Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.5153
Revised: December 5, 2013
Accepted: January 14, 2014
Published online: May 7, 2014
Processing time: 212 Days and 19.5 Hours
Plexiform neurofibroma (PN) of the digestive tract is very rare and usually part of the generalized syndrome of neurofibromatosis type 1 (von Recklinghausen disease). Solitary PN of the stomach is extremely rare and has not been reported in the literatures. Here we present a case of solitary PN of the stomach, which was not associated with von Recklinghausen disease. A 38-year-old male presented abdominal pain and distention for 7 d. The patient underwent endoscopy of the upper gastrointestinal tract, which revealed a 3.5 cm protruding and cauliflower-shaped mass with a shallow 1 cm central ulcer in the greater curvature of the stomach. The lesion was removed by laparoscopic surgery. Histological examination demonstrated characteristic histological findings of spindle-shaped cells. Immunohistochemical analysis showed that the tumor cells were positive for S-100 protein, but negative for CD34, KI-67, CD117, and actin. Based on histological findings, gastrointestinal stromal tumor could be excluded, and thus the case was confirmed as PN. We described the clinical features, physical examination, endoscopic findings, and histopathological examination of this case.
Core tip: Solitary plexiform neurofibroma (PN) is extremely rare. We report a case of solitary PN of the stomach in a 38-year-old male who underwent laparoscopic surgery. The case was not associated with von Recklinghausen disease, and the patient was in good condition 6 mo after surgery, with no tumor recurrence. To our knowledge, this is the first reported case of isolated stomach PN undergoing laparoscopic surgery. Endoscopic treatment is technically feasible and may be considered as the procedure of choice for solitary PN treatment. A long-term follow-up endoscopy of the upper gastrointestinal tract is greatly needed.
- Citation: Shi L, Liu FJ, Jia QH, Guan H, Lu ZJ. Solitary plexiform neurofibroma of the stomach: A case report. World J Gastroenterol 2014; 20(17): 5153-5156
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/5153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.5153
Plexiform neurofibroma (PN) is a rare benign peripheral nerve sheath tumor mainly associated with von Recklinghausen disease. PN is rarely presented as a sporadic lesion in a patient without features of generalized neurofibromatosis[1]. Up until now, there were no reports of solitary PN of the stomach without evidence of neurofibromatosis type 1 (NF1) in the literature. Here we describe a rare case of a solitary PN of the stomach in a patient without features of NF1.
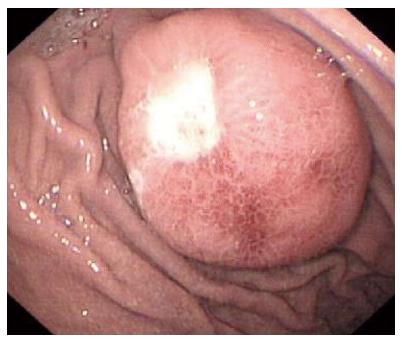
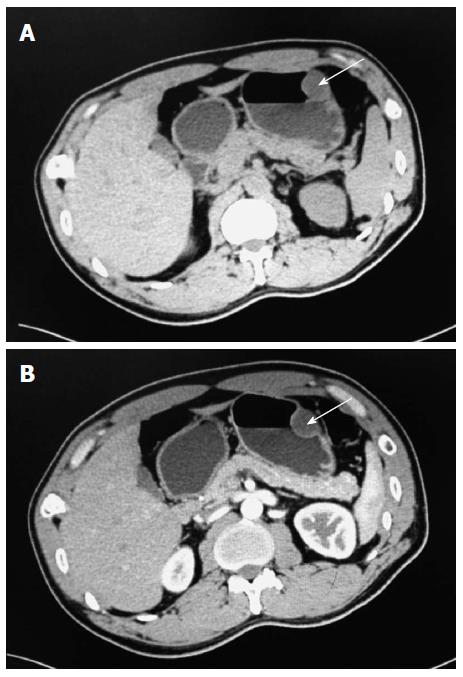
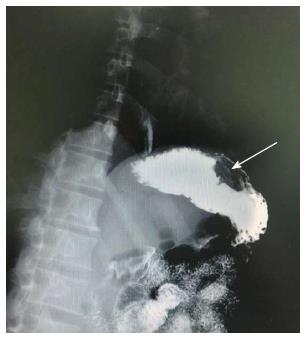
A 38-year-old man was admitted to our department due to abdominal pain and distention for 7 d. Informed consent was obtained from the patient. No other signs of von Recklinghausen disease were found in this patient or his family. Physical examination revealed moderate tenderness on palpation of the upper abdominal region. Physical examination of the lungs, heart, and other systems revealed no abnormal features. The patient had no significant medical background or family history. No abnormal findings were revealed by laboratory tests. Chest X-ray, electrocardiography, and abdominal sonogram showed no abnormal features. For diagnostic purposes, an endoscopy of the upper gastrointestinal tract was performed, which revealed a 3.5 cm protruding and cauliflower-shaped mass with a shallow 1 cm central ulcer in the greater curvature of the stomach (Figure 1). Further endoscopic examination revealed no lesions in the small bowel or colon. Computed tomography (CT) imaging with and without contrast medium revealed a soft tissue mass-like lesion about 2.5 cm × 3.5 cm in the greater curvature of the stomach (Figure 2). Upper gastrointestinal radiography showed a mass in the greater curvature of the stomach (Figure 3). The patient underwent an endoscopic submucosal dissection for the en-bloc resection of the lesion. Injection of saline into the submucosa during gastroscopy did not completely elevate the tumor. Therefore, local gastroscopic resection was not indicated. Laparoscopic surgery was successfully performed.
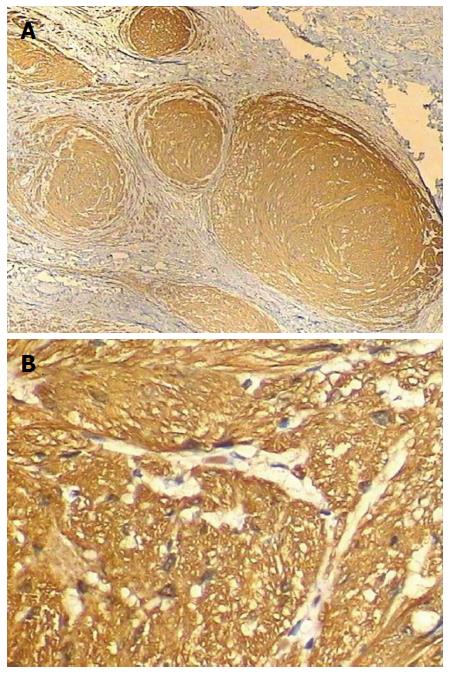
The tumor was diagnosed as sporadic PN, which rarely occurs in the digestive tract. The diagnosis was confirmed by laparoscopic surgery with complete resection. Histological examination demonstrated characteristic histological findings of spindle-shaped cells. Immunohistochemical analysis showed that the tumor cells were positive for S-100 protein (Figure 4), but negative for CD34, KI-67, CD117, and actin. Gastrointestinal stromal tumor (GIST) could thus be excluded in our case and confirmed as PN. Furthermore, we performed PCR and sequencing on a blood sample from the patient and found no any mutations in the NF1 gene (data not shown). We performed upper gastrointestinal endoscopy six months post-operatively for follow-up, and found no evidence of recurrence.
Neurofibromatosis is a benign peripheral nerve sheath tumor. It is a neurocutaneous disorder with two clinical forms: (1) NF1, described by von Recklinghausen; and (2) central type 2, described as a central neurofibromatosis that mainly affects the central nervous system[2]. NF1 is the most common type of neurofibromatosis and accounts for about 90% of all cases. An autosomal-dominant mutation of the neurofibromin (tumor suppressor) gene on chromosome 17 causes tumors of the peripheral nerves known as neurofibromas. NF2 is an autosomal-dominant disorder that induces tumor development. NF-1 is a systemic disorder that may affect any organ in the body, with clinical presentation depending upon the body system involved. Diagnosis of NF1 is based upon a series of clinical criteria as defined by the National Institutes of Health Consensus Statement[3]: (1) at least six so-called “café-au-lait” spots in post-pubertal patients; (2) at least two neurofibromas of any type or one PN; (3) axillary or groin freckling; (4) at least two so-called “Lisch nodules”; (5) optic glioma; (6) distinctive osseous bony dysplasia (such as thinning of the long bone cortex or sphenoid wing dysplasia; and (7) a close family member with NF1. The patient should have at least two of the previous criteria to be considered as NF1.
PN has a network-like growth involving multiple fascicles of a nerve, and may include multiple branches of a large nerve trunk. PN is a rare benign tumor developing from Schwann cells of the peripheral nervous system, often associated with NF1. Isolated PN in other locations without NF1 features has been very rarely encountered in clinical practice. The few cases have been reported so far have been in the oral cavity[4], submandibular salivary gland[1], lesser omentum[5], penis[6], cheek mucosa[7], and intraparotid facial nerve[8]. The specific cause and development of solitary PN are not known, but the possibility of mosaicism of NF-1 or a related gene has been considered. It is supposed that solitary PN may be caused by a sporadic localized mutation of the NF1 tumor suppressor gene[9]. However, in this case, we found no direct evidence of NF1 mutation.
To our knowledge, this is the first report on the rare case of PN in the stomach. The 38-year-old male patient was diagnosed as PN based on clinical appearance, physical examination, and histological features. Our case should be distinguished from GIST, which usually occurs in the small intestine as multiple and clinically indolent tumors. GIST is consistently CD117 (KIT)-positive, generally CD34-positive, occasionally actin-positive, and rarely S-100-positive[10]. According to immunohistochemical analysis, the tumor from this case was positive for S-100 protein, but negative for CD34, KI-67, CD117, and actin. Thus, GIST could be excluded in this case.
Although the cause and development of this rare case remain speculative, due to the malignancy potential, surgical resection, such as open surgery, combined endoscopic/laparoscopic intragastric resection, and laparoscopically gastroesophageal resection, is advocated[11]. The pre-operative diagnosis of PN is often difficult. As a result, PN usually needs to be resected surgically. The application of fine needle aspiration (FNA), including computed tomography guided, transabdominal ultrasonography guided, and endoscopic ultrasonography guided FNA, facilitates the diagnosis of GIST[12]. However, FNA evaluation should not replace subsequent examination of resected specimens[13].
With the wide application of novel techniques, including endoscopic mucosal resection and endoscopic submucosal dissection, endoscopic resection has recently been performed as a curative treatment for adenoma and early colorectal cancer[14]. Endoscopic resection provides a better quality of life for the patient than conventional open surgery, as it is minimally invasive, brings complete resection, and supports detailed histopathologic evaluation of the specimens.
Although PN is generally benign, prompt resection is recommended because of a risk of malignant transformation. We advocated that endoscopic treatment is technically feasible and may be considered as the procedure of choice for solitary PN. Furthermore, a long-term follow-up endoscopy of the upper gastrointestinal tract is greatly needed.
A 38-year-old male was admitted to their department on an emergency basis because of abdominal pain and distention for 7 d.
Physical examination revealed moderate tenderness on palpation of the upper abdominal region.
Immunohistochemical analysis showed that this case could be excluded as gastrointestinal stromal tumor.
No abnormal findings were revealed by routine laboratory tests.
Computed tomography and upper gastrointestinal radiography showed a mass in the greater curvature of the stomach.
Histological examination demonstrated characteristic histological findings of spindle-shaped cells.
Laparoscopic surgery was successfully performed.
Isolated plexiform neurofibroma (PN) in other body organs without features of NF1 has been very rarely encountered in clinical practice. The few cases of isolated PN that have been reported so far have been located in the oral cavity, submandibular salivary gland, lesser omentum, penis, cheek mucosa, and intraparotid facial nerve.
Plexiform neurofibroma is a rare benign peripheral nerve sheath tumor mostly associated with von Recklinghausen disease and rarely presenting as a sporadic lesion in a patient without features of generalized neurofibromatosis.
Immunohistochemical analysis should be performed to distinguish plexiform neurofibroma from gastrointestinal stromal tumors.
This is the first report on a rare case of plexiform neurofibroma in the stomach. The authors described the clinical features, physical examination, endoscopic findings, and histopathological examination of this case. However, further information on the expression of the NF1 gene and histological diagnosis will help us further understand the pathogenesis of this case.
P- Reviewers: Fang BL, Wagner CA S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Fu CY, Lin CH, Peng YJ, Yu TC, Lu TC, Chen TW. Acute abdominal pain caused by spontaneous hemorrhagic infarction of a solitary plexiform neurofibroma of lesser omentum. Z Gastroenterol. 2008;46:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Zwane NP, Noffke CE, Raubenheimer EJ. Solitary oral plexiform neurofibroma: review of literature and report of a case. Oral Oncol. 2011;47:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Tsutsumi T, Oku T, Komatsuzaki A. Solitary plexiform neurofibroma of the submandibular salivary gland. J Laryngol Otol. 1996;110:1173-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Garaffa G, Bettocchi C, Christopher N, Ralph D. Plexiform neurofibroma of the penis associated with erectile dysfunction due to arterial steeling. J Sex Med. 2008;5:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gómez-Oliveira G, Fernández-Alba Luengo J, Martín-Sastre R, Patiño-Seijas B, López-Cedrún-Cembranos JL. Plexiform neurofibroma of the cheek mucosa. A case report. Med Oral. 2004;9:263-267. [PubMed] |
| 7. | Souaid JP, Nguyen VH, Zeitouni AG, Manoukian J. Intraparotid facial nerve solitary plexiform neurofibroma: a first paediatric case report. Int J Pediatr Otorhinolaryngol. 2003;67:1113-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Wimmer K, Eckart M, Meyer-Puttlitz B, Fonatsch C, Pietsch T. Mutational and expression analysis of the NF1 gene argues against a role as tumor suppressor in sporadic pilocytic astrocytomas. J Neuropathol Exp Neurol. 2002;61:896-902. [PubMed] |
| 9. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] |
| 10. | Ke ZW, Chen DL, Cai JL, Zheng CZ. Extraluminal laparoscopic wedge-resection of submucosal tumors on the posterior wall of the gastric fundus close to the esophagocardiac junction. J Laparoendosc Adv Surg Tech A. 2009;19:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Arantes V, Logroño R, Faruqi S, Ahmed I, Waxman I, Bhutani MS. Endoscopic sonographically guided fine-needle aspiration yield in submucosal tumors of the gastrointestinal tract. J Ultrasound Med. 2004;23:1141-1150. [PubMed] |
| 12. | Logrono R, Bhanot P, Chaya C, Cao L, Waxman I, Bhutani MS. Imaging, morphologic, and immunohistochemical correlation in gastrointestinal stromal tumors. Cancer. 2006;108:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Hong SJ, Jang JY, Kim SE, Seol SY. Outcome after endoscopic submucosal dissection for early gastric cancer in Korea. World J Gastroenterol. 2011;17:3591-3595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [PubMed] |