Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5527
Revised: February 18, 2014
Accepted: March 6, 2014
Published online: May 14, 2014
Processing time: 137 Days and 17.7 Hours
AIM: To compare the esophagogastric junction (EGJ) areas observed in sedated and non-sedated patients during esophagogastroduodenoscopy (EGD).
METHODS: Data were collected prospectively from consecutive patients who underwent EGD for various reasons. The patients were divided into three groups according to the sedation used: propofol, midazolam, and control (no sedation). The EGJ was observed during both insertion and withdrawal of the endoscope. The extent of the EGJ territory observed was classified as excellent, good, fair, or poor. In addition, the time the EGJ was observed was estimated.
RESULTS: The study included 103 patients (50 males; mean age 58.44 ± 10.3 years). An excellent observation was achieved less often in the propofol and midazolam groups than in the controls (27.3%, 28.6% and 91.4%, respectively, P < 0.001). There was a significant difference in the time at which EGJ was observed among the groups (propofol 20.7 ± 11.7 s vs midazolam 16.3 ± 7.3 s vs control 11.6 ± 5.8 s, P < 0.001). Multivariate analysis showed that sedation use was the only independent risk factor for impaired EGJ evaluation (propofol, OR = 24.4, P < 0.001; midazolam, OR = 25.3, P < 0.001). Hiccoughing was more frequent in the midazolam group (propofol 9% vs midazolam 25.7% vs control 0%, P = 0.002), while hypoxia (SaO2 < 90%) tended to occur more often in the propofol group (propofol 6.1% vs midazolam 0% vs control 0%, P = 0.101).
CONCLUSION: Sedation during EGD has a negative effect on evaluation of the EGJ.
Core tip: The development of clinically important lesions located in the esophagogastric junction (EGJ), such as gastroesophageal reflux disease, Barrett’s esophagus, and esophageal adenocarcinoma are increasing. The vast majority of endoscopies are performed with sedation and this practice seems likely to continue. Therefore, it is clinically relevant to evaluate the impact of sedation on inspection of the EGJ during esophagogastroduodenoscopy (EGD). We found that sedation during EGD has a negative impact on EGJ evaluation. It remains to be seen whether this negative impact affects the detection rate of EGJ lesions.
- Citation: Kim ES, Lee HY, Lee YJ, Min BR, Choi JH, Park KS, Cho KB, Jang BK, Chung WJ, Hwang JS. Negative impact of sedation on esophagogastric junction evaluation during esophagogastroduodenoscopy. World J Gastroenterol 2014; 20(18): 5527-5532
- URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5527
Endoscopically, the esophagogastric junction (EGJ) is defined as where the distal ends of the longitudinal esophageal vessels meet the proximal ends of the longitudinal gastric mucosal folds[1]. The EGJ plays a crucial role as a gatekeeper against gastroesophageal reflux, and most of the clinically important lesions related to several diseases, including gastroesophageal reflux disease (GERD) and Barrett’s esophagus, are located in this area. GERD is a common chronic disorder prevalent in many countries[2,3], while Barrett’s esophagus is a premalignant condition arising from GERD due to direct caustic injury to the luminal surface of the squamous epithelium by the reflux of acidic gastric juice[4]. Esophagitis and Barrett’s esophagus are associated with an increased risk of developing esophageal adenocarcinoma with relative risks of 4.5 and 29.8, respectively[5]. Considering the high prevalence and increasing incidence of these diseases[6], the endoscopic detection and surveillance of these lesions at the EGJ is becoming increasingly important[7].
The vast majority of endoscopies are performed with the aid of intravenous sedation, and this practice seems likely to continue[8]. The purpose of sedation during esophagogastroduodenoscopy (EGD) is to increase the overall acceptance of the procedure by reducing patient anxiety and discomfort[9]. There are guidelines regarding the optimal use of sedatives during EGD[10-12]. However, no study has examined the effect of sedation on EGJ evaluation during EGD. Therefore, this study compared observation of the EGJ in subjects given different sedatives during EGD with unsedated subjects.
From May 2010 to February 2011, patients older than 18 years scheduled to undergo diagnostic EGD at Keimyung University Dongsan Hospital, Daegu, South Korea were enrolled in this study. Exclusion criteria included (1) an esophageal or gastric deformity due to surgery; (2) emergency endoscopy; (3) tumor at the EGJ; (4) pregnancy; (5) allergy to any drug; (6) poor general condition with an American Society of Anesthesiology classification ≥ 3; and (7) failure to give consent. Demographic and clinical variables were recorded, including body mass index, circumferences of the abdomen and hip, indication for EGD, and concomitant diseases. Patients were divided into three groups according to the sedation agent used; propofol, midazolam, and no sedation. The use and type of sedative agent were decided by the patients and written informed consent was obtained before the procedure. This study was approved by the Institutional Review Board of our hospital. The protocol was registered on the World Health Organization (WHO) International Clinical Trials Registry Platform (KCT0000060).
For the non-sedation group, local laryngeal anesthesia was achieved with 20 mL of lidocaine jelly (Sungkwang, Seoul, South Korea) before the procedure. Sedation was performed according to the guidelines of the American Society for Gastrointestinal Endoscopy[11,13]. For propofol (Jeil Pharm, Seoul, South Korea), an initial bolus of 40-60 mg was administered and additional 10-20-mg boluses could be given until conscious sedation was achieved. For midazolam (Bukwang, Seoul, South Korea), the initial dose was 2-3 mg and further 1-2-mg boluses were administered. Propofol and midazolam were administered by registered nurses under the supervision of an endoscopist. All medical professionals including endoscopists and nurses reviewed guidelines for the use of sedation for gastrointestinal (GI) endoscopy. Each endoscopy room was equipped with a full set of medications and a resuscitation kit. All sedated patients received supplemental oxygen (2 L/min via a nasal cannula), and the heart rate, oxygen saturation, and pulse rate were monitored using a pulse oximeter during the procedures.
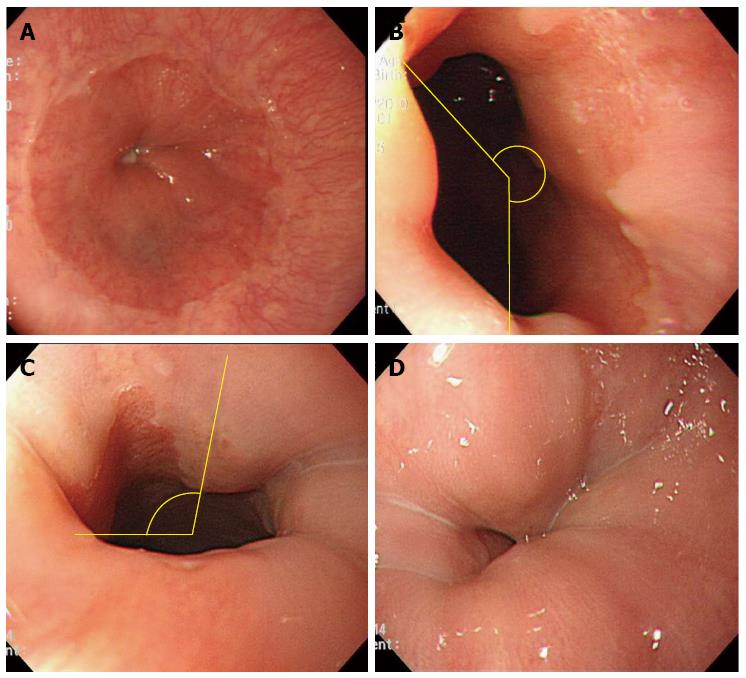
Two experienced endoscopists (Kim ES and Hwang JS) performed the EGD using single-channel upper gastrointestinal endoscopes (GIF Q260, Medical Systems, Tokyo, Japan). The EGJ was defined as the proximal margin of the gastric mucosal folds[14]. Patients were not asked regarding the respiration during EGD. The EGJ territory observed was assessed during both insertion and withdrawal of the endoscope and classified into four grades: excellent (100% of the EGJ), good (100% > EGJ ≥ 50%), fair (50% > EGJ), and poor (EGJ not visualized) (Figure 1). The observation time was defined as the time taken for the endoscopists to inspect the EGJ territory as clearly as possible. When the extent of the territory was not excellent (i.e., < 100%) during insertion of the endoscope, the endoscopists attempted to re-assess the EGJ territory during withdrawal with the purpose of achieving an excellent EGJ inspection.
The sample size was calculated on the assumption that the proportion of excellent observations in the non-sedation group would be 90%, while that in the sedation group would be 60%. This assumption was based on preliminary data from 20 subjects (10 from each group). With a two-tailed test of α = 0.05, 1 - β = 0.80, and 10% drop-out rate, 35 patients in each group were required.
The data management and statistical analysis were performed using SPSS (ver. 13.0 for Windows, Chicago, IL, United States). Differences in the categorical variables were analyzed using Fisher’s exact test or the χ2 test. The continuous data were expressed as the means ± SD and were compared using one-way analysis of variance (ANOVA). Multivariate analysis was performed using a logistic regression model to identify factors associated with a non-excellent observation after adjusting for other covariates which showed significant association (P < 0.1) on univariate analysis. P values < 0.05 were considered to indicate statistical significance.
Overall, 103 patients were included in the study (33 propofol, 35 midazolam, 35 no sedation). There were no significant differences in demographic and clinical characteristics among the groups (Table 1). The most common indication for performing EGD was screening, followed by epigastric symptoms, which did not differ significantly among the groups. With regard to adverse events during the procedures, hiccoughing was significantly more frequent in the midazolam group (P = 0.002). Hypoxia (SpO2 < 85%) was observed in only the propofol group (two cases, 6.1%, P = 0.101) (Table 2).
| No sedation | Midazolam | Propofol | P value | |
| (n = 35) | (n = 35) | (n = 33) | ||
| Age, yr | 61 ± 9.2 | 57.5 ± 10.5 | 56.5 ± 10.9 | 0.132 |
| Male | 17 (48.6) | 21 (60.0) | 12 (36.4) | 0.152 |
| Weight, kg | 60.6 ± 9.2 | 52.5 ± 8.9 | 63.5 ± 12.4 | 0.506 |
| Height, cm | 161.5 ± 6.7 | 163.2 ± 9.9 | 160.3 ± 8.1 | 0.340 |
| Abdomen, cm | 81.8 ± 10.0 | 85.8 ± 7.5 | 84.8 ± 10.6 | 0.194 |
| Hip, cm | 92.7 ± 5.8 | 94.4 ± 6.1 | 95.1 ± 6.3 | 0.260 |
| BMI | 23.2 ± 3.0 | 23.44 ± 2.6 | 24.5 ± 3.1 | 0.143 |
| Comorbidity | ||||
| Hypertension | 10 (28.6) | 7 (20.0) | 9 (27.3) | 0.717 |
| Diabetes mellitus | 2 (5.7) | 5 (14.3) | 8 (24.2) | 0.103 |
| Cardiovascular accident | 1 (2.9) | 1 (2.9) | 1 (3.0) | 1.000 |
| Ischemic heart disease | 2 (5.7) | 0 (0.0) | 2 (6.1) | 0.277 |
| Liver disease | 2 (5.7) | 1 (2.9) | 2 (6.1) | 0.867 |
| Malignancy | 1 (2.9) | 2 (5.7) | 0 (0.0) | 0.771 |
| No sedation | Midazolam | Propofol | P value | |
| (n = 35) | (n = 35) | (n = 33) | ||
| Indications | 0.084 | |||
| Screening | 28 (80.0) | 25 (71.4) | 17 (51.5) | |
| Epigastric discomfort/pain | 5 (14.3) | 6 (17.2) | 14 (42.6) | |
| Peptic ulcer disease follow-up | 1 (2.9) | 1 (2.9) | 2 (6.0) | |
| Others | 1 (2.9) | 3 (8.6) | 0 (0.0) | |
| Endoscopic findings | ||||
| Reflux esophagitis | 4 (11.4) | 2 (5.7) | 2 (6.1) | 0.726 |
| Hiatal hernia | 0 (0.0) | 3 (8.6) | 0 (0.0) | 0.105 |
| Peptic ulcer disease | 3 (8.6) | 2 (5.7) | 2 (6.1) | 1.000 |
| Gastric polyp | 2 (5.7) | 2 (5.7) | 1 (3.0) | 1.000 |
| Neoplasm | 0 (0.0) | 2 (5.7) | 0 (0.0) | 0.327 |
| Adverse event | ||||
| Hiccoughing | 0 (0.0) | 9 (25.7) | 3 (90.0) | 0.002 |
| Hypoxia | 0 (0.0) | 0 (0.0) | 2 (6.1) | 0.101 |
There was a significant (P < 0.001) difference in observation time of the EGJ among the groups. Observation time was longest in the propofol group (20.7 ± 11.7 s), followed by the midazolam (16.3 ± 7.3 s) and no sedation (11.6 ± 5.8 s) groups (Table 3). Regarding the extent of the EGJ territory observed during insertion, an excellent rating was less frequent in the sedation groups compared with the non-sedation group (propofol 27.3%, midazolam 28.6%, no sedation 91.4%, P < 0.001). We then reassessed the extent of EGJ territory observed during withdrawal of the endoscope in 52 patients with non-excellent observations during insertion. In approximately one quarter of the patients in each group, the observation territory improved during withdrawal compared with during insertion (Table 4).
| No sedation | Midazolam | Propofol | P value | |
| Observation time | 11.6 ± 5.8 | 16.3 ± 7.3 | 20.7 ± 11.7 | < 0.001 |
| Observed EGJ territory | < 0.001 | |||
| Excellent | 32 (91.4) | 10 (28.6) | 9 (27.3) | |
| Good | 1 (2.9) | 13 (37.3) | 12 (36.4) | |
| Fair | 1 (2.9) | 11 (31.4) | 11 (33.3) | |
| Poor | 1 (2.9) | 1 (2.9) | 1 (3.0) | |
| No sedation | Midazolam | Propofol | P = 0.291 | |
| (n = 3) | (n = 25) | (n = 24) | ||
| Extended | 1 (33.3) | 5 (20) | 6 (25) | |
| Same | 2 (66.7) | 10 (40) | 10 (41.7) | |
| Reduced | 0 | 10 (40) | 8 (33.3) |
We subjected the factors associated with a non-excellent EGJ observation territory during insertion to univariate analysis (Table 5). All patients were divided into two groups: excellent (n = 51) and non-excellent (n = 52) grades. A larger proportion of patients with sedation had non-excellent grades compared with those without sedation (propofol 43.2%, midazolam 48.1%, no sedation 5.8%, P < 0.001). In addition, the non-excellent group tended to have more patients with comorbid diabetes mellitus (P = 0.057) and a larger hip circumference (P = 0.073). However, in the multivariate analysis, the use of midazolam (OR = 25.32, 95%CI: 6.22-103.09) and propofol (OR = 24.42, 95%CI: 5.87-101.58, P < 0.001) was the only independent risk factor for incomplete inspection of the EGJ during EGD (Table 6).
| Excellent | Non-excellent | P value | |
| (n = 51) | (n = 52) | ||
| Age, yr | 59.39 ± 9.5 | 57.5 ± 11.1 | 0.355 |
| Male | 11 (21.6) | 23 (44.2) | 0.377 |
| Weight, kg | 61.29 ± 8.8 | 63.05 ± 11.5 | 0.384 |
| Height, cm | 161.29 ± 7.7 | 161.37 ± 9.6 | 0.706 |
| BMI | 23.33 ± 2.8 | 24.10 ± 3.1 | 0.185 |
| Abdomen, cm | 82.97 ± 9.5 | 85.27 ± 9.4 | 0.221 |
| Hip, cm | 92.98 ± 5.5 | 95.14 ± 6.5 | 0.073 |
| HW ratio | 0.89 ± 0.06 | 0.89 ± 0.05 | 0.704 |
| Comorbidity | |||
| Hypertension | 11 (21.6) | 15 (28.8) | 0.395 |
| Diabetes mellitus | 4 (7.8) | 11 (21.2) | 0.057 |
| Cardiovascular accident | 2 (3.9) | 1 (1.9) | 0.548 |
| Ischemic heart disease | 2 (3.9) | 3 (5.8) | 0.664 |
| Liver disease | 2 (3.9) | 3 (5.8) | 0.664 |
| Sedation | < 0.001 | ||
| No | 32 (62.7) | 3 (5.8) | |
| Midazolam | 10 (19.6) | 25 (48.1) | |
| Propofol | 9 (17.6) | 24 (43.2) |
| Variables | OR | 95%CI | P value |
| Hip | 1.044 | 0.961-1.133 | 0.307 |
| Diabetes mellitus | 1.984 | 0.472-8.337 | 0.350 |
| Sedation | |||
| No | 1.000 | ||
| Midazolam | 25.316 | 6.217-103.088 | < 0.001 |
| Propofol | 24.417 | 5.869-101.575 | < 0.001 |
This study demonstrated that sedation with midazolam or propofol made EGJ inspection more difficult, with longer examination times and poor visualization of the Z-line, indicating that sedation has a negative impact on EGJ evaluation during EGD. Although the mechanism of this is unclear, the inhibitory effect of sedation on the diaphragm is a possible explanation. The EGJ is supported by two sphincters: the smooth muscle lower esophageal sphincter (LES) and the skeletal muscle crural diaphragm[15]. Voluntary contraction of the diaphragm during inspiration causes an increase in the EGJ pressure, and this pinchcock-like action of the crural part of the diaphragm is easily observed during endoscopy[16]. It is this action of the diaphragm that enables an excellent view of the EGJ or Z-line during endoscopy by contracting the skeletal muscles during deep inspiration, which lowers the crural part of the diaphragm enabling a 100% Z-line view. The respiratory depression and muscle relaxant effects of propofol and midazolam might inhibit the pinchcock effect of the diaphragm, resulting in a non-excellent view of the EGJ during endoscopy[17]. Numerous studies have reported that anesthetic agents, including propofol and midazolam, decrease the LES pressure and cause more acid reflux, supporting the inhibitory effect of sedation on diaphragm contractility[18-20]. However, few studies have directly evaluated the effect of sedation on EGJ inspection during endoscopy.
An excellent view of the EGJ during insertion of the endoscope was possible only in 28.6% of the midazolam and 27.3% of the propofol groups, compared with 91.4% of the no-sedation group. Sedation was the only independent risk factor for a non-excellent view of the EGJ. This is clinically relevant as the importance of a thorough inspection of the EGJ has been highlighted recently. As the prevalence of diseases located at the EGJ, such as GERD, Barrett’s esophagus, and esophageal adenocarcinoma, is increasing[3,21,22], early diagnosis and surveillance using endoscopy are key to establishing an optimal management strategy for esophageal diseases. The guidelines of the American College of Gastroenterology recommend that every patient with gastroesophageal reflux symptoms be referred for Barrett’s esophagus screening endoscopy at least once in a lifetime[23]. Therefore, considerable attention has been paid to the EGJ using sophisticated endoscopic techniques, including enhanced magnification endoscopy and computed virtual chromoendoscopy[24,25]. Further study is needed to confirm whether the negative effect of sedation on EGJ evaluation reduces the accuracy of detecting esophageal diseases in clinical practice.
Of the 52 patients who had non-excellent views of the EGJ during insertion, we were able to improve the territory observed in almost one quarter (20% in midazolam, 25% in propofol, and 33.3% in no sedation) (Table 4). Therefore, an effort should be made to improve EGJ inspection during the withdrawal phase in the case of poor visualization of the EGJ during insertion of the endoscope.
There was no significant difference in EGJ evaluation during EGD between the propofol and midazolam groups, although propofol appeared to have a more negative impact on EGJ evaluation than midazolam (observation time 20.7 ± 11.7 s vs 16.3 ± 7.3 s; excellent EGJ territory 27.3% vs 28.6%, respectively). In accordance with our data, Meining et al[26] reported no difference in the assessment of the Z-line between propofol and midazolam, while propofol sedation resulted in significantly better scores for other parameters related to the quality of EGD than did midazolam. However, a larger sample size might be able to identify significant differences between these two agents in EGJ evaluation during EGD.
As the aim of this study was to evaluate EGJ area, the precise definition of EGJ is crucial. Indeed, it is quite difficult to endoscopically define EGJ because of the lack of authoritative guidance in the literature on how to locate the EGJ[14]. Furthermore, EGJ definition differs between Asian and Western endoscopists; the distal end of the lower-esophageal palisade vessels was used to define the EGJ in Japan[27], while the landmark was the upper end of the gastric folds in Western countries[14]. As the upper end of the gastric folds has been recognized as a more suitable landmark than palisade vessels for EGJ in the literature[14,28], we decided to use this definition in the study.
The main limitation of this study was that the design was neither randomized nor used a blinded approach to compare the results of each group. Therefore, the endoscopists might have had some preconceptions regarding the effect of sedation on endoscopy. In Korea, which has a high prevalence of gastric cancer, subjects over 40 years of age can have EGD screening every other year with the support of government as the national strategy for cancer control since 1999, which means most participants had previous experience of EGD. Therefore, patients selected sedation or no sedation based on their experience and it is difficult for us to allocate them to the sedation or non-sedation group against their will. Second, because the data were from a single center, it may be difficult to ensure consistency.
In conclusion, sedation during EGD has a negative impact on EGJ evaluation. Given that the goal of sedation is to achieve a balance between the benefits of sedation vs avoidable risks, endoscopists must bear in mind the effect of sedation when performing EGD in the presence of suspicious EGJ lesions, which require precise inspection of the Z-line area. However, these results should be confirmed by a randomized, blinded study. In addition, it remains to be seen whether this negative impact affects the detection rate of EGJ lesions.
Important lesions are often located near the esophagogastric junction (EGJ). The vast majority of endoscopies are performed with the aid of intravenous sedation, and this practice seems likely to continue.
Several studies have reported that anesthetic agents, including propofol and midazolam, decrease the lower esophageal sphincter pressure and cause more acid reflux, supporting the inhibitory effect of sedation on diaphragm contractility. However, the effect of sedation on evaluation of the EGJ during esophagogastroduodenoscopy (EGD) has not been elucidated.
This study demonstrated that sedation with midazolam or propofol made EGJ inspection more difficult, with longer examination times and poor visualization of the Z-line, indicating that sedation has a negative impact on EGJ evaluation during EGD.
Given that the goal of sedation is to achieve a balance between the benefits of sedation vs avoidable risks, endoscopists must bear in mind the effect of sedation when performing EGD in the presence of suspicious EGJ lesions, which require precise inspection of the Z-line area. It remains to be seen whether this negative impact affects the detection rate of EGJ lesions.
EGJ is the area where the distal ends of the longitudinal esophageal vessels meet the proximal ends of the longitudinal gastric mucosal folds. The observation time is defined as the time taken for the endoscopist to inspect the EGJ territory as clearly as possible.
This article presents interesting data concerning a very current discussion about the optimal sedation effect in endoscopies. Although non-randomization with non-blinded design is the main limitation, the concept of the study is quite important.
P- Reviewers: Gaertner W, Lee CL, Lee YY S- Editor: Gou SX L- Editor: Webster JR E- Editor: Ma S
| 1. | McClave SA, Boyce HW, Gottfried MR. Early diagnosis of columnar-lined esophagus: a new endoscopic diagnostic criterion. Gastrointest Endosc. 1987;33:413-416. [PubMed] |
| 2. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-87.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1467] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 3. | Wu JC. Gastroesophageal reflux disease: an Asian perspective. J Gastroenterol Hepatol. 2008;23:1785-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Vaezi MF, Richter JE. Role of acid and duodenogastroesophageal reflux in gastroesophageal reflux disease. Gastroenterology. 1996;111:1192-1199. [PubMed] |
| 5. | Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Estores D, Velanovich V. Barrett esophagus: epidemiology, pathogenesis, diagnosis, and management. Curr Probl Surg. 2013;50:192-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Louis H. Reflux disease and Barrett’s esophagus. Endoscopy. 2007;39:969-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Thomson A, Andrew G, Jones DB. Optimal sedation for gastrointestinal endoscopy: review and recommendations. J Gastroenterol Hepatol. 2010;25:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Abraham NS, Fallone CA, Mayrand S, Huang J, Wieczorek P, Barkun AN. Sedation versus no sedation in the performance of diagnostic upper gastrointestinal endoscopy: a Canadian randomized controlled cost-outcome study. Am J Gastroenterol. 2004;99:1692-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Faigel DO, Baron TH, Goldstein JL, Hirota WK, Jacobson BC, Johanson JF, Leighton JA, Mallery JS, Peterson KA, Waring JP. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc. 2002;56:613-617. [PubMed] |
| 11. | Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317-322. [PubMed] |
| 12. | Regula J, Sokol-Kobielska E. Sedation in endoscopy: when and how. Best Pract Res Clin Gastroenterol. 2008;22:945-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Training Committee. Training guideline for use of propofol in gastrointestinal endoscopy. Gastrointest Endosc. 2004;60:167-172. [PubMed] |
| 14. | Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & amp; M criteria. Gastroenterology. 2006;131:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 716] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 15. | Mittal RK, Shaffer HA, Parollisi S, Baggett L. Influence of breathing pattern on the esophagogastric junction pressure and esophageal transit. Am J Physiol. 1995;269:G577-G583. [PubMed] |
| 16. | Mittal RK, Rochester DF, McCallum RW. Effect of the diaphragmatic contraction on lower oesophageal sphincter pressure in man. Gut. 1987;28:1564-1568. [PubMed] |
| 17. | Fujii Y, Hoshi T, Takahashi S, Toyooka H. Propofol decreases diaphragmatic contractility in dogs. Anesth Analg. 1999;89:1557-1560. [PubMed] |
| 18. | Bechtold ML, Holly JS, Thaler K, Marshall JB. Bravo (wireless) ambulatory esophageal pH monitoring: how do day 1 and day 2 results compare? World J Gastroenterol. 2007;13:4091-4095. [PubMed] |
| 19. | Fung KP, Math MV, Ho CO, Yap KM. Midazolam as a sedative in esophageal manometry: a study of the effect on esophageal motility. J Pediatr Gastroenterol Nutr. 1992;15:85-88. [PubMed] |
| 20. | Turan A, Wo J, Kasuya Y, Govinda R, Akça O, Dalton JE, Sessler DI, Rauch S. Effects of dexmedetomidine and propofol on lower esophageal sphincter and gastroesophageal pressure gradient in healthy volunteers. Anesthesiology. 2010;112:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 21. | Coleman HG, Bhat S, Murray LJ, McManus D, Gavin AT, Johnston BT. Increasing incidence of Barrett’s oesophagus: a population-based study. Eur J Epidemiol. 2011;26:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103:2694-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 23. | Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 504] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 24. | Curvers WL, Alvarez Herrero L, Wallace MB, Wong Kee Song LM, Ragunath K, Wolfsen HC, Prasad GA, Wang KK, Subramanian V, Weusten BL. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett’s esophagus. Gastroenterology. 2010;139:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Fortun PJ, Anagnostopoulos GK, Kaye P, James M, Foley S, Samuel S, Shonde A, Badreldin R, Campbell E, Hawkey CJ. Acetic acid-enhanced magnification endoscopy in the diagnosis of specialized intestinal metaplasia, dysplasia and early cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2006;23:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Meining A, Semmler V, Kassem AM, Sander R, Frankenberger U, Burzin M, Reichenberger J, Bajbouj M, Prinz C, Schmid RM. The effect of sedation on the quality of upper gastrointestinal endoscopy: an investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy. 2007;39:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Eda A, Osawa H, Satoh K, Yanaka I, Kihira K, Ishino Y, Mutoh H, Sugano K. Aberrant expression of CDX2 in Barrett’s epithelium and inflammatory esophageal mucosa. J Gastroenterol. 2003;38:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Amano Y, Ishimura N, Furuta K, Takahashi Y, Chinuki D, Mishima Y, Moriyama I, Fukuhara H, Ishihara S, Adachi K. Which landmark results in a more consistent diagnosis of Barrett’s esophagus, the gastric folds or the palisade vessels? Gastrointest Endosc. 2006;64:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |