Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.5119
Revised: January 22, 2014
Accepted: March 8, 2014
Published online: May 7, 2014
Processing time: 197 Days and 12.1 Hours
AIM: To compare the efficacy and tolerance of ilaprazole compared with other proton pump inhibitors (PPIs) in the treatment of duodenal ulcer.
METHODS: An electronic database search of Medline, Embase, the Cochrane controlled trials register, Web of Science, PubMed, and the Chinese Biomedical Literature Database (updated to July 2013), and manual searches were conducted. A meta-analysis of randomized controlled trials comparing the efficacy and tolerance of ilaprazole and other PPIs in the treatment of duodenal ulcers was performed.
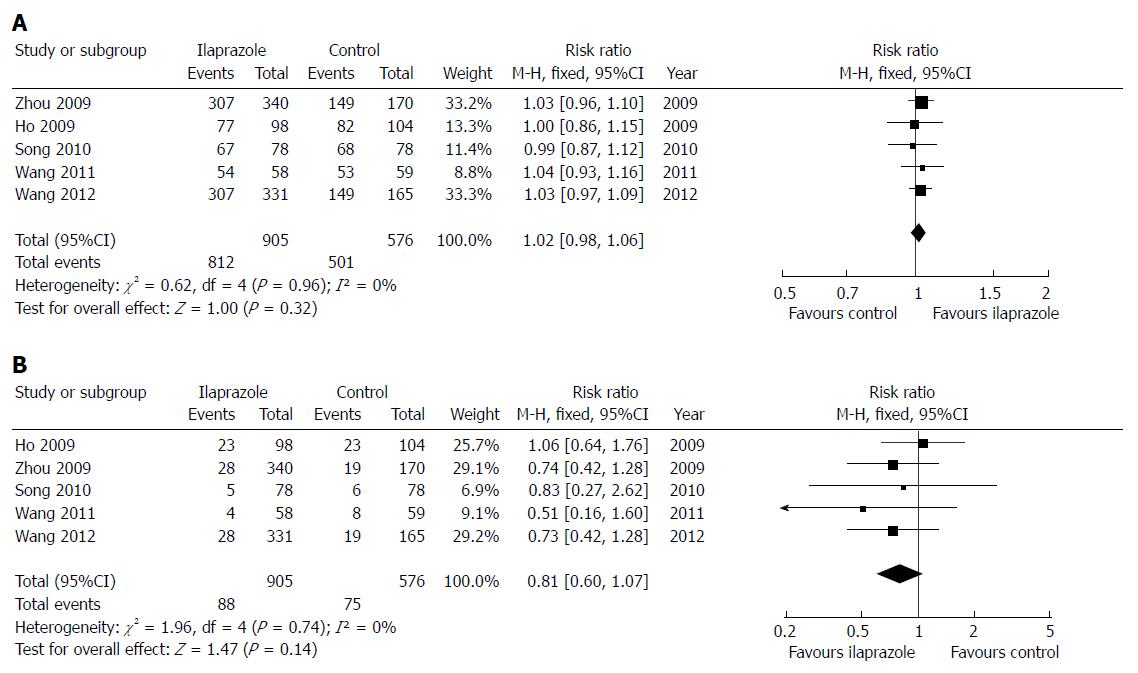
RESULTS: Five articles involving 1481 patients were included. The meta-analysis showed no difference in the 4-wk healing rate between ilaprazole and other PPIs [89.7% vs 87.0%; relative risk (RR) = 1.02; 95%CI: 0.98-1.06; Z = 1.00; P = 0.32]. The results did not change in the sensitivity analyses. The meta-analysis indicated that the adverse effect rate in the ilaprazole group was lower than that in the control group, but the difference was not significant (9.7% vs 13.0%; RR = 0.81; 95%CI: 0.60-1.07; Z = 1.47; P = 0.14).
CONCLUSION: Ilaprazole is a highly effective and safe PPI in the treatment of duodenal ulcers. Ilaprazole can be recommended as a therapy for acid-related disorders, especially in Asian populations.
Core tip: Ilaprazole, a proton pump inhibitor (PPI), is a newly developed medicine in the management of acid-related disorders. This meta-analysis showed that ilaprazole was a highly effective and safe PPI compared with other PPIs in the treatment of duodenal ulcer. Ilaprazole can be recommended as a therapy for acid-related disorders, especially in Asian populations.
- Citation: Ji XQ, Du JF, Chen G, Chen G, Yu B. Efficacy of ilaprazole in the treatment of duodenal ulcers: A meta-analysis. World J Gastroenterol 2014; 20(17): 5119-5123
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/5119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.5119
Duodenal ulcer (DU) is a very common digestive disease with a high incidence all over the world[1-4]. As the first proton pump inhibitor (PPI), omeprazole has been used therapeutically for many years, and shown great efficacy in treating peptic ulcers[5-7]. Currently, research is focused on more effective PPIs with a lower dose and comparative safety[8-11].
Ilaprazole (also known as IY-81149), the latest proton pump inhibitor (PPI), has been less well reported in clinical practice, as a newly developed medicine in the management of acid related disorders[12,13]. Ilaprazole is synthesized by Il-Yang (South Korea) and presently developed by Livzon Pharmaceutical Group Inc. (China), and has been approved by the State Food and Drug Administration of China (license ID: CN 1121714A) with a recommended dose of 10 mg/d for peptic ulcers. The mechanism of ilaprazole’s action to suppress gastric acid secretion is almost the same as omeprazole, in which the protonated substituted benzimidazoles suppress gastric acid secretion through inhibition of the H+/K+-ATPase at the secretory surfaces of gastric parietal cells[14,15].
Preclinical research found that ilaprazole had a more prolonged half-life and higher suppression of gastric acid secretion in a dose-dependent manner, and similar safety compared with omeprazole. A comparative pharmacodynamic study on patients with gastroesophageal reflux disease reported that ilaprazole, at a dose of 5 mg, provided gastric pH control comparable with the use of 20 mg omeprazole, and at doses of 10 and 20 mg it was found to have a more powerful and longer-lasting acid-suppressant effect than omeprazole at a dose 20 mg[16].
There have been several clinical trials comparing ilaprazole and other PPIs in the treatment of duodenal ulcer, which showed that ilaprazole had a high 4-wk healing rate[17-19]. The aim of the present study was to conduct a pooled meta-analysis of randomized controlled trials (RCT) comparing the efficacy and tolerance of ilaprazole with other PPIs in the treatment of duodenal ulcers.
Relevant studies were identified and selected by searching the databases, Medline (1990 to July 2013), Embase (1990 to July 2012), Cochrane controlled trials register (Cochrane Library Issue 2, 2013), Web of Science (1990 to July 2013), PubMed (updated to July 2013) and Chinese Biomedical Literature Database (1989 to July 2013) under the search term “ilaprazole”. We also performed a full manual search from the bibliographies of each peer-reviewed paper selected. No language or date limitations were imposed. Furthermore, there was no limitation in publication form.
The selection criteria for inclusion in the meta-analysis were: (1) RCT comparing ilaprazole 10 mg/d and other PPIs in the treatment of duodenal ulcers; (2) duodenal ulcers must have been diagnosed by upper gastrointestinal endoscopy; (3) the patients should not receive other medical therapies before the trial, except the standard triple therapy for Helicobacter pylori (H. pylori) eradication; and (4) the duration of the trials should be 4 wk, and ulcer healing was also assessed by endoscopy after 4 wk of therapy. The decision to include or exclude any trial was made by 2 researchers separately. The 2 lists were compared and discrepancies were resolved.
Data were independently abstracted from each trial by 2 researchers, and disagreement was resolved by consensus. Data were extracted with a pre-designed review form. Data to be extracted were as follows: study design, number of patients in each treatment arm, duration of treatment, drug regimen, percentage of adverse effects, and quality score.
The methodological quality of studies included in the meta-analysis was scored with the Jadad composite scale (including items of randomization, double-blinding, and description of withdrawal/dropouts)[20,21]. This is a 5-point quality scale, with low quality studies having a score of ≤ 2 and high quality studies a score of ≥ 3[21,22]. Methodological quality assessment was independently performed by 2 of the present authors. Each study was given an overall quality score based on the above criteria, which was then used to rank studies.
The meta-analysis was performed using the Mantel-Haenszel method (fixed effects model) or the DerSimonian and Laird method (random effects model) with Review Manager Software (RevMan 5.1, Cochrane Collaboration, Oxford, England). The relative risk (RR) for each clinical event was presented with 95% confidence interval (CI). Heterogeneity was tested using the χ2 test (with P≤ 0.05 indicating significant heterogeneity) and I2 test (25%, 50%, and 75%, represent low, moderate, and high heterogeneity, respectively). The RR for each clinical event was pooled with the fixed effects model, and if the χ2 test and I2 test for heterogeneity were significant, the analysis was also done with random effects model.
The search strategy generated 32 studies. From these, we identified 5 trials involving 1481 patients comparing ilaprazole with other PPIs in the treatment of duodenal ulcer, which fulfilled the criteria for the meta-analysis. Four papers were published as peer-reviewed articles, and one as a meeting abstract[18]. Four were published in English and the other was published in Chinese[19]. The baseline characteristics of the 5 articles are listed in Table 1. All the trials were based on intention-to-treat analysis. All trials were of high quality except one[18] (Table 1). The results of the 5 trials are shown in Table 2[17-19,23,24].
| Ref. | Language | Publication type | Time | Patients (n) | Duration (wk) | Jadad score |
| Ho et al[17], 2009 | English | Full text | 2002-2004 | 202 | 4 | 5 |
| Zhou et al[19], 2009 | Chinese | Full text | 2005-2006 | 510 | 4 | 5 |
| Song et al[18], 2010 | English | Abstract | Not reported | 156 | 4 | 2 |
| Wang et al[23], 2011 | English | Full text | 2004-2005 | 117 | 4 | 5 |
| Wang et al[24], 2012 | English | Full text | 2005-2006 | 496 | 4 | 5 |
| Ref. | Regimen | 4-wk healing rate | Rate of adverse effects |
| Ho et al[17], 2009 | I 10 mg/d | 78.6% (77/98) | 23.5% (23/98) |
| O 20 mg/d | 78.8% (82/104) | 22.1% (23/104) | |
| Zhou et al[19],2009 | I 10 mg/d | 90.3% (307/340) | 8.2% (28/340) |
| O 20 mg/d | 87.6% (149/170) | 11.2% (19/170) | |
| Song et al[18], 2010 | I 10 mg/d | 85.9% (67/78) | 6.4% (5/78) |
| E 40 mg/d | 87.2% (68/78) | 7.5% (6/78) | |
| Wang et al[23], 2011 | I 10 mg/d | 93.1% (54/58) | 6.9% (4/58) |
| O 20 mg/d | 89.8% (53/59) | 13.6% (8/59) | |
| Wang et al[24], 2012 | I 10 mg/d | 95.0% (307/331) | 8.5% (28/331) |
| O 20 mg/d | 90.0% (149/165) | 11.6% (19/165) |
We first compared ilaprazole at the standard dose 10 mg/d with other PPIs on the 4-wk healing rate and rate of adverse effects (Figure 1). There was no statistical heterogeneity in the 4-wk healing rate among the 5 trials and the fixed effects model was used (χ2 = 0.62; P = 0.96; I2 = 0%) The meta-analysis showed no difference between the ilaprazole and other PPIs in 4-wk healing rate (89.7% vs 87.0%; RR = 1.02; 95%CI: 0.98-1.06; Z = 1.00; P = 0.32). Regarding adverse effects, there was no statistical heterogeneity found by the χ2 test (χ2 = 1.96; P = 0.74) or I2 test (I2 = 0%), and the fixed effects model was used. The meta-analysis indicated that the rate of adverse effects in the ilaprazole group was lower than that in the control group, but the difference was not significant (9.7% vs 13.0%; RR = 0.81; 95%CI: 0.60-1.07; Z = 1.47; P = 0.14).
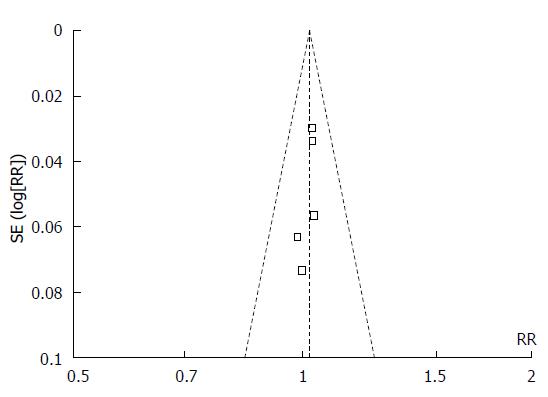
The funnel plots for the 4-wk healing rate comparing ilaprazole at a dose of 10 mg/d with other PPIs showed some asymmetry, suggesting the possibility of publication bias (Figure 2). Thus, we further performed a sensitivity analysis to assess the stability and reliability of the results of the primary meta-analysis (Table 3). The sensitivity analysis only included the 4 trials of high quality (Jaded score ≥ 3). The analysis indicated no difference in the 4-wk healing rate between 10 mg/d ilaprazole and other PPIs (RR = 1.02; 95%CI: 0.98-1.07; Z = 1.16; P = 0.25).
| Analysis | n | RR (95%CI) | P value |
| High quality studies | 4 | 1.02 (0.98–1.07) | 0.25 |
| English studies | 4 | 1.02 (0.97–1.07) | 0.54 |
| Studies using omeprazole as control | 4 | 1.02 (0.98–1.07) | 0.25 |
Four trials were published in English and the other was published in Chinese. A further sensitivity analysis was made only including the studies published in the English. The analysis revealed no difference between the 10 mg/d ilaprazole and other PPIs in the trials published in English (RR = 1.01; 95%CI: 0.97-1.07; Z = 0.61; P = 0.54).
A final sensitivity analysis was performed only including trials using omeprazole as control. The analysis indicated no difference between the ilaprazole at a dose of 10 mg/d and omeprazole (RR = 1.02; 95%CI: 0.98-1.07; Z = 1.16; P = 0.25).
PPIs are highly effective medications widely used in the management of peptic diseases including gastric and duodenal ulcers, gastroesophageal reflux disease and Zollinger-Ellison syndrome[25]. Many new therapeutic drugs with similar structures and better therapeutic outcomes have been developed since omeprazole first entered the market, including rabeprazole, pantoprazole, lansoprazole, esomeprazole, and the new molecule we studied in this analysis, ilaprazole. Because ilaprazole was currently only approved in a number of Asian countries, the clinical studies on ilaprazole were not regularly reported in international journals, and most were conducted in China and published in Chinese. Thus, this study aimed to perform a systematic review and meta-analysis on the effect of ilaprazole on the healing of duodenal ulcers.
The current standard dose of ilaprazole recommended for the management of peptic diseases is 10 mg/d. The meta-analysis showed no difference between 10 mg/d ilaprazole and other PPIs with standard or higher doses. In addition, the sensitivity analyses also confirmed the results of the primary meta-analysis. The meta-analyses documented that ilaprazole was a highly effective PPI compared with other PPIs.
Ilaprazole shows major suppression of gastric acid secretion. As an inhibitor of acid output, ilaprazole is more powerful than omeprazole. An experimental study in a surgically-induced rat reflux esophagitis model showed that ilaprazole had a much lower ED50 than omeprazole[26]. Ilaprazole at a dose of 5 mg provided gastric pH control comparable with 20 mg omeprazole[16].
As for the safety and tolerability profile, the meta-analysis on adverse effects also revealed fewer adverse effects in the ilaprazole group, though the difference was not significant. Wang et al[23] reported that ilaprazole at a dose of 5, 10, or 20 mg/d is comparable to 20 mg/d omeprazole. Considering the rate of adverse effects of PPIs is low, and the adverse effects are usually mild, we may conclude that ilaprazole is a safe drug with minor adverse effects.
There were several limitations in this study. First, the low quality of 2 individual trials was a major limitation. Second, due to the fact that ilaprazole is only approved in Asian countries, the trials included in this study all come from Asian countries, and thus further trials are needed in Western populations. Third, there were few trials comparing ilaprazole at a dose of 5 mg/d with other PPIs.
In conclusion, ilaprazole is a highly effective and safe PPI in the treatment of duodenal ulcers. Ilaprazole can be recommended as a therapy for acid-related disorders, especially in Asian populations.
Ilaprazole, the latest proton pump inhibitor (PPI), is a newly developed medicine in the management of acid related disorders.
There have been several clinical trials comparing ilaprazole and other PPIs in the treatment of duodenal ulcers which showed that ilaprazole had a high 4-wk healing rate.
The authors conducted a meta-analysis of randomized controlled trials comparing the efficacy and tolerance of ilaprazole with other PPIs in the treatment of duodenal ulcer.
Ilaprazole is a highly effective and safe PPI in the treatment of duodenal ulcer. Ilaprazole can be recommended as a therapy for acid-related disorders, especially in Asian populations.
This study evaluated the efficacy and tolerance of ilaprazole with other PPIs in the treatment of duodenal ulcer by conducting a meta-analysis. The findings are useful in the management of duodenal ulcer.
P- Reviewers: Celinski K, Koch TR, Kate V, Rodrigo L S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Zhang DN
| 1. | Lam SK. Differences in peptic ulcer between East and West. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Lau JY, Barkun A, Fan DM, Kuipers EJ, Yang YS, Chan FK. Challenges in the management of acute peptic ulcer bleeding. Lancet. 2013;381:2033-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Milosavljevic T, Kostić-Milosavljević M, Jovanović I, Krstić M. Complications of peptic ulcer disease. Dig Dis. 2011;29:491-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Najm WI. Peptic ulcer disease. Prim Care. 2011;38:383-394, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 6. | Leong RW. Differences in peptic ulcer between the East and the West. Gastroenterol Clin North Am. 2009;38:363-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Pilotto A, Franceschi M, Maggi S, Addante F, Sancarlo D. Optimal management of peptic ulcer disease in the elderly. Drugs Aging. 2010;27:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Bohidar NP, Krishna K, Panda BK, Patel C. Ilaprazole: Is this a superior proton pump inhibitor for duodenal ulcer? Trop Gastroenterol. 2013;34:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS One. 2013;8:e56060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56:931-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Devlin JW, Welage LS, Olsen KM. Proton pump inhibitor formulary considerations in the acutely ill. Part 1: Pharmacology, pharmacodynamics, and available formulations. Ann Pharmacother. 2005;39:1667-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | de Bortoli N, Martinucci I, Giacchino M, Blandizzi C, Marchi S, Savarino V, Savarino E. The pharmacokinetics of ilaprazole for gastro-esophageal reflux treatment. Expert Opin Drug Metab Toxicol. 2013;9:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | DU YQ, Guo WY, Zou DW, Zhan XB, Li Z, Hu JH, Gong YF, He J, Lu JP, Li ZS. Acid inhibition effect of ilaprazole on Helicobacter pylori-negative healthy volunteers: an open randomized cross-over study. J Dig Dis. 2012;13:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Kim EJ, Lee RK, Lee SM, Kim DY. General pharmacology of IY-81149, a new proton pump inhibitor. Arzneimittelforschung. 2001;51:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Kwon D, Chae JB, Park CW, Kim YS, Lee SM, Kim EJ, Huh IH, Kim DY, Cho KD. Effects of IY-81149, a newly developed proton pump inhibitor, on gastric acid secretion in vitro and in vivo. Arzneimittelforschung. 2001;51:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Periclou AP, Goldwater R, Lee SM, Park DW, Kim DY, Cho KD, Boileau F, Jung WT. A comparative pharmacodynamic study of IY-81149 versus omeprazole in patients with gastroesophageal reflux disease. Clin Pharmacol Ther. 2000;68:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ho KY, Kuan A, Zaño F, Goh KL, Mahachai V, Kim DY, Yoon HM. Randomized, parallel, double-blind comparison of the ulcer-healing effects of ilaprazole and omeprazole in the treatment of gastric and duodenal ulcers. J Gastroenterol. 2009;44:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Song J, Guo B, Yao L, Tang J. The clinical study of ilaprazole on duodenal ulcer, a randomize study compared with esomeprazole. Gastroenterology. 2010;138:S166. |
| 19. | Zhou LY, Ilaprazole research group. Effect of ilaprazole on duodenal ulcer and the influence of CYP2C19 polymorphisms: a multicenter clinical trial. Zhongguo Xiaohua Neijing Zazhi. 2009;26:475-479. [DOI] [Full Text] |
| 20. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12887] [Article Influence: 444.4] [Reference Citation Analysis (1)] |
| 21. | Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1870] [Cited by in RCA: 1713] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 22. | Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 2654] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 23. | Wang L, Zhou L, Lin S, Hu H, Xia J. A new PPI, ilaprazole compared with omeprazole in the treatment of duodenal ulcer: a randomized double-blind multicenter trial. J Clin Gastroenterol. 2011;45:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Wang L, Zhou L, Hu H, Lin S, Xia J. Ilaprazole for the treatment of duodenal ulcer: a randomized, double-blind and controlled phase III trial. Curr Med Res Opin. 2012;28:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Mullin JM, Gabello M, Murray LJ, Farrell CP, Bellows J, Wolov KR, Kearney KR, Rudolph D, Thornton JJ. Proton pump inhibitors: actions and reactions. Drug Discov Today. 2009;14:647-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Kil BJ, Kim IW, Shin CY, Jeong JH, Jun CH, Lee SM, Kim DY, Huh IH, Sohn UD. Comparison of IY81149 with omeprazole in rat reflux oesophagitis. J Auton Pharmacol. 2000;20:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |