Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.5141
Revised: January 10, 2014
Accepted: March 7, 2014
Published online: May 7, 2014
Processing time: 206 Days and 18.9 Hours
Multiple lymphomatous polyposis (MLP) is an uncommon type of gastrointestinal lymphoma characterized by the presence of multiple polyps along the gastrointestinal tract. Most of this entity is in fact considered the counterpart of gastrointestinal tract involvement for mantle cell lymphoma (MCL). To our knowledge, there have been no reports on [fluorine-18]-fluorodeoxy-glucose (18F-FDG)-positron emission tomography (PET)/computed tomography (CT) imaging for gastrointestinal MCL with MLP. We present the results of 18F-FDG PET/CT imaging in a patient with gastrointestinal tract involvement of MCL showing continuous MLP from the stomach to the rectum and intestinal intussusception. FDG-PET/CT findings were false negative in typical MLP spreading widely over the gastrointestinal tract, but uptake was noted in large lesions with deep infiltration considered atypical as MLP. On FDG-PET/CT imaging, the Ki-67 proliferative index, which is a cell proliferation marker, showed neither correlation with the presence of uptake nor the maximum standardized uptake value.
Core tip: To the present, there have been no reports on [fluorine-18]-fluorodeoxy-glucose (18F-FDG)-positron emission tomography (PET)/computed tomography (CT) imaging of gastrointestinal mantle cell lymphoma (MCL) with multiple lymphomatous polyposis (MLP). In this report, we present the results of 18F-FDG PET/CT imaging in such a MCL patient showing continuous MLP from the stomach to the rectum. We also compared FDG-PET/CT imaging with the Ki-67 proliferative index, an index of cell proliferation, and found that their relationship was inconsistent. The findings of FDG-PET/CT were false negative in typical MLP, but uptake was noted in larger lesions with deep infiltration considered atypical MLP, regardless of the Ki-67 proliferative index.
- Citation: Saito M, Miyazaki M, Tanino M, Tanaka S, Miyashita K, Izumiyama K, Mori A, Irie T, Tanaka M, Morioka M, Tsukamoto E. 18F-FDG PET/CT imaging for a gastrointestinal mantle cell lymphoma with multiple lymphomatous polyposis. World J Gastroenterol 2014; 20(17): 5141-5146
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/5141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.5141
Multiple lymphomatous polyposis (MLP) is a term applied to a specific lymphoma characterized by a distinctive pattern of gastrointestinal tract involvement in which long segments of the intestine are superficially infiltrated by multiple white nodular or polypoid tumors, ranging in size from 0.2 to 2 cm[1,2]. In most of MLP, lymphoma cells originate in the mantle zone of the lymphoid follicle, so this disease is actually considered a subtype of malignant lymphoma called mantle cell lymphoma (MCL)[3,4]. Since Cornes’ report in 1961, various documented cases of MLP have been reported in the literature[5,6]. However, to the present, there have been no reports on [fluorine-18]-fluorodeoxy-glucose (18F-FDG)-positron emission tomography (PET)/computed tomography (CT) of gastrointestinal MCL cases with MLP. We encountered a rare case of gastrointestinal MCL showing continuous MLP from the stomach to the rectum and intestinal intussusception, and present the results of 18F-FDG PET/CT imaging in this report. Additionally, we closely evaluated its relationship with FDG-PET/CT imaging and the Ki-67 proliferative index, a cell proliferation marker. This is the world’s first report studying the causes of the low sensitivity of FDG-PET/CT for the detection of MCL with gastrointestinal tract involvement.
A 49-years-old Japanese female who had noted lumps in the cervical and inguinal regions for about half a year to 1 year developed right abdominal discomfort and constipation for 2 wk. She was suspected to have malignant lymphoma on contrast-enhanced CT and was referred to our hospital. Examinations on admission revealed mild anemia (hemoglobin: 10.9 g/dL), hypoproteinemia (total protein: 5.4 g/dL), and hypoalbuminemia (albumin: 3.1 g/dL), and the soluble interleukin 2 receptor level was elevated at 4460 U/mL (normal range: 124-466 U/mL). Inguinal lymph node biopsy was performed, immunophenotype with double-color flow cytometry showed strong positivity (80%-90% ≤) for CD5, CD19, and CD20, and fluorescence in situ hybridization showed IgH/CCND1 (bcl1) in 97.0%. As below mentioned, a histopathological diagnosis of MCL was made. She received hyper-CVAD chemotherapy (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone combined with high dose cytarabine and methotrexate) with rituximab for 8 courses. Lymphoma lesion in the duodenal bulb was perforated after chemotherapy for 3 courses, however, a favorable response to hyper-CVAD chemotherapy was obtained. She is alive at present for more than 2.6 years after initial therapy, with no recurrence.
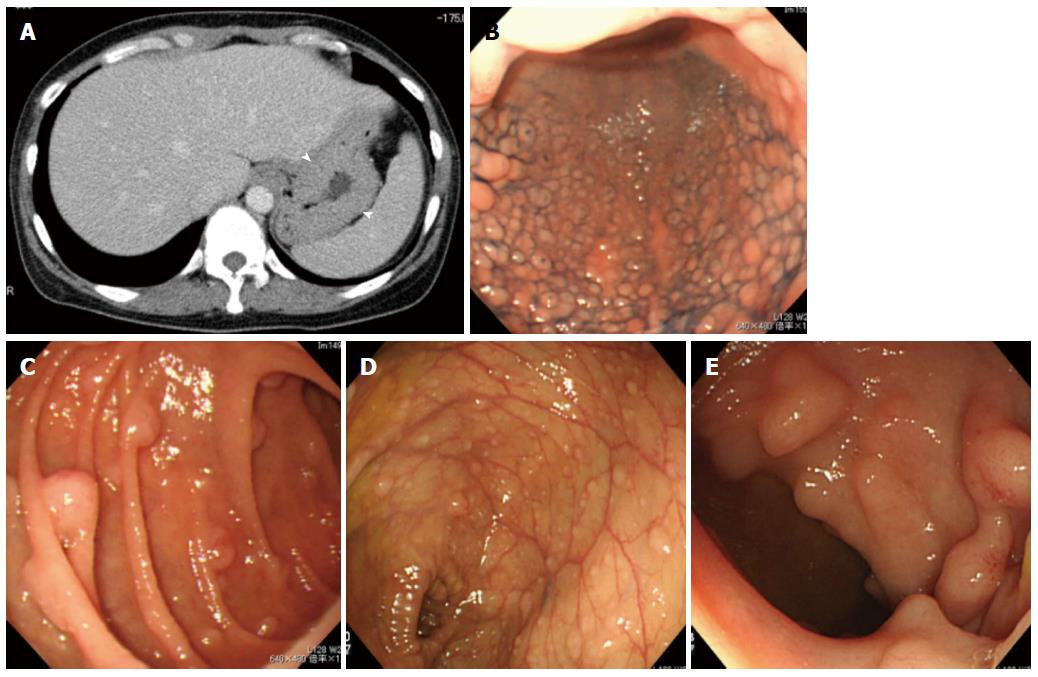
CT showed enlargement of systemic lymph nodes, and also revealed tumorous lesions in the gastrointestinal tract, as well as clear thickening of the gastric wall (Figure 1A).
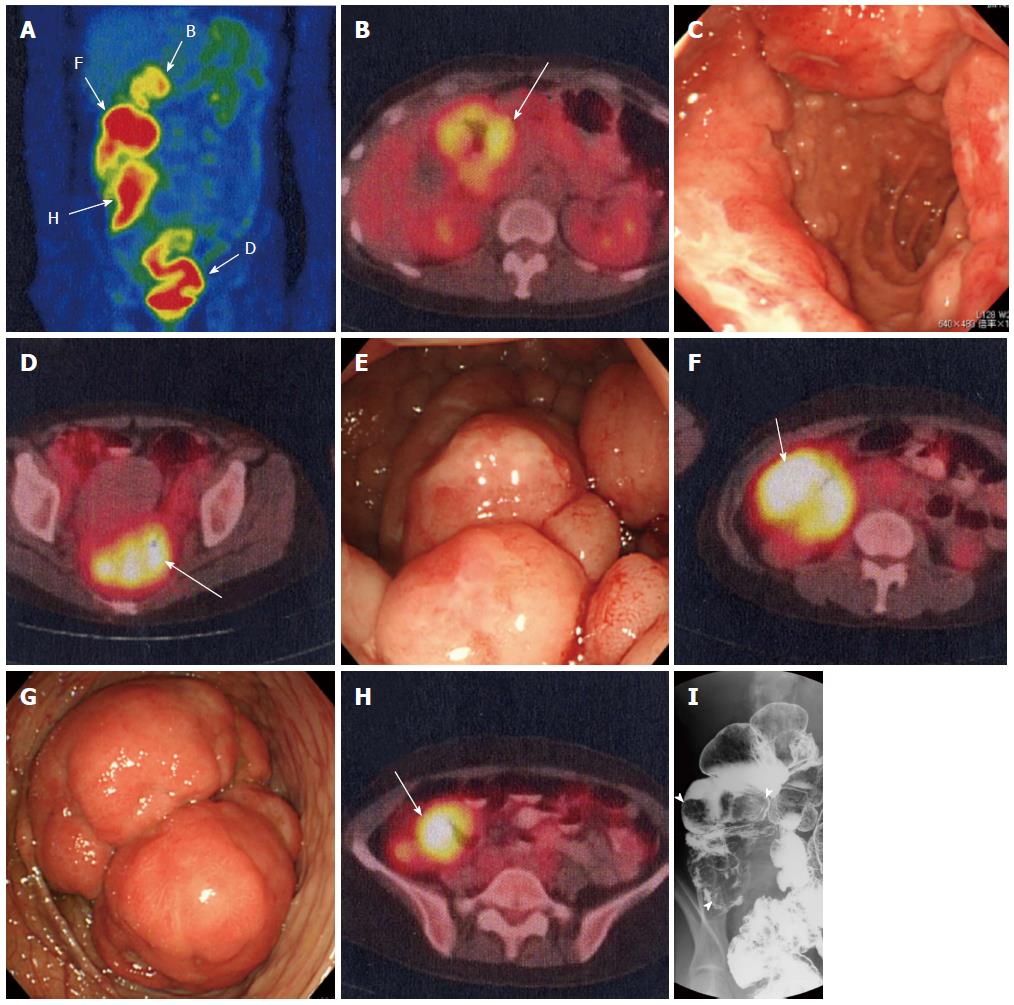
Whole-body 18F-FDG PET/CT was performed using a PET/CT system (Gemini-GXL 16; Philips Medical Systems, Inc., Cleveland, Ohio). She was instructed to fast for 6 h before the injection of 18F-FDG. The serum glucose level before injection was 85 mg/dL. The injected dose of 18F-FDG was 199.6 MBq. A scan was performed 70 minutes after injection. An expert nuclear medicine physician (Tsukamoto E) interpreted the PET/CT imaging. The maximum standardized uptake value (SUVmax) was measured semi-quantitatively. On FDG-PET/CT, as well as in various lymph nodes, uptake of SUVmax 5-7 was observed in the tumorous lesions in the duodenal bulb, ileocecal region, ascending colon, and rectum (Figure 2A, Table 1), which were also observed on CT. Except for these lesions, no uptake was noted in the other gastrointestinal tract.
| PET (-) | PET (+) | |||
| Portion | Ki-67 | Portion | SUVmax | Ki-67 |
| Stomach | 43.8% | Duodenum (bulb) | 5.1 | 50.2% |
| Duodenum (descending) | 32.7% | Ascending colon | 7.7 | 30.1% |
| Transverse colon | 34.7% | Rectum | 6.9 | 32.1% |
Gastrointestinal endoscopy showed MLP spreading over a wide area of the stomach (Figure 1B). Three large tumorous lesions with central depressions were observed in the duodenal bulb (→PET positive, Figure 2B and C), and MLP extended continuously on its anal side (Figure 1C).
Before chemotherapy, colonoscopy showed MLP consisting of large nodules in the rectum (→PET positive, Figure 2D and E). The ascending colon was intussuscepted due to a large tumor (→PET positive, Figure 2F and G), and MLP spread from the anal side of this site to the rectum (Figure 1D).
X-ray imaging of the small intestine yielded findings compatible with MLP mainly in the ileum (not shown). X-ray imaging of the colon showed large tumors in the ascending colon and cecum (→both were PET positive, Figure 2F, H and I).
After chemotherapy, insertion of the colonoscope to the ileocecal region became possible and MLP was also confirmed in the terminal ileum (Figure 1E). It was found that there were intussuscepted at tumors in the ascending colon arising at and around the ileocecal valve and the cecal tumor arose near the appendiceal orifice (not shown).
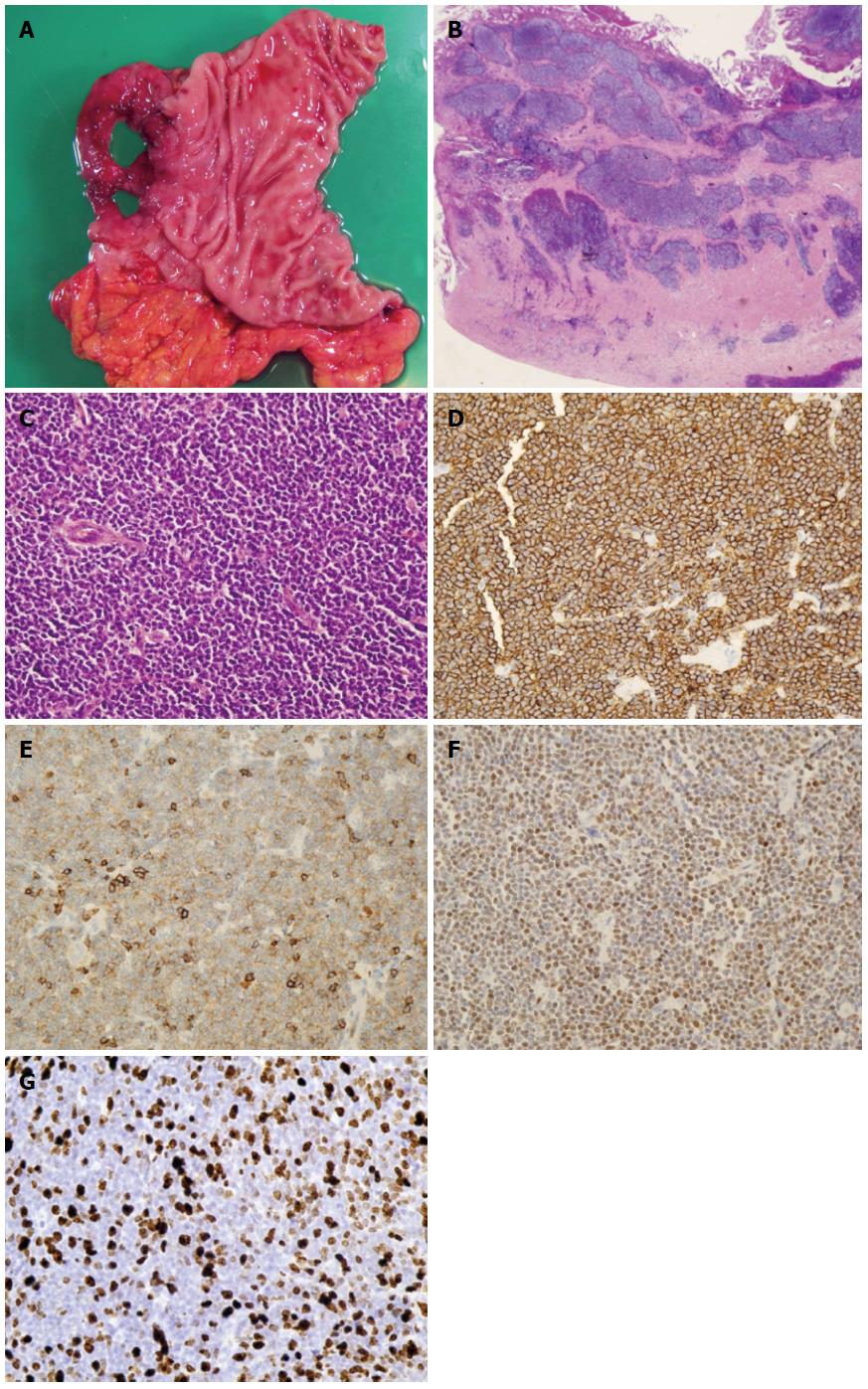
Duodenal bulb was perforated and surgically resected (Figure 3A). Histopathological examination showed that lymphoma lesion invaded deep into the muscularis propria (Figure 3B), and tumor cells varying in size from small to middle-sized proliferated densely (Figure 3C). Lymphoma cells were positive for CD20, CD5, and Cyclin D1 on immunohistochemical staining (Figure 3D-F). These features were compatible with MCL.
Proliferation indices were assessed by staining representative slides with the monoclonal MIB1 antibody (DAKO, Tokyo, Japan) directed against the Ki-67 antigen. Before chemotherapy, biopsy was performed from each of the 6 gastrointestinal lesions shown in the figure, and the Ki-67 proliferative index was evaluated (Figure 3G). It was 43.8%, 32.5%, and 34.7% in the stomach, duodenum (descending portion), and transverse colon, respectively, which were compatible with typical type MLP negative on PET. On the other hand, Ki-67 proliferative index was 50.2%, 30.1%, and 32.1%, and SUVmax was 5.1, 7.7, and 6.9, respectively, in the lesions of the duodenum (bulb), ascending colon, and rectum, which were positive on PET and appeared atypical as MLP (Table 1). The Ki-67 proliferative index was neither related to the presence or absence of uptake nor the SUVmax on FDG-PET/CT imaging.
This report presented a rare case of gastrointestinal MCL showing continuous MLP from the stomach to the rectum. Gastrointestinal tract involvement for MCL presents a variety of lesions[7], ranging from the characteristic MLP to mucosal changes that are too vague to be identified endoscopically and can only be diagnosed through biopsy[8,9]. We previously advocated that lymphoma cells of MCL had invaded the lamina propria to submucosal layer before MLP developed[10].
FDG-PET is a non-invasive imaging technique, commonly performed on patients with malignant lymphoma and recommended for initial staging in diffuse large B-cell and Hodgkin lymphoma[11]. The usefulness of FDG-PET in MCL was also reported, with pretreatment PET scans being positive in 94%-100%[12,13], however, PET scans have too low of a sensitivity (11%-20%) to detect gastrointestinal tract involvement for MCL[13,14]. These reports did not discuss the causes of the low sensitivity of FDG-PET for the detection of MCL with gastrointestinal tract involvement, and the morphological characteristics of a picked up lesion as those of MLP. Our case was the first report to present PET/CT imaging of MCL case with multiple MLP from the stomach to the rectum. The findings of FDG-PET/CT were false negative in typical MLP, particularly, gastric lesions were detected by CT, and the sensitivity of FDG-PET/CT was lower than that of CT. Meanwhile, we do not deny the possible contribution of not administering a H2-blocker or proton pump inhibitor, the duodenal bulb had been perforated in the present case because lymphoma cells had deeply invaded the muscularis propria. Uptake was noted on FDG-PET/CT imaging in atypical type MLP, probably because the lesions were larger and infiltrated deeper than in typical type MLP regardless of the histologic malignant potential, the Ki-67 proliferative index of lymphoma cells. Gastrointestinal MCL lesions are generally shallow[1,10]. This may reduce the sensitivity of FDG-PET/CT for gastrointestinal MCL.
It has also recently been reported that a relationship between SUVmax in 18F-FDG PET/CT imaging and Ki-67-positive tumor cells was revealed in untreated patients with non-Hodgkin lymphoma including MCL[15]. On the other hand, there have been no reports of evaluation of the Ki-67 proliferative index in gastrointestinal MCL showing MLP. In this study, the lack of statistical relationship between SUVmax and the percentage of Ki-67-positive cells may be explained by the fact that the highest SUVmax is determined by analysis on the whole body, while Ki-67 immunostaining is restricted to tissue biopsies in which sampling errors are possible.
Gastrointestinal lesions, including MLP, frequently reflect the pathogenesis of MCL. However, 18F-FDG PET/CT is not useful for the accurate initial staging of MCL before treatment because of the low detection rate of gastrointestinal lesions. In the future, more gastrointestinal MCL patients should be examined the findings of 18F-FDG PET/CT uptake, which is observed exclusively in large and deeply infiltrating lesions as shown in the present study.
In conclusion, the findings of FDG-PET/CT were false negative in typical MLP, but uptake was noted in larger lesions with deep infiltration considered atypical MLP, regardless of the Ki-67 proliferative index.
A 49-years-old female with lumps in the cervical and inguinal regions developed right abdominal discomfort and constipation.
Bilateral cervical and inguinal lymph nodes were palpable and mild tenderness was found in the right-sided abdomen.
Colon cancer with lymph nodes metastases.
Hemoglobin 10.9 g/dL; total protein 5.4 g/dL; albumin 3.1 g/dL; soluble interleukin 2 receptor 4460 U/mL; other laboratory data were within normal limits.
(fluorine-18)-fluorodeoxy-glucose (18F-FDG)-positron emission tomography (PET)/computed tomography (CT) showed tumorous lesions in the duodenal bulb, ileocecal region, ascending colon, and rectum with SUVmax of 5-7, as well as in various lymph nodes, in addition to multiple lymphomatous polyposis (MLP) in the other gastrointestinal tract with no FDG activity.
Gastrointestinal biopsy and resected duodenal bulb revealed mantle cell lymphoma (MCL), positive for CD20, CD5, and Cyclin D1.
The patient was treated with hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone combined with high dose cytarabine and methotrexate) with rituximab for 8 courses.
18F-FDG PET/CT have too low of a sensitivity (11%-20%) to detect gastrointestinal tract involvement for MCL and there have been no reports on 18F-FDG PET/CT imaging for gastrointestinal MCL with MLP. On the other hand, there have been no reports of evaluation of the Ki-67 proliferative index in gastrointestinal MCL showing MLP.
Ki-67 proliferative index is a cell proliferation marker. According to recently report, a relationship between SUVmax in 18F-FDG PET/CT imaging and Ki-67-positive tumor cells was revealed in untreated patients with non-Hodgkin lymphoma including MCL
18F-FDG PET/CT findings were false negative in typical MLP spreading shallow, but uptake was noted in large lesions with deep infiltration considered atypical as MLP regardless of the Ki-67 proliferative index of lymphoma cells.
This report presented a rare case of gastrointestinal MCL with MLP, and the presentation on 18F-FDG PET/CT imaging was shown and well discussed.
P- Reviewers: Caboclo JLF, Lee TY, Misra SP S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Cornes JS. Multiple lymphomatous polyposis of the gastrointestinal tract. Cancer. 1961;14:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Isaacson PG. Gastrointestinal lymphoma. Hum Pathol. 1994;25:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 233] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392. [PubMed] |
| 4. | Ruskoné-Fourmestraux A, Delmer A, Lavergne A, Molina T, Brousse N, Audouin J, Rambaud JC. Multiple lymphomatous polyposis of the gastrointestinal tract: prospective clinicopathologic study of 31 cases. Groupe D’étude des Lymphomes Digestifs. Gastroenterology. 1997;112:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Remes-Troche JM, De-Anda J, Ochoa V, Barreto-Zuñiga R, Arista-Nasr J, Valdovinos MA. A rare case of multiple lymphomatous polyposis with widespread involvement of the gastrointestinal tract. Arch Pathol Lab Med. 2003;127:1028-1030. [PubMed] |
| 6. | Chung Kim Yuen C, Tomowiak C, Yacoub M, Barrioz T, Barrioz C, Tougeron D. A rare case of mantle cell lymphoma as lymphomatous polyposis with widespread involvement of the digestive tract. Clin Res Hepatol Gastroenterol. 2011;35:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Iwamuro M, Okada H, Kawahara Y, Shinagawa K, Morito T, Yoshino T, Yamamoto K. Endoscopic features and prognoses of mantle cell lymphoma with gastrointestinal involvement. World J Gastroenterol. 2010;16:4661-4669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Romaguera JE, Medeiros LJ, Hagemeister FB, Fayad LE, Rodriguez MA, Pro B, Younes A, McLaughlin P, Goy A, Sarris AH. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003;97:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Salar A, Juanpere N, Bellosillo B, Domingo-Domenech E, Espinet B, Seoane A, Romagosa V, Gonzalez-Barca E, Panades A, Pedro C. Gastrointestinal involvement in mantle cell lymphoma: a prospective clinic, endoscopic, and pathologic study. Am J Surg Pathol. 2006;30:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Saito M, Mori A, Irie T, Tanaka M, Morioka M, Ozasa M, Kobayashi T, Saga A, Miwa K, Tanaka S. Endoscopic follow-up of 3 cases with gastrointestinal tract involvement of mantle cell lymphoma. Intern Med. 2010;49:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3311] [Cited by in RCA: 3634] [Article Influence: 201.9] [Reference Citation Analysis (0)] |
| 12. | Brepoels L, Stroobants S, De Wever W, Dierickx D, Vandenberghe P, Thomas J, Mortelmans L, Verhoef G, De Wolf-Peeters C. Positron emission tomography in mantle cell lymphoma. Leuk Lymphoma. 2008;49:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Hosein PJ, Pastorini VH, Paes FM, Eber D, Chapman JR, Serafini AN, Alizadeh AA, Lossos IS. Utility of positron emission tomography scans in mantle cell lymphoma. Am J Hematol. 2011;86:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Bodet-Milin C, Touzeau C, Leux C, Sahin M, Moreau A, Maisonneuve H, Morineau N, Jardel H, Moreau P, Gallazini-Crépin C. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from the GOELAMS group. Eur J Nucl Med Mol Imaging. 2010;37:1633-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Papajík T, Mysliveček M, Sedová Z, Buriánková E, Procházka V, Koranda P, Raida L, Kubová Z, Palová M, Kučerová L. Standardised uptake value of 18F-FDG on staging PET/CT in newly diagnosed patients with different subtypes of non-Hodgkin's lymphoma. Eur J Haematol. 2011;86:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |