INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs), also known as islet cell tumors, are a rare form of endocrine neoplasms, accounting for a reported 1%-4% of all pancreatic tumors[1-3]. These tumors are associated with an annual incidence of one per 100000 population, and their diagnosis has increased over the past 40 years, most likely due to advances in imaging and histopathological methods[4-6]. PNETs can manifest at any age, however, most present during the 4th to 6th decades of life. When considered as a general entity, no gender predilection is demonstrated, but the various subtypes when observed separately do show slight gender predilection[7]. Although the majority of cases are sporadic, 10%-30% have been shown to be associated with multiple endocrine neoplasia (MEN) 1 syndrome, and < 1% with (Von Hippel-Lindau) VHL disease[8,9]. Other genetic syndromes in which PNETs may present include neurofibromatosis type 1 and tuberous sclerosis[4]. PNETs can be classified as functional and nonfunctional, the latter being far more common and typically presenting late during disease evolution, with symptoms related to mass effect, invasion into surrounding structures, or metastasis[3].
The most common form of functional PNET is insulinoma, accounting for 70%-80% of cases. Ninety percent are benign and solitary, and they are predominantly located in the body and the tail of the pancreas (65%-80%). Due to the fact that symptoms of hypoglycemia dominate the clinical picture early in the course of the disease, the majority are small in size (< 2 cm) at the time of presentation, and compatible with surgical resection[10-12]. This contrasts with gastrinomas, another type of functional PNET, which in more than 50% are extrapancreatic, tend to be larger in size, and present with metastasis in 60%-70% of cases. Other rare types include glucagonomas and vasoactive intestinal peptide-producing tumors (VIPomas), the majority of which are also malignant (80% and 60%, respectively). Somatostatinomas are typically large neoplasms, causing mass effect around the pancreatic head or periampullary region. The majority of these tumors are malignant (70%)[13].
It is due to the above-mentioned characteristics that the “non-insulinoma PNETs” are less suitable for surgical resection. That said, surgery remains the only curative modality for neuroendocrine neoplasms of the pancreas, and is the treatment of choice when technically feasible, even in the presence of malignancy and occasionally locally advanced or metastatic disease[14-19].
With advances in minimally invasive surgery, laparoscopic resection has become a well-accepted modality in the management of pancreatic tumors, with an increasing number of surgeons utilizing these techniques[18]. The use of laparoscopy in pancreatic surgery was initially introduced in 1994 by Gagner et al[20] and Cuschieri[21], and two years later, Gagner et al[22] reported on their early experience with laparoscopic resection of islet cell tumors. Since then, several publications have described laparoscopic pancreatic surgery, however, only a small number of large series have described laparoscopic surgery in the setting of PNETs[13,18,23,24]. The purpose of this article is to review the available literature on laparoscopic resection of PNETs, with a focus on the various surgical techniques, and compare laparoscopic surgery to open pancreatic surgery in terms of results and complications.
PREOPERATIVE LOCALIZATION
Preoperative localization of the neuroendocrine tumor is of utmost importance in the management of these neoplasms. Prior to considering laparoscopy, an expected surgical strategy must be contemplated in accordance with the findings on imaging studies.
Imaging studies provide information regarding the location of the tumor, the extent of local invasion, and the presence of metastatic lesions[7]. Localization studies commonly used include ultrasonography, computed tomography (CT) scanning, and magnetic resonance imaging (MRI). CT is generally the initial test used by clinicians to localize PNETs. On CT scans, these tumors typically appear hyperdense on arterial phase. Although there is great variation in the literature regarding the reported usefulness of this modality for the detection of PNETs, it is generally accepted that it has a sensitivity of less than 50%-60%[25-27]. Nevertheless, one study reported a sensitivity of 94% for CT in the detection of PNETs[28]. MRI has the advantage of decreased radiation when compared with CT, and is commonly used to detect small PNETs and to assess local invasion[29]. A sensitivity ranging from 30% up to 95% has been reported in the literature for the detection of PNETs[30,31].
A study that has gained popularity due to increased accuracy is endoscopic ultrasound (EUS), however, the disadvantage of this technique is operator dependence[32-35]. It has a higher success rate in localizing tumors of the head and body than those of the tail. This modality has been associated with a sensitivity of 80% to 88% and a specificity of 95%[13]. One study reported a sensitivity of 82% and a specificity of 95% for EUS in the localization of PNETs not identified by CT or angiography[36]. It is also worth noting that the combination of EUS with biphasic helical CT scanning has been demonstrated to increase the diagnostic accuracy to 97%[13]. Although EUS has been shown to be effective in the detection of regional lymph node involvement, its usefulness in the diagnosis of liver metastasis is largely limited[37].
Angiographic techniques with portal vein sampling are invasive methods, and are typically reserved for patients in whom other less invasive diagnostic tests have failed to localize the pathology. These methods have been shown to provide accurate regionalization (but not exact localization) in up to 90% of cases[38].
A functional study commonly utilized is somatostatin receptor scintigraphy (octreotide scan)[39]. A relatively new modality shown to be superior to the octreotide scan in the diagnosis of neuroendocrine tumors is gallium-68 somatostatin receptor PET scan, which utilizes radio-labeled tracers with affinity to somatostatic receptors to localize these tumors[40]. A recently published meta-analysis demonstrated that this imaging modality has a sensitivity of 93% (when 68Ga-DOTATOC is utilized) and 96% (when 68Ga-DOTATATE is utilized). The specificity was shown to be 85% and 100%, respectively[41]. It should be noted that the diagnostic yield of these tests is reduced in insulinomas, which may not express somatostatin receptors. The use of FDG-PET CT for the diagnosis of PNETs is limited, mainly due to the slow-growing nature of these tumors[42]. However, the ability of this test to detect more aggressive tumors (due to the fact that less differentiated tumors consume more glucose) has been proposed[43].
It appears that after establishing the localization of a lesion by more than one noninvasive study (for example, CT scan and MRI), it is reasonable to explore the patient laparoscopically, and to perform an intraoperative ultrasound (discussed below)[44]. Due to the relative safety and diagnostic accuracy of modern laparoscopic techniques along with the use of intraoperative ultrasound, many recommend that the utilization of more invasive preoperative diagnostic methods, such as angiography, be reserved for equivocal cases only[13].
DETERMINATION OF SURGICAL TECHNIQUE
Surgery remains the cornerstone of management of PNETs, with increased utilization of the laparoscopic approach demonstrated over the past two decades[45]. The planned surgical approach is governed largely by the findings in preoperative localization studies, but may commonly change in accordance with intraoperative findings.
There is no general consensus regarding the indications for and limitations of laparoscopic surgery for PNETs. Although some have claimed that the presence of a malignant PNET is a contraindication for laparoscopic resection[46], others have shown the feasibility and safety of laparoscopic surgery in these malignant tumors[24].
Laparoscopic enucleation is utilized in lesions less than 3 cm in size which are noninvasive and located peripherally and thereby do not involve the main pancreatic duct. When the above criteria are fulfilled, this procedure may be applicable for tumors located in the pancreatic head, body, or tail[47]. When the tumor is in proximity to the Wirsung duct, enucleation is not suitable due to the elevated risk of pancreatic fistula development[48]. Due to the fact that insulinomas are typically small, single and benign lesions, the use of laparoscopic enucleation for surgical management of these tumors has been widely described in the literature. The use of intraoperative ultrasound, however, is essential in order to rule out the presence of other lesions before the decision to perform enucleation is made, and to assess the proximity of these tumors to the pancreatic duct and vascular structures[48].
When the PNET is not compatible with enucleation, pancreatic resection is necessary[47]. In tumors involving the head of the pancreas, pancreaticoduodenectomy (Whipple Operation) is indicated. This procedure is not widely performed laparoscopically worldwide, due to the associated technical difficulties. However, many studies have shown the effectiveness and safety of this procedure, when performed by sufficiently-trained hepatobiliary or laparoscopic surgeons[49-52].
In tumors that are located in the body or the tail and are not suitable for enucleation, laparoscopic distal pancreatectomy is the treatment of choice. This surgery can be further divided into three different entities: spleen-preserving distal pancreatectomy with splenic vessel preservation, spleen-preserving distal pancreatectomy without splenic vessel preservation, and distal pancreatectomy with splenectomy[53]. The main factors which dictate the procedure chosen are the location of the tumor within the pancreatic body or tail, and its relation to the splenic vessels and splenic hilum. In addition, the presence or suspicion of malignant neuroendocrine tumors generally favors more radical approaches, with the resection of splenic vessels in order to enable adequate lymph node sampling. Ligation of splenic vessels is also advocated when uncontrolled bleeding from the vessels at the upper border of the pancreas is demonstrated intraoperatively[13]. This procedure is less technically demanding and is associated with shorter operation time. Ligation and transection of the splenic vessels is performed at the level of the pancreatic resection and at the splenic hilum. Postoperatively, the spleen receives its vascular supply from the short gastric vessels and left gastroepiploic vessels[13].
When possible, spleen-preserving distal pancreatectomy with splenic vessel preservation is performed; however, this procedure requires higher technical expertise, with separation of the splenic vessels from the pancreatic parenchyma, and the dissection and ligation of the branching vessels supplying the pancreas. As a result, this procedure is associated with a longer operating time[54-57]. Splenic preservation in these PNETs is generally encouraged when it is technically feasible; however, the occasional presence of hilar fibrosis due to previous inflammation can make splenic preservation difficult, and in these cases, en bloc pancreaticosplenectomy appears to be the safest option[58]. This is also true in malignant PNETs that involve or are adjacent to the hilum of the spleen and in these cases, the need for a complete oncologic resection supersedes the benefits of splenic preservation. That said, the avoidance of splenectomy can be achieved in the majority of cases[54-56]. In Assalia and Gagner’s publication, the rate of successful splenic salvage in laparoscopic distal pancreatectomy for islet cell tumors approached 85%[13].
It is worth mentioning that in the presence of a functioning PNET, medical control of the patient’s symptoms prior to surgical intervention is of utmost importance. Although a detailed discussion of these treatments is beyond the scope of this review, this generally entails strict regulation of blood glucose levels in insulinomas, proton pump inhibitor treatment in gastrinomas, etc. In addition, a multidisciplinary approach involving the endocrinologist, surgeon, and anesthesiologist is essential in order to ensure safe resection.
TECHNICAL ASPECTS OF LAPAROSCOPIC SURGERY FOR PNETS
Various surgical techniques for laparoscopic pancreatic surgery have been described in the literature with some modifications that are based on surgeons’ experience and preferences[13,22,48,53,55,59]. The following descriptions outline the important aspects and steps that are the basis for laparoscopic resections of PNETs. It is to be noted that the procedural description of laparoscopic pancreaticoduodenectomy (Whipple Operation) will not be described in this review.
Enucleation of tumors of the pancreatic head
After appropriate exposure of the pancreas, intraoperative laparoscopic ultrasonography is performed. Due to the lack of tactile sensation in laparoscopic surgery, this imaging tool is of utmost importance. It helps localize the tumor, rules out the presence of multiple lesions, and identifies the tumor’s relation to and distance from surrounding structures[60]. Depending on the location of the tumor, focal dissection is continued in order to provide maximal exposure prior to enucleation (Figure 1A shows a PNET involving the posterior aspect of the pancreatic head, following appropriate exposure). Laparoscopic ultrasound is used to again identify the exact location of the tumor and its relation to the Wirsung duct and the superior mesenteric vein (SMV). Under extensive care not to damage these structures (which would lead to postoperative leak in the case of damage of the pancreatic duct), electrocautery with the hook coagulator is utilized to dissect the parenchyma surrounding the tumor and perform enucleation. In tumors located at the inferior surface of the pancreatic head, the LigaSure device (Tyco, United States Surgical Volleylab, Boulder, Co. United States) may be used to incise the plane between the pancreas and the tumor. A surgical drain is left at the excision bed[48].
Figure 1 Tumors of the pancreatic head.
A: A pancreatic neuroendocrine tumor (PNET) involving the posterior aspect of the pancreatic head, after adequate exposure by extensive kocherization and medial retraction of the pancreatic head, prior to enucleation; B: A PNET involving the inferior border of the body/tail of the pancreas. Resection is being performed using the LigaSure device; C: PNETs located in the posterior aspect of the body/tail occasionally require partial resection of the splenic vein in order to perform successful enucleation; D: The intraoperative appearance after performance of spleen-preserving distal pancreatectomy with splenic vessel preservation; E: The intraoperative appearance after performance of spleen-preserving distal pancreatectomy without splenic vessel preservation. Figure 1D and E represent patients with multiple endocrine neoplasia-1, with an additional PNET located in the head. This synchronous tumor will be excised by enucleation.
Enucleation of tumors of the pancreatic body and tail
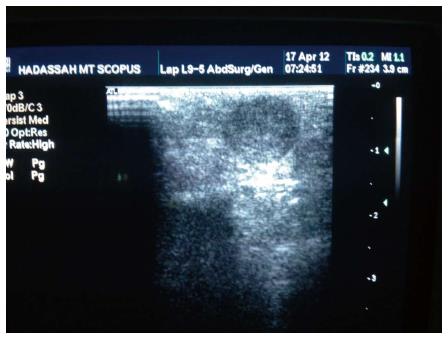
After sufficient surgical exposure and mobilization of the pancreatic body and tail, laparoscopic ultrasonography is performed for tumor localization and to identify the relationship with surrounding structures (the pancreatic duct and splenic vessels). Figure 2 demonstrates the intraoperative sonographic appearance of an insulinoma involving the pancreatic tail.
Figure 2 An image from intraoperative ultrasound demonstrating an insulinoma involving the pancreatic tail.
When the tumor is located anteriorly, an incision is made in the pancreatic capsule using electrocautery, and delicate dissection is carried out between the tumor and the normal pancreatic parenchyma until successful enucleation of the mass is achieved. Bleeding is controlled by clips and cautery.
Tumors located at the inferior pancreatic border are commonly resected by hemostatic dissection using the LigaSure device (Figure 1B).
Posteriorly-located tumors are commonly partially covered by the splenic vein. The inferior border of the pancreas is lifted up, allowing exposure of the posterior pancreatic surface. Occasionally enucleation is only possible after local resection of the adjacent portion of the vein (Figure 1C). In this process, injury to the splenic artery must be avoided. After enucleation, the tumor bed must be examined for evidence of pancreatic duct injury.
Tumors located in the distal portion of the tail of the pancreas are in very close proximity to the Wirsung Duct, and therefore enucleation of these tumors is commonly not recommended[48].
Spleen-preserving distal pancreatectomy with splenic vessel preservation
Exposure and mobilization of the body and tail of the pancreas is performed, as is mobilization of the splenic flexure. Adhesions are divided between the posterior surface of the stomach and the pancreas; however, care should be taken not to divide the short gastric and the left gastroepiploic vessels. After detaching the inferior pancreatic margin from the retroperitoneum, visualization of the posterior aspect of the pancreas is now feasible, as is identification of the SMV and the splenic vein forming the portal vein. Blunt dissection around the splenic vein is performed, with ligation of the small bridging vessels that reach the pancreas. After identification and preservation of the splenic artery, the pancreas is divided using an endoscopic stapler device. The body/tail of the pancreas is then anteriorly retracted, allowing further separation of small bridging vessels reaching the pancreas from the splenic artery and vein. The resection is completed after reaching the splenic hilum, and a surgical drain is left in proximity to the pancreatic stump[48] (Figure 1D).
Spleen-preserving distal pancreatectomy without splenic vessel preservation
This procedure follows the same course mentioned above until visualization of the posterior aspect of the pancreas and the splenic vein entering the SMV to form the portal vein. At this stage, clips are applied to the splenic vein and it is divided. The pancreas is then divided by endoscopic stapler, followed by ligation and division of the splenic artery. The pancreatic body and tail are retracted upwards (along with the attached splenic artery and vein), and these vessels are clipped and divided between the pancreatic tail and the splenic hilum. After this procedure, the sole remaining blood supply to the spleen is from the short gastric vessels and left gastroepiploic vessels, indicating the importance of their preservation in earlier steps[48] (Figure 1E).
Distal pancreatectomy with splenectomy
This procedure is similar to the previous technique (Spleen-preserving distal pancreatectomy without splenic vessel preservation) with a few exceptions. Unlike in spleen-preserving procedures, the short gastric and left gastroepiploic vessels can be ligated. In addition to mobilizing the splenic flexure (thereby exposing the inferior splenic border), the spleen’s lateral aspect is also mobilized, up to the left crus of the diaphragm. The splenic vessels can be divided along with the pancreas or separately. The specimens are typically extracted from the abdomen in two separate specimen bags. As in the previous procedure, a surgical drain is left in situ[13]. Note that some surgeons use different methods to seal the bed of tumor enucleation or the margins of resection, including adhesive biologic materials or sutures.
SURGERY IN PANCREATIC NEUROENDOCRINE CARCINOMA
According to the WHO 2010 classification, neuroendocrine carcinomas (NECs) are defined histopathologically as neuroendocrine tumors with a Ki-67 index above 20%[45]. These tumors are extremely invasive and aggressive, and fortunately they are rare, accounting for only 2%-3% of PNETs[45,61,62]. Radical surgery is generally indicated for locally advanced disease, followed by adjuvant chemotherapy. When there is evidence or suspicion of malignant disease, or when the tumor size is greater than 5 cm, it is recommended that a modified strasberg operation be performed[24]. This entails radical lymph node dissection of the peri-pancreatic, portal, hepatic, and superior mesenteric areas.
Not only does the literature support surgical excision of locally invasive disease, but a survival benefit has also been demonstrated after excision of metastases (in addition to the primary tumor) when technically feasible. Therefore, in selected patients with localized liver metastasis, it is recommended that a synchronous resection of the primary tumor and liver metastases should be attempted[14-18,63-68]. Nevertheless, the role of laparoscopy for these complicated procedures is yet to be clarified. Despite case reports of successful laparoscopic synchronous excision of the primary tumor and metastases this issue remains controversial as opponents claim that laparoscopic surgery may jeopardize the oncologic outcome and a planned open procedure must be carried out. As randomized controlled studies are unlikely this controversy will remain a matter of debate and it is reasonable to limit these procedures to highly experienced laparoscopic pancreatic/hepatobiliary surgeons.
SURGERY IN PNETS ASSOCIATED WITH MEN-1
More than 75% of patients with PNETs and MEN-1 have multiple pancreatic tumors, therefore enucleation alone in this clinical setting is likely to be inadequate[24,48]. Generally accepted indications for surgery in MEN-1 include the presence of a functioning PNET, in addition to nonfunctioning tumors of more than 2 cm in size[9,69]. However, some authors consider the diameter of 1 cm to be a safer cutoff, and advocate the surgical resection of nonfunctioning PNETs of more than 1 cm in diameter[69,70]. Smaller nonfunctioning tumors must be closely observed, and their rate of growth may subsequently provide an indication for surgical resection. The recommended surgical procedure for these patients seems to be intraoperative laparoscopic ultrasonography, followed by subtotal distal pancreatectomy (usually with splenic preservation), along with enucleation of any lesions in the pancreatic head (Figures 1D and E).
OUTCOMES OF LAPAROSCOPIC PANCREATIC SURGERY FOR PNETS
As previously mentioned, surgery is the curative modality of choice for PNETs, improving survival across all stages of the disease. Recent years have shown a significant increase in the laparoscopic approach in these surgeries[18]. In several centers worldwide, almost all patients with suspicious PNET of the pancreatic body or tail undergo laparoscopic surgery[24,48,71,72].
In a recently published series, 75 laparoscopic procedures for PNETs were documented, of which 65 pancreatic resections or enucleations were performed[47]. The most common operation performed was distal pancreatectomy with splenectomy (n = 28), and this was followed by distal pancreatectomy without splenectomy (n = 23). The status of splenic vessel preservation was not clarified. Enucleation of a PNET of the head was performed in 7 cases, and of the body or tail in another 7 patients. The most common surgical complication was found to be post-operative pancreatic fistula (POPF), occurring in 21% of patients. This complication was more common in patients undergoing enucleation (50%) a finding that has been repeatedly shown in the literature, with a reported incidence of 13%-50% of POPF following enucleation[23,73-75]. Other “non-fistula” surgical complications had an incidence of 21%, and no perioperative mortality was demonstrated. In this study, a 5-year disease-specific survival of 90% was demonstrated, which can be compared to another series of 125 patients who underwent open surgical treatment of PNET and were found to have a 5-year survival of 65%[76]. However, issues of selection bias in these two different retrospective studies must be considered. DiNorcia et al[18] published a retrospective series in which 45 laparoscopic procedures for PNETs were compared to 85 open surgeries that were performed at the same institution. The two groups were similar with respect to gender, age, and race; however, as expected, a statistically significant difference was observed with regard to pathological characteristics of the tumors, with the laparoscopically operated group having smaller, lower-grade tumors, with less local and lymph node invasion. This study showed no statistically significant difference in overall morbidity rate between the two groups (48.9% vs 57.6%, P = 0.34, laparoscopic vs open operations, respectively); however, major complications were more prevalent in the open surgery group (11.1% vs 28.2%, P = 0.03). No perioperative mortality was seen in the laparoscopic group, while in patients who underwent open surgery, the perioperative mortality rate was 3.5% (P = 0.55). Median length of hospital stay was found to be significantly shorter in the laparoscopy group (6 d vs 9 d). Within the 25.4 mo follow-up period of the laparoscopic group, a 4.4% recurrence rate was demonstrated, compared to a 15.3% recurrence after a median follow-up of 42.7 mo in the open surgery group. In the study by Fernández-Cruz et al[24] which included 49 patients undergoing laparoscopic surgery for PNETs, a higher perioperative complication rate among patients undergoing laparoscopic enucleation compared to those who underwent laparoscopic distal pancreatectomy (42.8% vs 22%, respectively, P < 0.001) was observed[24]. The main complication was POPF which also occurred more frequently in the enucleation group (38% vs 8.7%, P < 0.001). However, all fistulas following enucleation were successfully managed conservatively. No perioperative mortality was demonstrated.
Assalia et al[13] reported their experience with 17 cases of laparoscopically treated PNETs, and demonstrated a perioperative complication rate of 23%, and a POPF rate of 15.3%. No mortality or recurrence was shown, although their series did not include patients with malignant neuroendocrine tumors. In the same publication, a review of an additional 93 reported cases of laparoscopically managed PNETs from the literature was also presented. These cases demonstrated a perioperative complication rate of 28%, and a POPF rate of 17.9%. Following enucleation, the fistula rate was higher than that following distal pancreatectomy (30.7% vs 5.1%, respectively). Fistulas were managed mainly by drainage alone (11/14), with a combination of drainage and ERCP with stenting (1/14), and two cases required reoperation. No mortality was observed.
In the literature, the reported rate of “conversion to open” in laparoscopic pancreatic surgery ranges from 8%-33%[24,77-82]. Reasons for conversion include intraoperative complications such as bleeding, inability to localize the tumor, or location of the tumor in close proximity to vital structures (such as the main pancreatic duct or portal vein) in which continuation of laparoscopic resection would either jeopardize those structures or would prevent an appropriate oncologic resection. However, as previously mentioned, the presence or suspicion of a malignant lesion in itself is not an indication for conversion or open surgery. A multi-center study compared open distal pancreatectomy with laparoscopic distal pancreatectomy for adenocarcinoma, and similar short- and long-term oncologic outcomes were demonstrated between the two groups[83]. Unfortunately, similar studies are not yet available for pancreatic neuroendocrine carcinomas.
CONCLUSION
Laparoscopy is a safe modality for the surgical treatment of PNETs. Retrospective studies demonstrated similar overall complication rates in comparison with open pancreatic surgery for these tumors; however, there is evidence that the rate of major complications is higher in those undergoing open surgery. Laparoscopy, although considered to be more technically demanding, is not associated with a compromise in terms of oncologic outcome, and provides the benefits of decreased postoperative pain, better cosmetic results, shorter hospital stay, and a shorter postoperative recovery period. Further prospective, multi-center, and randomized trials are required for the analysis of these minimally invasive surgical techniques for the treatment of PNETs and their comparison to traditional open pancreatic surgery.