Published online May 7, 2014. doi: 10.3748/wjg.v20.i17.4994
Revised: September 4, 2013
Accepted: September 16, 2013
Published online: May 7, 2014
Processing time: 344 Days and 16.6 Hours
AIM: To determine calprotectin release before and after colorectal cancer operation and compare it to tumor and histopathological parameters.
METHODS: The study was performed on patients with diagnosed colorectal cancer admitted for operation. Calprotectin was measured in a single stool sample before and three months after the operation using an enzyme-linked immunosorbent assay (ELISA). Calprotectin levels greater than or equal to 50 μg/g were considered positive. The compliance for collecting stool samples was assessed and the value of calprotectin was correlated to tumor and histopathological parameters of intra- and peri-tumoral inflammation. Surgical specimens were fixed in neutral buffered formalin and stained with hematoxylin and eosin. Staging was performed according to the Dukes classification system and the 7th edition tumor node metastasis classification system. Intra- and peri-tumoral inflammation was graded according to the Klintrup criteria. Immunohistochemical quantification was performed for MPO, CD45R0, TIA-1, CD3, CD4, CD8, CD57, and granzyme B. Statistical significance was measured using Wilcoxon signed rank test, Kruskal Wallis test and Spearman’s rank correlation coefficient as appropriate.
RESULTS: Between March 2009 and May 2011, 80 patients with colorectal cancer (46 men and 34 women, with mean age of 71 ± 11.7 years old) were enrolled in the study. Twenty-six patients had rectal carcinoma, 29 had left-side tumors, 23 had right-side tumors, and 2 had bilateral carcinoma. In total, 71.2% of the patients had increased levels of calprotectin before the operation (median 205 μg/g, range 50-2405 μg/g) and experienced a significant decrease three months after the operation (46 μg/g, range 10-384 μg/g, P < 0001). The compliance for collecting stool samples was 89.5%. Patients with T3 and T4 tumors had significantly higher values than those with T1 and T2 cancers (P = 0.022). For all other tumor parameters (N, M, G, L, V, Pn) and location, no significant difference in calprotectin concentration was found. Furthermore, the calprotectin levels and histological grading of both peri- and intra-tumoral inflammation was not correlated. Additional testing with specific markers for lymphocytes and neutrophils also revealed no statistically significant correlation.
CONCLUSION: Fecal calprotectin decreases significantly after colorectal cancer operation. Its value depends exclusively on the individual T-stage, but not on other tumor or histopathological parameters.
Core tip: Colorectal cancer (CRC) patients have a significant increase of fecal calprotectin release. The mechanisms for this observation are unclear. In our study, we examined the calprotectin release before and after operation of 46 CRC patients. This is the first study that assessed the correlation of calprotectin with both tumor as well as histopathological parameters. Our study contains the following new information: (1) the release of calprotectin is exclusively correlated to the T-stage, but to no histopathological parameters; and (2) except the T-stage, all other tumor characteristics assessed by the seventh edition of the tumor node metastasis classification are not correlated.
- Citation: Lehmann FS, Trapani F, Fueglistaler I, Terracciano LM, von Flüe M, Cathomas G, Zettl A, Benkert P, Oertli D, Beglinger C. Clinical and histopathological correlations of fecal calprotectin release in colorectal carcinoma. World J Gastroenterol 2014; 20(17): 4994-4999
- URL: https://www.wjgnet.com/1007-9327/full/v20/i17/4994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i17.4994
Colorectal cancer (CRC) is the third most common malignancy in the world and accounts for more than 10% of all cancer deaths[1,2]. Recent studies have shown that stool parameters such as calprotectin and lactoferrin are increased in many CRC patients[3-8]. The increase in calprotectin in CRC patients is, however, highly variable with levels ranging from insignificant to 100% sensitivity[9]. In fact, a meta-analysis by von Roon et al[10] revealed the increase of calprotectin in CRC patients was not recommended as screening tool for CRC. Calprotectin is a small calcium-binding protein consisting of two heavy and one light polypeptide chains. It is found in abundance in neutrophilic granulocytes, where it accounts for 60% of the cytosolic fraction, as well as in monocytes and macrophages[11,12].
CRC is associated with a local acute inflammatory reaction of variable intensity. The recruitment of neutrophils to the tumor site is hypothesized to be due to the local release of chemotactic factors[4,6,7]. Calprotectin enters the bowel lumen by migration rather than by bleeding or shedding of tumor cells. The neutrophilic infiltrate is variable and might be related to the tumor size, suggesting calprotectin would be a less sensitive marker in smaller tumors[13].
To date, no correlation of calprotectin and tumor parameters including tumor localization, size or stages has been found, as assessed by the Dukes classification or older TNM classifications[6,7]. In our study, we used the seventh edition of the TNM classification introducing additional components (G, L, V and Pn)[14]. In addition, our study contains the first systematic assessment of different histopathological markers to examine the correlation of calprotectin and parameters of peri- as well as intratumoral inflammation. We measured fecal calprotectin concentrations in patients with proven CRC before and after operation and correlated the results to tumor and histopathological parameters. We hypothesized that increased calprotectin levels were related to the T-stage, as well as to the grading of peri- and intratumoral inflammation and specific neutrophil markers.
Eighty patients with proven CRC, admitted for treatment to one of the following hospitals: University Hospital of Basel, St. Claraspital Basel and the Bruderholzspital, Switzerland, were included. The study was carried out according to the Principles of the Declaration of Helsinki and the protocol was accepted by the local ethical committee. All patients gave written informed consent before participating in any protocol-specific procedures.
Calprotectin was measured in a single stool sample from each patient collected in the hospital 24 h prior to the operation and 3 mo after hospital discharge. Samples were stored at 4 °C before transfer to the laboratory (Viollier Laboratories, Basel, Switzerland) within 48 h for analysis. Calprotectin is stable up to seven days at room temperature[3].
Fecal calprotectin levels were determined using an enzyme-linked immunosorbent assay (ELISA) (Viollier Laboratories, Basel, Switzerland). Aliquots of approximately 100 mg feces were homogenized in 5 mL extraction buffer. Two mL of the homogenate was centrifuged for 5 min at 3000 g and 100 μL of the diluted supernatant (1:50 with incubation buffer) were incubated at room temperature onto a microtiter plate coated with a monoclonal capture antibody highly specific to the calprotectin heterodimeric and polymeric complexes. After incubation, washing, a second incubation with a specific detection antibody, and a further washing step, tetramethylbenzidine (blue color formation) followed by a stop solution (change to yellow color) were added. The absorption was determined at an optical density of 450 nm. The measuring range of the test was 10-600 μg calprotectin/g feces with an intra- and inter-assay coefficient of 4.7% and 4.1%, respectively. Calprotectin levels greater than or equal to 50 μg/g were considered positive. All fecal samples were processed within 72 h after collection. The laboratory personnel carrying out the analysis was blinded to the clinical history of the patients.
Surgical specimens were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin. Staging was performed according to the Dukes classification and the 7th edition of the TNM classification by the Union for International Cancer Control[14]. Blinded senior pathologists examined all specimens.
Peri- and intratumoral inflammation were graded from 1-3 according to the Klintrup criteria as used by Richards et al[15]. Immunohistochemical quantification (score 0-3) was performed for MPO, CD45R0, TIA-1, CD3, CD4, CD8, CD57 and granzyme B. Grading and immunohistochemistry were performed in 49 patients. Sections (4 μm) of paraffin embedded tissue were immunostained for the antibody (Table 1). Staining was carried out according to the manufacturer’s protocol (Table 1). Negative controls for all proteins consisted of omission of the primary antibody. Three microscopic images (× 40) from each sample were obtained as representative of tissue type, distinct from lymphoid aggregation and within the area of most positive staining. The number of positive cells was counted in tumor and stromal tissue to give a score of inflammatory cellular infiltrate.
| Antibody | Clone | Dilution | Technique |
| CD 45R0 (DAKO) | UHCL-1 | 1:1600 | ABC |
| TIA-1 (IMMUNOTECH) | 2G9A10F5 | 1:1000 | ABC |
| CD3 (VENTANA) | 2GV6 | Pre-diluted | Benchmark XT |
| CD4 (VENTANA) | SP-35 | Pre-diluted | Benchmark XT |
| CD8 (VENTANA) | SP-57 | Pre-diluted | Benchmark XT |
| CD57 (VENTANA) | NK-1 | Pre-diluted | Benchmark XT |
| Granzyme B (VENTANA) | Polyclonal | Pre-diluted | Benchmark XT |
Immunostaining was performed as described previously[16,17]. Briefly, after dewaxing and rehydration in dH2O, sections for immunostaining were subjected to heat antigen retrieval in a microwave oven (1200 W, 60 min) in 0.01 mol/L citrate buffer pH 7.0 for TIA-1. Endogenous peroxidase activity was blocked using 0.5% H2O2. After transfer to a humidified chamber, the sections were incubated with 10% normal goat serum (Dako Cytomation) for 20 min and incubated with primary antibody overnight at 4 °C (CD45RO and TIA-1) Sections were then incubated with peroxidase-labeled polymer {K4005, EnVision + System-Horseradish Peroxidase (HRP) [3-amino-9-ethylcarbazole (AEC)]; DakoCytomation} for 30 min at room temperature.
For visualization of the antigen, sections were immersed in AEC + substrate-chromogen [K4005, EnVision + System-HRP (AEC); DakoCytomation] for 30 min and lightly counterstained with Harris’s hematoxylin), Ventana BenchMark XT system was used for immunohistochemical analysis.
The proportion of patients with pathological calprotectin concentrations was estimated together with the 95%CI. Pre- and post-operative calprotectin concentrations were compared by a Wilcoxon signed rank test. For various descriptors, calprotectin concentrations between patients with different factor levels were compared using Kruskal-Wallis test. The correlation between calprotectin concentration and various histopathological variables was assessed graphically as well as based on Spearman’s rank correlation coefficient. All analyses were performed with R (version 2.13.2). A two-sided P value < 0.05 was considered significant.
Eighty patients with proven CRC (46 men, 34 women, 71 +/- 11.7 years old) were included in the final analysis. A second calprotectin level 3 mo after the operation was only determined if the first concentration was > 50 μg/g. A second assay was not possible in nine patients, six denied a second test, one was not operated on and two could not be asked for ethical reasons. The compliance for collecting stool samples was 89.5%. Baseline characteristics are shown in Table 2.
| Characteristics | Statistics | |
| Calprotectin | (n = 80) | |
| Demographic data | ||
| Males | 46 (57.5) | |
| Age (yr) | 71.1 ± 11.7 | |
| Tumor location | ||
| Left | 29 (36.2) | |
| Right | 23 (28.7) | |
| Rectum | 26 (32.5) | |
| Two locations | 2 (2.5) | |
| Tumor classification | ||
| T1 | 5 (6.2) | |
| T2 | 12 (15.0) | |
| T3 | 49 (61.3) | |
| T4 | 13 (16.2) | |
| N0 | 44 (55.0) | |
| N1 | 16 (20.0) | |
| N2 | 18 (22.5) | |
| M0 | 71 (88.8) | |
| M1 | 8 (10.0) | |
| G1 | 1 (1.2) | |
| G2 | 57 (71.2) | |
| G2-3 | 3 (3.8) | |
| G3 | 13 (16.2) | |
| V0 | 62 (77.5) | |
| V1 | 14 (17.5) | |
| Pn0 | 64 (80.0) | |
| Pn1 | 12 (15.0) | |
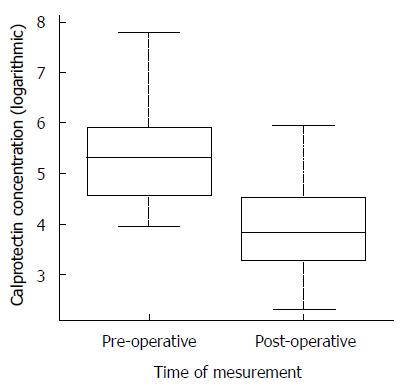
In 57 of 80 patients (71.2%, 95%CI: 60.1%-80.3%), calprotectin was significantly increased (> 50 μg/g). The median fecal calprotectin concentration was 205 μg/g (range 50-2405 μg/g) before and 46 μg/g (range 10-384 μg/g) three months after the operation (P < 0.001, Figure 1).
Twenty-six patients had rectal carcinoma, 29 had tumors of the left side, 23 had right-side tumors and two had a double carcinoma. No significant difference in calprotectin concentration was found between the three locations. Patients with T3 and T4 tumors had significantly higher calprotectin values than those with T1 and T2 stages (P = 0.022). For all other tumor parameters (N, M, G, L, V, Pn), no significant difference in calprotectin concentration was found between the factor levels of the individual parameters. Further, no difference in calprotectin release was found between Dukes B (n = 39) and Dukes C (n = 29) stages (P = 0.132).
Peri- and intratumoral inflammation was graded from 1 - 3 according to the Klintrup criteria as used by Richards et al[15]. Calprotectin levels and histological grading of both peri- and intratumoral inflammation were not correlated. Additional testing with specific markers for lymphocytes and neutrophils such as CD3, CD4, CD8, CD45, TIA-1, granzyme B and myeloperoxidase also revealed no statistically significant correlation (Table 3).
| Spearman’s rho | P value | |
| CD45.R.intra | -0.19 | 0.18 |
| CD45.peri | 0.05 | 0.74 |
| CD3.intra | 0.03 | 0.85 |
| CD3.peri | -0.1 | 0.47 |
| CD8.intra | -0.04 | 0.76 |
| CD8.peri | -0.14 | 0.31 |
| CD4.intra | -0.07 | 0.64 |
| CD4.peri | -0.06 | 0.66 |
| TIA1.itra | 0.06 | 0.66 |
| TIR1.peri | 0.05 | 0.71 |
| Granzyma.intra | -0.08 | 0.57 |
| Granzyma.peri | 0.09 | 0.52 |
| CD57.intra | 0.02 | 0.91 |
| CD57.peri | -0.14 | 0.31 |
| MPO.intra | 0.12 | 0.4 |
| MPO.peri | 0.09 | 0.55 |
We examined the calprotectin release before and after operation of 46 colorectal cancer patients. This is the first study that assessed the correlation of calprotectin with tumor and histopathological parameters. We found the release of calprotectin is exclusively correlated to the T-stage, but not to histopathological parameters. Further, we found all tumor characteristics assessed by the 7th edition of the TNM classification, with the exception of the T-stage, are not correlated with calprotectin.
Our results are in line with most previous studies showing significantly increased levels of fecal calprotectin in CRC patients[3-7]. While patients with active Crohn’s disease (CD) or ulcerative colitis (UC) exhibit more consistent elevated calprotectin levels, those from CRC patients are highly variable. In CRC, the sensitivity of calprotectin varies between 100%[9] and not significant[10], indicating it is not a suitable tool for CRC screening.
The significant fall in fecal calprotectin after surgical tumor removal was first described by the study group of Kristinsson et al[5] and Johne et al[18], although their participant numbers were smaller than in our study. Interestingly, that there is no corresponding decrease of elevated calprotectin after polypectomy[19].
We found T3 and T4 cancers significantly induce higher levels of calprotectin. This could be explained by the hypothesis that they attract more neutrophils than T1 and T2 tumors[13]. A correlation of fecal calprotectin and tumor size or T-stage has not been shown[7], with Kristinsson et al[5] providing the only indication that T1 and T2 tumors may be associated with lower calprotectin concentrations than T3 and T4 cancers. The lack of correlation between calprotectin and tumor localization, grading, as well as clinical stages in our patients is in line with findings from previous studies[5-7]. In our analysis, tumor characteristics have been assessed for the first time by the 7th edition of the TNM classification[14]. Previous studies used either Dukes or older TNM classifications. Our data revealed no difference in calprotectin release between Dukes B (n = 39) and Dukes C (n = 29) stages (P = 0.132). It would be of interest to correlate the calprotectin release with additional variables such as ESR, plasma CRP, blood platelets, LDH, as well as patient outcomes data (survival, time-to recurrence). However, these analyses were beyond the scope of this study.
Our study contains the first systematic assessment of different histopathological markers to examine the correlation of calprotectin and parameters of peri- as well as intratumoral inflammation. Various inflammatory cells, mainly along the invasive margin, infiltrate human CRC tissue. In colorectal tumors, calprotectin reactivity is found in granulocytes and macrophages, but not in neoplastic cells[4]. Increased amounts of granulocytes have been described in the stool of patients with CRC, possibly due to shedding from the ulcerated tumor[20,21]. It has been postulated that circulating leukocytes may actively migrate through neoplastic tissues in response to intraluminal antigens[4]. Interestingly, the immunohistochemical expression of calprotectin correlates with the degree of neutrophilic infiltration[22]. However, these studies are hampered by lack of a specific tissue marker. Kim et al[23] did show significant expression of the two calprotectin subunits S100A8 and S100A9 in tumor infiltrating lymphocytes.
In UC, calprotectin correlates significantly with clinical, endoscopic and histological parameters of disease activity[3,24,25]. The level of calprotectin seems to correlate more closely with the grading of histological than of endoscopic findings[26]. The concentration of calprotectin is directly proportional to the intensity of the neutrophilic infiltrate in the gut mucosa[26]. Active UC is characterized by a 10-fold or more increased migration of neutrophils from the circulation to the inflamed colon mucosa. Røseth et al[24] demonstrated the microscopic inflammation was graded 0 (normal mucosa) to 3 (extensive crypt injury with crypt abscesses and ulcerations). The correlation of histological grading and calprotectin concentration was statistically significant (P < 0001). In our study, the Klintrup score was used for histological grading of peri- and intratumoral inflammation. Several clinical studies have clearly shown that the grading of local inflammation as assessed by the Klintrup criteria is an independent predictor of survival in colon and rectal cancers[15,27,28]. In contrast to the findings in patients with UC, grading of tumor-associated inflammation was not correlated with calprotectin concentrations. The lack of correlation applies to the individual markers for lymphocytes and neutrophils as well. There are several explanations for this obvious discrepancy: (1) peri- and intratumoral inflammation are local. This is also expressed by the lower calprotectin concentration in CRC in comparison to active IBD[10]; (2) the local inflammatory reaction in CRC is of variable intensity; and (3) tumor-associated inflammation is not uniformly characterized by a significant amount of neutrophils[9]. This is in line with the observation that leukocyte scintigraphy is only sometimes positive in CRC patients[21].
In a current review of Gisbert et al[29], it has been questioned whether the need to collect one or several fecal samples might be a disadvantage for clinical use of calprotectin. This could not be confirmed in our study. The compliance rate in our study was 89.5% with only 6 out of 57 patients with increased calprotectin denying a second stool test. This is in the same range as the compliance rate of 96% in 602 patients referred for colonoscopy described by Tibble et al[30]. For a longer follow-up period, the compliance rate might be lower.
In summary, we have shown that most colorectal cancer patients have increased levels of fecal calprotectin, which is followed by a significant fall after the operation. Patients with T3 and T4 tumors have significantly higher calprotectin values than those with T1 and T2 stages.
Previous studies have shown a significant, but highly variable increase of fecal calprotectin release in patients with colorectal cancer (CRC). The mechanisms for this observation are not fully elucidated. CRC is associated with a significant recruitment of neutrophils to the tumor site. Activated neutrophils, monocytes and macrophages are believed to be the cellular source of calprotectin release.
The increase of calprotectin in CRC is highly variable. The analysis of fecal calprotectin may have the potential for clinical CRC surveillance. In most previous studies, no correlation of calprotectin and tumor parameters assessed by older classification systems could be found. To better characterize the role of calprotectin in CRC, its correlation with tumor as well as histopathological parameters should be examined.
The study contains the following relevant information: (1) calprotectin shows a significant decrease three months after the operation; (2) the release of calprotectin is exclusively correlated to the T-stage, but not to histopathological parameters; and (3) except the T-stage, all other tumor characteristics assessed by the 7th edition of the TNM classification are not correlated.
The study results clearly indicate that fecal calprotectin cannot be used for colorectal cancer screening. In patients with initially elevated calprotectin, its role could be tested in the clinical follow up of CRC patients.
Calprotectin is a small calcium-binding protein consisting of two heavy and one light polypeptide chains. It is found in abundance in neutrophilic granulocytes, in which it accounts for 60% of the cytosolic fraction, as well as in monocytes and macrophages. It is a simple, rapid, sensitive, inexpensive and non-invasive marker to detect and monitor intestinal inflammation, but is not disease-specific.
This is a simple study with a clear message that at least in some cases the tumor node metastasis classification may have advantages over the traditional Dukes or Stage classification of CRC which are the dominant systems, even in the reporting of clinical trials. The authors make the link between granulocytes (neutrophils) and degree and extent of inflammation. It would be interesting to learn if the authors measured some other variables in this context, such as the ESR, or plasma CRP.
P- Reviewer: Breimer LH S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | Kievit J, Bruinvels DJ. Detection of recurrence after surgery for colorectal cancer. Eur J Cancer. 1995;31A:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Davies RJ, Miller R, Coleman N. Colorectal cancer screening: prospects for molecular stool analysis. Nat Rev Cancer. 2005;5:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 492] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 4. | Røseth AG, Kristinsson J, Fagerhol MK, Schjønsby H, Aadland E, Nygaard K, Roald B. Faecal calprotectin: a novel test for the diagnosis of colorectal cancer? Scand J Gastroenterol. 1993;28:1073-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Kristinsson J, Røseth A, Fagerhol MK, Aadland E, Schjønsby H, Børmer OP, Raknerud N, Nygaard K. Fecal calprotectin concentration in patients with colorectal carcinoma. Dis Colon Rectum. 1998;41:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Tibble J, Sigthorsson G, Foster R, Sherwood R, Fagerhol M, Bjarnason I. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut. 2001;49:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Kristinsson J, Armbruster CH, Ugstad M, Kriwanek S, Nygaard K, Tøn H, Fuglerud P. Fecal excretion of calprotectin in colorectal cancer: relationship to tumor characteristics. Scand J Gastroenterol. 2001;36:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Uchida K, Matsuse R, Tomita S, Sugi K, Saitoh O, Ohshiba S. Immunochemical detection of human lactoferrin in feces as a new marker for inflammatory gastrointestinal disorders and colon cancer. Clin Biochem. 1994;27:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Damms A, Bischoff SC. Validation and clinical significance of a new calprotectin rapid test for the diagnosis of gastrointestinal diseases. Int J Colorectal Dis. 2008;23:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (3)] |
| 10. | von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (2)] |
| 11. | Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer’s patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993;34:1357-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 384] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | D'Incà R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG, Martines D, Sturniolo GC. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Sobin LH, Brierley J. Colon and Rectum. in Sobin LH, Gospodarowicz MK, Wittekind Ch, editors. TNM Classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell 2009; . |
| 15. | Richards CH, Roxburgh CS, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M, Terracciano L. CD8+ lymphocytes/ tumour-budding index: an independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br J Cancer. 2009;101:1382-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Lugli A, Tzankov A, Zlobec I, Terracciano LM. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod Pathol. 2008;21:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Johne B, Kronborg O, Tøn HI, Kristinsson J, Fuglerud P. A new fecal calprotectin test for colorectal neoplasia. Clinical results and comparison with previous method. Scand J Gastroenterol. 2001;36:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kronborg O, Ugstad M, Fuglerud P, Johne B, Hardcastle J, Scholefield JH, Vellacott K, Moshakis V, Reynolds JR. Faecal calprotectin levels in a high risk population for colorectal neoplasia. Gut. 2000;46:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Becker W, Schäffer R, Börner W. Sigmoid carcinoma mimicking an intra-abdominal abscess in an 111In-labeled white blood cell scan. Eur J Nucl Med. 1985;11:283-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Saverymuttu SH, Maltby P, Batman P, Joseph AE, Maxwell D. False positive localisation of indium-111 granulocytes in colonic carcinoma. Br J Radiol. 1986;59:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Luley K, Noack F, Lehnert H, Homann N. Local calprotectin production in colorectal cancer and polyps--active neutrophil recruitment in carcinogenesis. Int J Colorectal Dis. 2011;26:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Kim HJ, Kang HJ, Lee H, Lee ST, Yu MH, Kim H, Lee C. Identification of S100A8 and S100A9 as serological markers for colorectal cancer. J Proteome Res. 2009;8:1368-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 258] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Ricanek P, Brackmann S, Perminow G, Lyckander LG, Sponheim J, Holme O, Høie O, Rydning A, Vatn MH. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery for colon and rectal cancers. J Gastrointest Surg. 2009;13:2011-2018; discussion 2018-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Powell AG, Wallace R, McKee RF, Anderson JH, Going JJ, Edwards J, Horgan PG. The relationship between tumour site, clinicopathological characteristics and cancer-specific survival in patients undergoing surgery for colorectal cancer. Colorectal Dis. 2012;14:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Gisbert JP, McNicholl AG. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (2)] |