Epub ahead of print. [PubMed] |
| 24. | Zhang J, Gu C, Lawrence DA, Cheung AK, Huang Y. A PAI-1 mutant retards diabetic nephropathy in db/db mice through protecting podocytes. Exp Physiol. 2014;Epub ahead of print. [PubMed] |
| 25. | Thapa B, Kim YH, Kwon HJ, Kim DS. The LRP1-independent mechanism of PAI-1-induced migration in CpG-ODN activated macrophages. Int J Biochem Cell Biol. 2014;49:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kim ER, Yang MH, Lim YJ, Lee JH, Chang DK, Kim YH, Son HJ, Kim JJ, Rhee JC, Kim JY. Association between Plasma Levels of Plasminogen Activator Inhibitor-1 and Colorectal Neoplasms. Gut Liver. 2013;7:519-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol Cancer Ther. 2013;12:2697-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |