Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.5051
Revised: January 5, 2014
Accepted: February 17, 2014
Published online: May 7, 2014
Processing time: 166 Days and 14.1 Hours
AIM: To evaluate the success rates, procedural time and adverse event rates of the modified methods in endoscopic ultrasonography-guided hepaticogastrostomy (EUS-HGS).
METHODS: Twenty-eight patients in a prospective case series who underwent EUS-HGS (phase I). Forty-six patients in a matched case-control study (phase II). The simplified technique for fistula dilation was the primary use of a 4 mm balloon catheter with a stainless steel stylet. The stent deployment was modified by deploying the metal stent inside a bile duct (half of the stent) under EUS and fluoroscopic guidance and gently pulling the echoendoscope after full deployment of the stent inside the echoendoscope channel (remaining portion of the stent) under fluoroscopic guidance. This cohort was compared with a matched historical cohort.
RESULTS: In phase I, the technical and clinical success with the modified method was 96% (27/28) and 89% (24/27 as per-protocol analysis). The overall adverse event rate was 7%. In phase II, there was no difference in technical and clinical success, stent patency and overall adverse events in each group. However, the procedural time (15.3 ± 5.2 min vs 22.3 ± 6.0 min, P < 0.001) and early adverse events (0% vs 26%, P = 0.02) were statistically improved in case cohort compared with control cohort.
CONCLUSION: Compared with the conventional EUS-HGS technique, the procedural time was shorter and early adverse events were less frequent with our simplified and modified technique.
Core tip: Endoscopic ultrasonography-guided hepaticogastrostomy (EUS-HGS) with direct transluminal stenting is a complex procedure in terms of guidewire manipulation, fistula dilation and stent deployment. We prospectively evaluated our simplified and modified EUS-HGS technique; fistula dilation with a 4 mm balloon dilation catheter with a stainless steel stylet and stent deployment maneuver with an 8 mm fully covered metal stent with dual flaps. The technical and clinical success was 96% (27/28) and 89% (24/27). The overall adverse event rate was 7%. Compared with the conventional EUS-HGS technique, the procedural time was shorter and early adverse events were less frequent with our modified technique.
- Citation: Paik WH, Park DH, Choi JH, Choi JH, Lee SS, Seo DW, Lee SK, Kim MH, Lee JB. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J Gastroenterol 2014; 20(17): 5051-5059
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/5051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.5051
Endoscopic ultrasonography-guided biliary drainage (EUS-BD) is an emerging alternative to percutaneous transhepatic biliary drainage (PTBD) or surgery after failed endoscopic retrograde cholangiopancreatography (ERCP)[1-3]. EUS-BD can be completed in 3 ways: EUS-guided hepaticogastrostomy, choledochoduodenostomy, and rendezvous therapy[2,4-10]. EUS-guided rendezvous therapy seems to be the safest of all 3 approaches[11]. However, this technique is not always successful. Although an enhanced guidewire manipulation protocol has been introduced, 44% of patients may still require EUS-guided hepaticogastrostomy or choledochoduodenostomy after failed ERCP[12]. EUS-guided hepaticogastrostomy with direct transluminal stenting (EUS-HGS) may be a viable option for patients with surgically altered anatomy, proximal bile duct obstruction, and duodenal invasion after failed ERCP[2]. EUS-guided hepaticogastrostomy is one of the most complex procedures in terms of guidewire manipulation, fistula dilation, and stent deployment[11,13]. With regard to guidewire manipulation, the intrahepatic approach appears to present a challenge because the overall technical success rate of EUS-guided rendezvous and antegrade biliary stenting/balloon dilation has been reported to be lower than that of the extrahepatic rendezvous method[14]. Thus, a substantial number of patients in which the intrahepatic approach of guidewire manipulation was used may eventually require EUS-HGS[12].
For fistula dilation in EUS-HGS, graded dilation with a 4 F cannula and a 6 F and 7 F bougie dilator may be preferred because this step-by-step procedure seems to be safe[12]. However, this procedure is not always successful. The procedural time may also be increased because of the need for accessory changes and difficulties with the advancement of each bougie dilator. Furthermore, accidental loss of the guidewire may occur during this step-by-step maneuver. A needle knife may eventually have to be used in some cases due to the difficulty of graded dilation of the fistula tract. The use of a needle knife for fistula dilation in EUS-BD may be associated with postprocedure adverse events[4]. Another difficulty in EUS-HGS is transgastric stent deployment[4,15]. Scope position will be backward for identification of the distal end of the deploying stent, and the stent will be placed in a more inner side of the intrahepatic duct during stent deployment[16]. This placement may result in proximal stent migration after stent deployment[2]. Furthermore, distal stent migration in EUS-HGS may occur during follow-up periods[9].
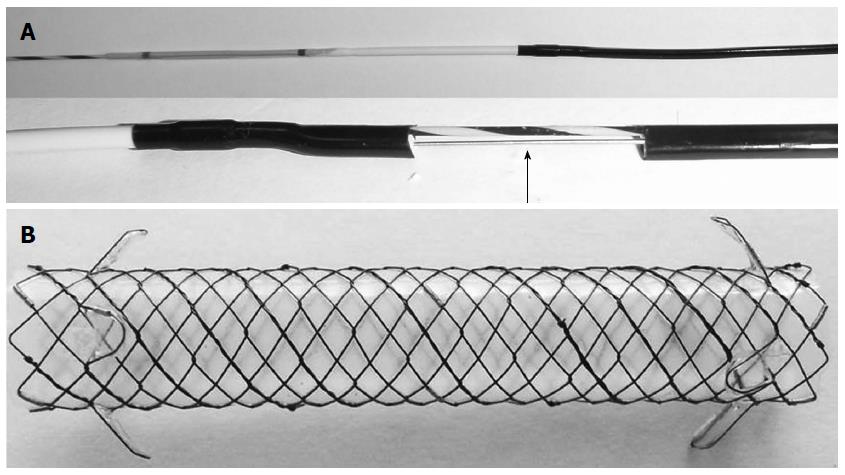
Thus, a simple step for fistula dilation, troubleshooting of stent deployment, and a stent with a modified design are needed to prevent proximal or distal migration in EUS-HGS. During EUS-guided drainage of pancreatic pseudocysts, we found that a direct 4 mm balloon catheter (Hurricane RX, Boston-Scientific, Natick, MA) was usually successful for fistula dilation. This device has a low-profile 5.8 F balloon shaft designed to reduce resistance and increase pushability. It also features a 4 F tip and stainless steel stiffening stylet inserted in the proximal portion of the catheter shaft, thereby ensuring less trauma and pushability (Figure 1A). We introduced this device for fistula dilation in EUS-HGS. In our previous study[17], a fully covered self-expandable metal stent (FCSEMS) with an anchoring flap showed excellent antimigration compared with FCSEMS with flared ends in patients with benign distal biliary stricture. In the present study, we prospectively evaluated the role of a 4 mm balloon dilation catheter with a stainless steel stylet and modified stent deployment maneuver with an 8 mm fully covered metal stent with dual flaps for EUS-HGS (Figure 1B). To determine whether this simplified and modified technique affects the procedural time or adverse events, we also performed a case-control study.
Between August 2012 and August 2013, 2042 ERCPs were carried out by a single experienced endoscopist (Park DH). In this study period, 1109 cases needed biliary decompression for benign or malignant biliary obstruction. Of these 62 (5.6%) patients were candidates for alternative or complementary techniques other than ERCP for biliary decompression. Among the 62 patients, 59 underwent EUS-BD as follows: 28 hepaticogastrostomy, 11 antegrade biliary stenting, 9 rendezvous technique, 8 choledochoduodenostomy, and 3 hepaticoduodenostomy. Trainees were not involved in the ERCP. The EUS-BD was performed at the time of a failed ERCP in the same session.
The inclusion criteria for EUS-HGS were failure of initial biliary cannulation or bile duct decompression through ERCP or EUS-guided rendezvous because of accompanying duodenal obstruction, surgically altered anatomy, high-grade hilar biliary stricture, or failed guidewire manipulation in EUS-guided rendezvous or antegrade therapy. Patients who refused PTBD were also included. The exclusion criteria were (1) refusal to participate in the study protocol; (2) pregnancy; and (3) patient age younger than 18 years. All enrolled patients were given antibiotics pre- and postprocedure. CO2 insufflation was routinely applied during ERCP and EUS before the commencement of this study (January 2010).
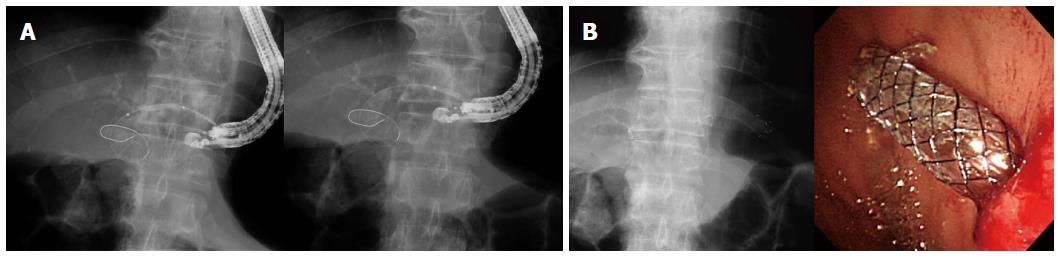
EUS was performed by using a GF-UCT 240 linear-array echoendoscope (Olympus Medical System, Tokyo, Japan). The echoendoscope was placed in the cardia or in the lesser curvature of the stomach and was oriented to view the intrahepatic duct. Color Doppler imaging was used to identify the regional vasculature. Dilated intrahepatic bile duct puncture was carried out with a 19-gauge needle (EUSN-19-T, Cook Endoscopy, Winston-Salem, NC), and bile juice was aspirated. Then, a contrast medium was injected into the punctured bile duct to confirm successful biliary access. Tract dilation was then carried out. To simplify the tract dilation, a 4 mm balloon dilation catheter was used initially. For the facilitation of the advance of a balloon catheter to intrahepatic bile duct, the balloon catheter was in a plane with the axis of the wire as it entered the bile duct on EUS. If the guidewire was placed in the right intrahepatic duct, hilum, or proximal left intrahepatic duct, the 4 mm balloon dilation catheter was directly used for fistula dilation. If the guidewire was placed toward the left peripheral intrahepatic duct instead of the hilum, a 4 F cannula (Glo-tip; Cook Medical, Winston-Salem, NC) was used as a stiff instrument to advance through the fistula tract and into the left intrahepatic duct[12]. When the guidewire was placed into the desired intrahepatic duct (proximal left, right intrahepatic duct, or hilum), the 4 mm balloon catheter was applied for fistula dilation. After full expansion was confirmed under fluoroscopy, the balloon was kept for 5-10 s. This 4 mm balloon catheter was dilated in two points (between the left hepatic duct and the hepatic parenchyma and between the hepatic parenchyma and the stomach) to facilitate the deployment of the metal stent (Figure 2). In the case of resistance to the advance of the 4 mm balloon catheter, graded dilation with 4 F cannula and tapered biliary dilators was applied. If 4 F cannula was failed to advance repeatedly, needle knife was used finally to prevent the loss of mounted guidewire.
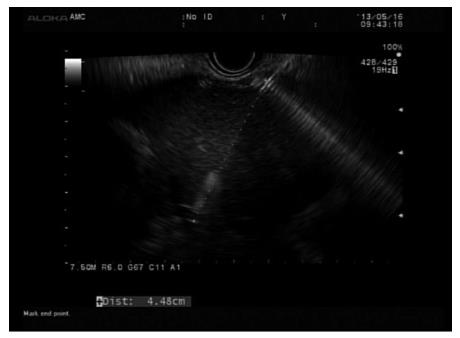
To stabilize the position/attachment of the endoscope in the high body of the stomach or the cardia during insertion of the stent in EUS-HGS, we deployed the front one-half of the dual-flap metal stent (8 mm in diameter, 5-10 cm in length, dual-flap fully covered metal stent, M.I. Tech, Gyeonggi-do, South Korea) with a 8.5 F introducer under echoendoscopic and fluoroscopic guidance. After deploying the remainder of the stent within the channel of echoendoscope under fluoroscopic guidance, the echoendoscope was pulled out gently, and the stent was left in the hepaticogastrostomy site. The length of a stent was determined by our formula [the length (cm) of an EUS needle between gastric wall and punctured left hepatic duct on the EUS (representative as approximately half of a stent in hepatic parenchyma) multiply two (representative as remaining half of a stent in deploying inside the echoendoscope, including possible stent shortening) plus 1 cm] (Figure 3). This 1 cm was considered as the intrahepatic bile duct portion of a metal stent. To reduce the resistance of stent deployment inside the channel of echoendoscope, an 8 mm in diameter FCSEMS was applied. Clinical data, including the technical success, adverse events, and other variables, were prospectively recorded and evaluated.
We followed the patients in phase I after the procedure until October 2013. To check migration of the stent, biochemical parameters and a simple abdominal film were assessed 1, 3 and 6 mo after the EUS-HGS. Follow-up data were collected prospectively.
The procedural time was defined as the time between the puncture of the intrahepatic bile duct with the EUS needle and the placement of FCSEMS. The procedural time is important because it represents the technical difficulty. Shortening the procedural time would help to increase success rates and reduce procedure-related adverse events. Overall adverse events included early adverse events (up to 14 d) and late adverse events (later than 14 d). Adverse events included complications of abdominal pain, pneumoperitoneum, bleeding and stent migration. Abdominal pain was defined as pain not caused by pancreatitis or perforation[18]. Proximal stent migration was defined as any migration of the FCSEMS into the bile duct or abdominal cavity, preventing its easy removal[19]. Distal stent migration was classified as spontaneous or gastric migration. Spontaneous migration was defined as distal migration without the stent becoming lodged in the bowel[17]. Gastric migration was defined as a partially migrated stent impacting in the distal hepaticogastrostomy site.
Technical success was defined as follows: (1) success of fistula dilation with the 4 mm balloon catheter and deployment of the metal stent with the modified method; and (2) the passage of the metal stent across the stomach or esophagus, along with the flow of contrast medium and/or bile through the stent. Functional success was defined as a decrease of bilirubin to < 75% of the pretreatment value within the first month[7]. The functional success rate was calculated for the patients for whom the procedure was technically successful (as per-protocol analysis). Stent occlusion was defined as the recurrence of jaundice and cholestasis and/or evidence of a dilated biliary system on images, which in all cases would require biliary intervention.
Conventional method was performed as follows. Graded dilation with 4 F cannula and tapered biliary dilators (6 F, and 7 F, catheter tip, 4 F; Cook) was applied initially for fistula dilation. In the case of resistance to the advance of the bougie dilator, graded dilation with a 4 F cannula was repeated again. At that time, a needle knife was used only when repeated graded dilation failed and the risk of failing guidewire maintenance was increased because our previous study found that the use of a needle knife was a risk factor for postprocedure adverse event after EUS-BD[4]. The metal stent was deployed under echoendoscopic and fluoroscopic guidance as per previous studies[4,7].
All the patients provided written informed consent to participate in this study. For the two phases of the study, our institutional review board approved the study protocol (phase I, IRB No. 2012-0608) and the retrieval and analysis of previous collected data for comparison with the prospective cohort (phase II, matched case-control study, IRB No. 2013-0664).
In phase I, our previous study showed 74% of successful graded dilation for fistula dilation in EUS-HGS[4]. To detect a 21% increase in successful fistula dilation with the 4 mm balloon dilation catheter in EUS-HGS, we needed 25 patients to have an 80% chance of rejection the hypothesis of no difference at the 0.05 level (2-tailed). As a 10% dropout rate was considered, 28 patients were invited to participate in this study. This sample size was then used as the stopping rule for patient recruitment in the phase I study. For the phase II study, clinical data were retrieved on 36 patients who underwent EUS-HGS with conventional technique from January 2010 to July 2012 (historical cohort). The patients in historical cohort were 1:1 blindly matched with our prospective cohort by age and etiology of biliary obstruction by a statistician (Lee JB) who was unaware of the purpose of this study. Finally, 23 patients in each cohort were enrolled in phase II study. As the focus of this research was to highlight potential differences, the resultant significant P values were not corrected for the multiple testing of data. Categorical and binary variables were tested using the χ2 test or Fisher’s exact test according to the EUS-HGS methods. The Mann-Whitney U test or Wilcoxon signed-rank test was used for continuous variables. Stent patency and overall survival were compared using the Kaplan-Meier method with a log-rank test. All statistical analyses were performed with SPSS v.18.0 (IBM, Armonk, NY), with results considered significant at a P value < 0.05.
In phase I, 28 patients underwent EUS-HGS with the 4 mm balloon dilation catheter with a stainless steel stiffening stylet and an 8 mm fully covered metal stent with dual flaps. The median age of the study population was 63 years (range, 29 to 87 years), and the male to female ratio was 2.5 (Table 1). The indications for EUS-HGS are described in Table 1. The technical and clinical success of EUS-HGS with the modified method was 96% (27/28) and 89% (24/27) as per-protocol analysis (Table 2). The fistula dilation with the 4 mm balloon catheter was successful in 27 patients. A needle knife was used in one patient since the 4 mm balloon catheter and 4 F cannula were not able to be advanced into the puncture site. The 4 F cannula was initially used in 7 patients (25%) to manipulate the guidewire in place in the desired intrahepatic duct. In the remaining patients, direct use of the 4 mm balloon catheter for fistula dilation was performed after the guidewire was placed in the desired left hepatic duct. Serum total bilirubin decreased significantly within 1 mo after EUS-HGS (10.3 ± 9.4 to 3.7 ± 5.1 mg/dL, P < 0.001). During mean follow-up period of 5.2 mo, no patients received the second treatments such as pancreaticoduodenectomy or gastrojejunostomy after EUS-HGS.
| Characteristics | EUS-BD (n = 28) |
| Median age (range, yr) | 63 (29-87) |
| Male | 20 (71) |
| Indication | |
| Malignant (n = 25) | |
| Cholangiocarcinoma | 10 |
| Pancreatic cancer | 5 |
| Gallbladder cancer | 2 |
| Stomach cancer | 2 |
| Ampulla of Vater cancer | 1 |
| Colon cancer | 1 |
| Duodenal cancer | 1 |
| Hepatocellular carcinoma | 1 |
| Intraductal papillary neoplasm of bile duct | 1 |
| Lymphoma | 1 |
| Benign (n = 3) | |
| Benign IHD stricture | 1 |
| Postoperative anastomosis site stricture | 1 |
| IgG4-related sclerosing cholangitis | 1 |
| Surgically altered anatomy | 6 (21) |
| Outcome | EUS-HGS (n = 28) |
| Technical success rate | 27 (96) |
| Clinical success rate | 24 (89) |
| Median length of stent (range), cm | 8.5 (5-10) |
| Procedure time, mean ± SD, min | 15.6 ± 5.8 |
| Median stent patency (95%CI), d | 150 (5-295) |
| Early adverse event | 0 |
| Late adverse event | 2 (7) |
| Overall survival (95%CI), mo | 7.5 (5.0-12.0) |
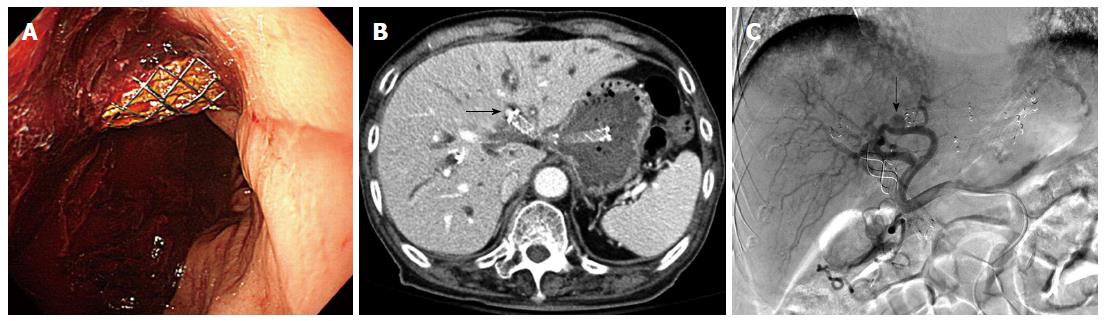
The overall adverse event rate was 7% (2/28). There were no early adverse events after EUS-HGS with the modified technique. However, two late adverse events were reported: gastric migration of the stent and bleeding from a pseudoaneurysm. The partially migrated stent was removed successfully with a rat tooth forceps, and a new FCSMES was inserted through the fistula tract. The patient with pseudoaneurysm was presented as hematemesis 8 mo after EUS-HGS. There was huge fresh blood clot attached to stent in endoscopic finding (Figure 4A). Since pseudoaneurysm from left hepatic artery was noted around the proximal end of hepaticogastrostomy stent in CT (Figure 4B), hemostasis was achieved by urgent embolization of the feeding vessel from the left hepatic artery (Figure 4C). After embolization, the patient fully recovered and remained alive without any biliary re-intervention 6 mo later.
In the age and etiology matched case-control study, there was no difference in baseline characteristics, technical success, clinical success, median stent patency, and overall adverse events in each group (Table 3). However, the procedural time (15.3 ± 5.2 vs 22.3 ± 6.0 min, P < 0.001) and early adverse events (0% vs 26%, P = 0.02) were statistically improved in the case cohort compared with the control cohort (Table 3).
| Characteristics | Modified method(n = 23) | Conventional method(n = 23) | P value |
| Age mean ± SD, yr | 62.9 ± 14.6 | 64.1 ± 12.8 | 0.88 |
| Male | 17 (74) | 12 (52) | 0.13 |
| Etiology of bile duct obstruction | > 0.99 | ||
| Benign | 1 | 1 | |
| Perihilar lesion | 15 | 15 | |
| Periampullary lesion | 5 | 5 | |
| Peribiliary or metastatic lymph node | 2 | 2 | |
| Surgically altered anatomy | 6 | 6 | > 0.99 |
| Failed guidewire manipulation during ERCP or EUS-guided rendezvous | 9 | 7 | 0.54 |
| Technical success rate1 | 22 (96) | 21 (91) | > 0.99 |
| Functional success rate2 | 20 (91) | 16 (76) | 0.24 |
| % of needle knife use in two groups | 1 (4) | 1 (4) | > 0.99 |
| Procedure time, mean ± SD, min | 15.3 ± 5.2 | 22.3 ± 6.0 | < 0.001 |
| Median stent patency (95%CI), d | 216 (73-359) | 129 (64-194) | 0.73 |
| Total adverse event (%) | 2 (9) | 8 (35) | 0.07 |
| Early | 0 | 6 (26)3 | 0.02 |
| Late | 2 (9) | 2 (9)4 | > 0.99 |
| Overall survival (95%CI), mo | 7.5 (5.6-9.4) | 4.3 (1.8-6.8) | 0.27 |
Although many studies of EUS-BD have shown promising results in terms of the technique’s efficacy and safety[4,6,7,20], studies on standard techniques or dedicated devices for EUS-BD are lacking. The absence of such studies may limit the general popularity of EUS-BD and cause it to be performed only by experts in a few tertiary centers. Graded dilation may be preferred to cautery dilation for the creation and dilation of the fistula in EUS-BD in terms of safety. However, graded dilation is not always successful, and it can result in a longer procedural time. Although needle knife cautery may reduce the procedural time, postprocedure adverse events may occur[4]. In the present study, one-stage fistula dilation was possible in 96% of cases by using a 4 mm-balloon dilation catheter with a stainless steel stylet. This simple step may shorten the procedural time in EUS-HGS without increasing postprocedure adverse events. We used a modified deployment technique with a metal stent with dual flaps for the prevention of proximal and distal migration during the procedure and follow-up periods. As in our previous study[17], this stent showed a reliable antimigration effect.
In spite of repeated graded tract dilations with bougie catheter were done to prevent the use of a needle knife in patients with conventional method, the adverse events were not decreased compared to previous study[4]. The dilation force of a bougie dilator or a needle knife is axial. The axial force by bougie dilator may widen the gap between the liver and the stomach during fistula dilation[21], which can lead to increase the stent migration or pneumoperitoneum. Actually, almost postprocedural adverse events occurred in conventional group were stent migration (3/6) or self-limited pneumoperitoneum (2/6). In contrast, because the dilation force of a balloon catheter is radial, the separation of tissue planes is minimized and the tract dilation can be done stably within a short period of time. Since the radial force tends to increase the risk of perforation[21], we performed the tract dilation with a balloon catheter in limited points shortly and used CO2 insufflation during procedure. Furthermore, this minimal dilation in the fistula tract may result in easy deployment of the metal stent because 4 mm balloon in the fistula tract and fistula site may obviate the resistance of the deployed metal stent and reduce the distance between the gastric wall and liver during a stent placement. This may minimize the chance of a bile leak or pneumoperitoneum.
Our previous two studies showed a comparable procedural time and no reduction in the procedural time, despite possible technical proficiency with time trends[6,7]. The simple dilation method in the present study with the modified stent deployment, which shortened the procedural time, may be used by endoscopists with various experiences of EUS-BD. Although there are various sizes of balloon catheter, we chose the smallest, a 4 mm (12 F) balloon catheter, because the minimal fistula dilation obtained with this size of catheter is sufficient to insert any commercially used catheters or stent delivery devices without resistance.
During stent deployment, echoendoscopic and fluoroscopic findings may be more important than endoscopic findings because the latter in the high body of the stomach or cardia during stent deployment may lead to instability of the proximal and distal position of the metal stent. In this study, we used an 8 mm diameter metal stent with dual flaps for stent deployment inside the bile duct (half of metal stent) and echoendoscope channel (remaining portion of the metal stent). This modified deployment maneuver may secure the stable position of the deployed metal stent and reduce the chance of proximal and distal migration. Usually, longer stents (up to 12 cm in length) are used to prevent proximal or distal migration[2]. However, such stents may result in the need for stent revision or encourage sludge formation[22]. Using the modified technique of stent deployment described herein, a stent with a more appropriate length may be placed.
In one patient with malignant hilar stricture, hematemesis from a left hepatic artery pseudoaneurysm occurred 8 mo after successful EUS-HGS (8 mm in diameter, 7 cm in length). Urgent feeding artery embolization was performed. As a hepatic artery pseudoaneurysm may occur after PTBD and after PTBD-assisted metal stent placement[23-25], EUS-HGS with long-dwelling metal stents may be associated with left hepatic artery pseudoaneurysm. After intrahepatic biliary decompression, the relatively large diameter of FCSEMS may erode the intrahepatic bile duct, resulting in a left hepatic artery pseudoaneurysm. The presence of pseudoaneurysm at the tip of the stent may suggest the possibility of its development due to compression of the arterial wall by the metal stent. Further larger studies of metal stents with a modified proximal tip (e.g., an uncovered portion without flared ends or a flap or with a smaller diameter) may be needed to address this issue.
This study has some limitations. In phase II, our prospective case series was retrospectively compared with a matched control. Although a matched case-control study may eliminate various confounding factors, it may provide less evidence for causal inference than randomized controlled trials. As our previous study showed a relatively higher postprocedural adverse event rate with the use of a needle knife when graded dilation was not feasible for fistula dilation and one intraperitoneal stent migration with a conventional stent deployment[12], a randomized trial comparing our modified with conventional method in EUS-BD may be impractical. Therefore, we performed a matched case-control study rather than a randomized trial.
The difference of stent design might have affected the postprocedural stent migration. With regard to the antimigration effect of FCSEMS for benign biliary stricture, the anchoring flap design was superior to the flared end design[17]. However, the FCSEMS with a flared end may be enough preventing migration for very tight biliary stricture[6,7]. There is a waist in the middle of the stent at the site of hepaticogastrostomy site when the metal stent is initially inserted. Therefore, it seems that the difference of stent design would not be a significant factor affecting early stent migration in EUS-HGS. However, if the hepaticogastrostomy site is dilated by the expansion of metal stent, there could be a difference of stent migration between anchoring flap and flared end like benign biliary strictures. Since the follow-up period was not enough in this study, there was no significant difference of late stent migration between two groups. In order to verify about this issue, further long-term studies will be required.
The procedural time and adverse event rate may be associated with proficiency in time trend. After about 60 cases of EUS-BD were performed, cohort control with prospectively maintained database were collected before the phase I period. Therefore, the effect of the difference in the technical proficiency of the operator on procedural time trends is likely minimal. Furthermore, CO2 insufflation was applied in both the case and the control cohort. These efforts may minimize the selection bias from the two groups in terms of safety.
PTBD is still standard care in patients with unsuccessful biliary decompression of ERCP[26]. For randomized trials of EUS-BD and PTBD for biliary decompression after failed ERCP, a standard protocol with a dedicated device for EUS-BD may be a prerequisite because previous multicenter studies with nonstandardized protocols and dedicated devices for EUS-BD showed relatively higher adverse event rates[27-29]. EUS-BD is still an evolving technique. Thus, standard and internationally accepted protocols for EUS-BD may be required before randomized trials of EUS-BD and PTBD.
In summary, our simplified and modified technique for EUS-HGS with a 4 mm balloon catheter with a stainless steel stylet and stent deployment maneuver inside the bile duct and echoendoscope channel appears to shorten the procedural time and reduce postprocedure adverse events compared with the conventional method. A multicenter study may be warranted to confirm our results. Based on our experience, a 4 mm balloon catheter with a stainless steel stylet may be considered as a platform for a dedicated EUS-HGS device for fistula dilation.
Dr. Sang Soo Lee provided the concepts of dual flap of metal stent.
Endoscopic ultrasonography-guided hepaticogastrostomy with direct transluminal stenting (EUS-HGS) is a complex procedure in terms of guidewire manipulation, fistula dilation, and stent deployment.
EUS-guided biliary drainage is an emerging alternative to percutaneous transhepatic biliary drainage or surgery after failed endoscopic retrograde cholangiopancreatography. A substantial number of patients in which the intrahepatic approach of guidewire manipulation was used may eventually require EUS-HGS. However, the standard technique for EUS-HGS has not been established yet.
To increase the success rate and to decrease the adverse events rate in EUS-HGS, maintaining the scope position during the procedure and shortening the procedural time are important. The simplified and modified technique for EUS-HGS with a 4 mm balloon catheter with a stainless steel stylet and stent deployment maneuver inside the bile duct and echoendoscope channel appears to shorten the procedural time and reduce postprocedure adverse events compared with the conventional method.
The study results suggest that a 4 mm balloon catheter with a stainless steel stylet may be considered as a platform for a dedicated EUS-HGS device for fistula dilation.
EUS-HGS: The dilated intrahepatic bile duct is punctured under EUS-guidance. Then bile duct is opacified by injection of contrast medium under fluoroscopic guidance. After identifying the biliary obstruction, the fistula is dilated and a stent is placed across the fistula to drain the bile juice.
The authors suggested the simplified and modified EUS-HGS technique, and compared with conventional method. The simplified technique for fistula dilation with 4 mm balloon catheter with stainless steel stylet and modified stent deployment maneuver in EUS-HGS was associated with shortened procedural time and reduced postprocedure adverse event.
P- Reviewers: Hashimoto N, Ikemoto T, Teoh AYB S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Park do H. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am. 2012;22:271-80, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 5. | Eum J, Park do H, Ryu CH, Kim HJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos). Gastrointest Endosc. 2010;72:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010;71:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Giovannini M, Bories E. EUS-Guided Biliary Drainage. Gastroenterol Res Pract. 2012;2012:348719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Savides TJ, Varadarajulu S, Palazzo L. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest Endosc. 2013;78:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided transhepatic antegrade balloon dilation for benign bilioenteric anastomotic strictures in a patient with hepaticojejunostomy. Gastrointest Endosc. 2012;75:692-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Varadarajulu S. EUS followed by endoscopic pancreatic pseudocyst drainage or all-in-one procedure: a review of basic techniques (with video). Gastrointest Endosc. 2009;69:S176-S181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Itoi T, Isayama H, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tanaka R. Stent selection and tips on placement technique of EUS-guided biliary drainage: transduodenal and transgastric stenting. J Hepatobiliary Pancreat Sci. 2011;18:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Park do H, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, Park SH, Lee SK, Kim MH, Kim SJ. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc. 2011;73:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1851] [Article Influence: 123.4] [Reference Citation Analysis (1)] |
| 19. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] |
| 20. | Khashab MA, Valeshabad AK, Modayil R, Widmer J, Saxena P, Idrees M, Iqbal S, Kalloo AN, Stavropoulos SN. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: rendezvous versus direct transluminal techniques (with videos). Gastrointest Endosc. 2013;78:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Hamada T, Nakai Y, Isayama H, Saito K, Kogure H, Sasaki T, Yamamoto N, Hirano K, Tada M, Koike K. Trimming a covered metal stent during hepaticogastrostomy by using argon plasma coagulation. Gastrointest Endosc. 2013;78:817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Gückel C, Steinbrich W. Arterial pseudoaneurysm as a complication of percutaneous transhepatic biliary drainage: treatment by embolization. Z Gastroenterol. 1995;33:602-604. [PubMed] |
| 24. | Almogy G, Bloom A, Verstandig A, Eid A. Hepatic artery pseudoaneurysm after liver transplantation. A result of transhepatic biliary drainage for primary sclerosing cholangitis. Transpl Int. 2002;15:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Rai R, Rose J, Manas D. Potentially fatal haemobilia due to inappropriate use of an expanding biliary stent. World J Gastroenterol. 2003;9:2377-2378. [PubMed] |
| 26. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, Abadia MA, Pérez-Millán A, González-Huix F, Gornals J, Iglesias-Garcia J, De la Serna C, Aparicio JR. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012;76:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Dhir V, Artifon EL, Gupta K, Vila JJ, Maselli R, Frazao M, Maydeo A. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: Choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Kawakubo K, Isayama H, Kato H, Itoi T, Kawakami H, Hanada K, Ishiwatari H, Yasuda I, Kawamoto H, Itokawa F. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |