Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4453
Revised: August 23, 2013
Accepted: September 4, 2013
Published online: April 21, 2014
Processing time: 281 Days and 23.5 Hours
Barrett’s oesophagus (BO) is a usually indolent condition that occasionally requires endoscopic therapy. Radiofrequency ablation (RFA) is an effective endoscopic treatment for high grade dysplasia (HGD) and intramucosal cancer in BO. It has a good efficacy, durability and safety profile although complications can occur. Here we describe a case of RFA in a patient with high grade dysplasia. Although the response to treatment was initially very good with the development of neosquamous epithelium, the patient very rapidly developed a squamous cell cancer of the oesophagus confirmed on radiology, histology and immunohistochemistry. Sanger sequencing confirmed that the original HGD and the squamous cell cancer (SCC) were derived from separate clonal origins. The report highlights the fact that SCC of the oesophagus has been noted after endoscopic ablation for BO previously and suggest that ablation of BO may encourage the clonal expansion of cells carrying carcinogenic mutations once a dominant clonal population has been eradicated.
Core tip: The development of squamous cell cancer of the oesophagus after endoscopic ablation can happen and may add further weight to the argument for continued surveillance after radiofrequency ablation for Barrett’s related pathologies.
- Citation: Zeki SS, Haidry R, Justo-Rodriguez M, Lovat LB, Wright NA, McDonald SA. Squamous cell carcinoma after radiofrequency ablation for Barrett's dysplasia. World J Gastroenterol 2014; 20(15): 4453-4456
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4453.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4453
Radiofrequency ablation (RFA) is an effective and safe treatment for the eradication of Barrett’s oesophagus (BO) related high grade dysplasia (HGD) and intramucosal cancer[1]. Recent evidence suggests that Barrett’s related pathology can still recur after RFA[2,3]. There are also case reports on the occurrence of squamous carcinomas occurring after endoscopic ablation. Some evidence suggests that the a stem cell origin of Barrett’s mucosa may be common between squamous and columnar lined epithelium[4] although this relationship has not been demonstrated either with synchronous or metachronous Barrett’s and squamous carcinoma. Here we describe the first documented case of squamous carcinoma occurring after radiofrequency ablation and investigate its clonal relationship to the pre-treatment Barrett’ related HGD.
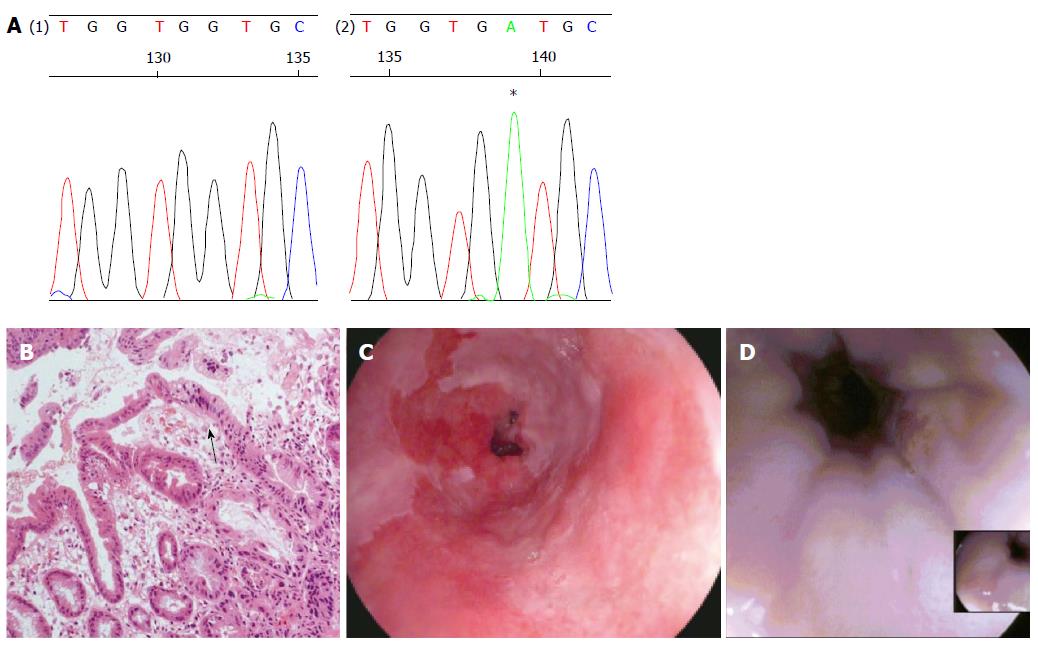
A 52-year-old man was admitted to the endoscopy unit in November 2008 for routine surveillance of Barrett’s oesophagus which he had undergone since 2005. The Barrett’s segment extended between 26 and 38 cm. Quadrantic biopsies demonstrated columnar-lined non-dysplastic epithelium throughout the segment apart from biopsies taken at 38 cm which demonstrated a high grade dysplasia. This was confirmed on subsequent endoscopies in December 2008 and February 2009.
In April 2009 the patient underwent focussed radiofrequency ablation [RFA (HALO™ 90)] with a further session of circumferential RFA in June 2009. Further focal RFA was applied to several Barrett’s islands in December 2009 and June 2010. A follow up gastroscopy in September 2010 with quadrantic biopsies showed normal, non-dysplastic squamous re-epithelialisation in all biopsies between 28 to 38 cm apart from two isolated areas at 35 and 38 cm which showed intestinal metaplasia only on histology.
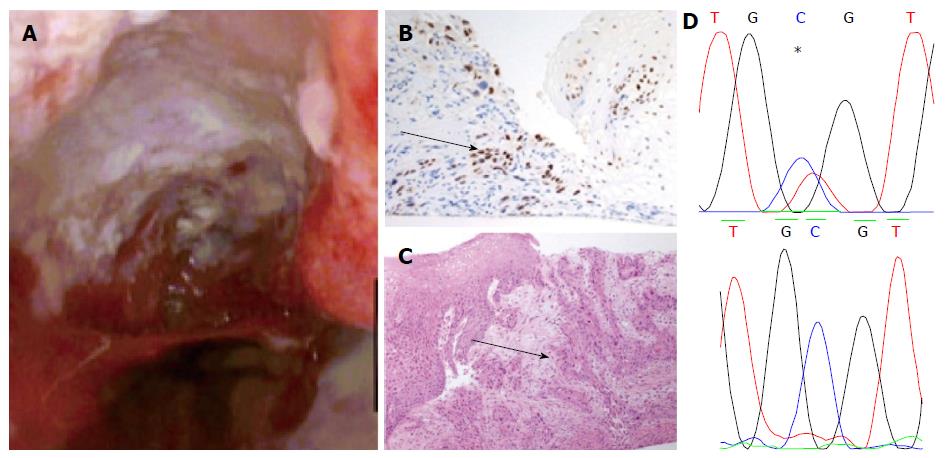
The patient underwent a further surveillance gastroscopy in August 2011. A large, ulcerating mass was found between 35 to 39 cm involving the GOJ. No intestinal metaplasia was seen.
Apart from two short tongues of Barrett’s that were separate to the ulcerating lesion the entire oesophagus was otherwise squamous-lined (Figure 1). The ulcerating lesion was characterized histologically as a squamous carcinoma (Figure 2) and confirmed immunophenotypically by p63 positivity (Figure 2B). A staging computed tomography scan confirmed the squamous cell cancer to involve loco-regional lymph nodes without distant metastases. The patient died prior to undergoing chemo-radiotherapy due to an unrelated pneumonia.
In order to further characterise the genotypic features of the Barrett’s related HGD and the squamous carcinoma, the samples underwent laser microdissection and sequencing of exons 5-9 of TP53 and exon 2 of CDKN2A as previously described[5]. These are known to be commonly mutated in both squamous cell cancer and Barrett’s HGD[6].
The biopsy samples of HGD demonstrated a mutation of CDKN2A c.286 G>A (p.V100M) (Figure 1A) only. However the squamous cell cancer did not contain the mutation detected in HGD, but demonstrated a different mutation of TP53 (c.817 C>T p. R273C) (Figure 2D).
A number of therapeutic modalities are now available for the treatment of Barrett’s oesophagus. Endoscopic therapies are now widely used and robust analyses have demonstrated efficacy in the treatment of HGD and intramucosal cancer[3,7]. Radiofrequency ablation of Barrett’s mucosa is a relatively safe technique with perforation and stricturing occurring less commonly than other endoscopic ablation techniques[1].
Our case is unique in that it shows the first demonstration of a squamous carcinoma occurring rapidly in an oesophageal segment recently treated with radiofrequency ablation for high-grade dysplasia.
There is a literature on the development of subsquamous Barrett’s after RFA with case reports demonstrating the rare development of adenocarcinoma in these areas[8]. There are also reports of squamous carcinoma occurring after ablation[9] although we have described the first case after RFA. The presence of squamous carcinoma in this and other reports so soon after a normal endoscopy subsequent to a successful ablation of Barrett’s related pathology indicates that patients may still warrant surveillance of neo-squamous epithelium after RFA. We have previously found somatic mutations in one case of normal squamous epithelium after radiofrequency ablation (accepted for publication by Am J Gastro). The fact that this may be a complication of RFA itself is indicated firstly by the absence of risk factors for squamous carcinoma development in this patient and secondly by the rapidity of its growth after RFA thermal injury. Thermal injury is already implicated as a causative factor in squamous carcinoma development[10,11] albeit over longer time courses.
Several studies have recently demonstrated a potential common progenitor for squamous and columnar epithelium by using mitochondrial and DNA mutations[4,5,12]. The fact that the two cancers are not clonally related on targeted sequencing of genes commonly mutated in both adenocarcinoma and squamous cell cancer indicates a different clonal origin of the two neoplasias. Given the squamous tumour was found at the same location as the previous Barrett’s HGD and morphologically was not a mixed adenosquamous type tumour, an alternative and interesting conjecture is that the eradication of Barrett’s dysplasia has allowed a protumorigenic clonal squamous population to proliferate. Such clonal competition has been suggested to occur in a number of different neoplasias including Barrett’s oesophagus[5]. An interesting and related issue also pertains to why metachronous squamous carcinoma and Barrett’s is so uncommon given the number of shared risk factors[13] and adds further weight to the idea that the clonal populations that characterise BO, or the phenotype of the tissue itself may protect against the development of squamous carcinoma.
P- Reviewers: Chai JY, Nagahara H, Tarnawski AS, Redondo-Cerezo E S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Bulsiewicz WJ, Kim HP, Dellon ES, Cotton CC, Pasricha S, Madanick RD, Spacek MB, Bream SE, Chen X, Orlando RC. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Korst RJ, Santana-Joseph S, Rutledge JR, Antler A, Bethala V, DeLillo A, Kutner D, Lee BE, Pazwash H, Pittman RH. Patterns of recurrent and persistent intestinal metaplasia after successful radiofrequency ablation of Barrett’s esophagus. J Thorac Cardiovasc Surg. 2013;145:1529-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, Wang KK, Fleischer DE, Sharma VK, Eisen GM, Fennerty MB, Hunter JG. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011;141:460-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 330] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 4. | Nicholson AM, Graham TA, Simpson A, Humphries A, Burch N, Rodriguez-Justo M, Novelli M, Harrison R, Wright NA, McDonald SA. Barrett’s metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2012;61:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, Harrison R, Novelli MR, Jankowski JA, Wright NA. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Catalogue of Somatic Mutations in Cancer. Available from: http://cancer.sanger.ac.uk/cancergenome/projects/cosmic. |
| 7. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 972] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 8. | Titi M, Overhiser A, Ulusarac O, Falk GW, Chak A, Wang K, Sharma P. Development of subsquamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett’s esophagus. Gastroenterology. 2012;143:564-566.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Allende D, Dumot J, Yerian L. Esophageal squamous cell carcinoma arising after endoscopic ablation therapy of Barrett’s esophagus with high-grade dysplasia. Report of a case. Dis Esophagus. 2013;26:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ribeiro U, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174-1185. [PubMed] |
| 11. | Islami F, Boffetta P, Ren JS, Pedoeim L, Khatib D, Kamangar F. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer. 2009;125:491-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 12. | Paulson TG, Xu L, Sanchez C, Blount PL, Ayub K, Odze RD, Reid BJ. Neosquamous epithelium does not typically arise from Barrett’s epithelium. Clin Cancer Res. 2006;12:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Streppel MM, Siersema PD, de Leng WW, Morsink FH, Vleggaar FP, Maitra A, Montgomery EA, Offerhaus GJ. Squamous cell carcinoma in Barrett’s esophagus: field effect versus metastasis. Dis Esophagus. 2012;25:630-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |