Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4433
Revised: December 25, 2013
Accepted: February 20, 2014
Published online: April 21, 2014
Processing time: 164 Days and 19.8 Hours
AIM: To report the devised anatomic liver resection of segments 6, 7 and 8 to improve the resection rate for patients with right liver tumors.
METHODS: We performed anatomic liver resection of segments 6, 7 and 8 to guarantee the maximum preservation of the remaining normal liver tissue. Segment 5 was determined by two steps of Glissonean pedicle occlusion. And a “┏┛” shaped broken resection line was marked upon the diaphragmatic surface of the liver. Selective right hemihepatic inflow occlusion was used to reduce blood loss during parenchymal transection between segments 6 and 5 and between segments 8 and 5. If needed, total hepatic Glissonean pedicle occlusion was used during parenchymal transection between segment 8 and the left liver.
RESULTS: Compared to right hemihepatectomy, the percentage of future liver remnant volume was increased by an average of 13.9% if resection of segments 6, 7 and 8 was performed. Resection of segments 6, 7 and 8 was completed uneventfully. After hepatectomy, the inflow and outflow of segment 5 were maintained. There was no perioperative mortality, postoperative abdominal bleeding or bile leakage in this group. Alpha-fetoprotein (AFP) returned to the normal range within 2 mo after the operation in all the patients. One patient died 383 d postoperatively due to obstructive suppurative cholangitis. One patient suffered from severe liver dysfunction shortly after surgery and had intrahepatic recurrence 4 mo postoperatively. Postoperative lung metastasis was found in one patient. No tumor recurrence was found in the other patients and the parameters including liver function and AFP level were in the normal range.
CONCLUSION: Anatomic liver resection of segments 6, 7 and 8 can be a conventional operation to improve the overall resection rate for hepatocellular carcinoma.
Core tip: Hepatic resection is the only curative treatment for patients with huge and multifocal tumors. However, patients with huge or multifocal tumors in the right liver and with a small volume of left liver cannot undergo right hemihepatectomy because of the possibility of postoperative liver failure, thus leading to a low overall resection rate for hepatocellular carcinoma. To increase the number of resectable patients and improve the overall resection rate, we devised anatomic liver resection of segments 6, 7 and 8 in patients with right liver tumors.
- Citation: Jia CK, Weng J, Chen YK, Fu Y. Anatomic resection of liver segments 6-8 for hepatocellular carcinoma. World J Gastroenterol 2014; 20(15): 4433-4439
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4433
Hepatic resection is the only curative treatment for patients with huge and multifocal tumors. These patients are less likely to benefit from liver transplantation or local ablative therapy[1-4]. In many such cases, complete resection of hepatocellular carcinoma (HCC) can only be achieved by hepatectomy that removes a significant proportion of the liver parenchyma. Recent advances in preoperative imaging, understanding of liver anatomy, surgical techniques, and anesthetic monitoring have increased the safety of liver resection[5-7]. Unfortunately, in some cases, hepatic failure occurs after major liver resection and carries poor prognosis due to the lower remnant liver volume[8]. So it restrains those patients who have much less remnant liver volume from radical operations. For example, patients with huge or multifocal tumors in the right liver and with a small volume of left liver cannot undergo right hemihepatectomy because of possible postoperative liver failure. To increase the number of resectable patients, we devised anatomic liver resection of segments 6, 7 and 8 using the technique of selective occlusion of hepatic inflow in patients with right liver tumors. This is a radical operation and benefits patients with huge or multifocal tumors in the right lobe.
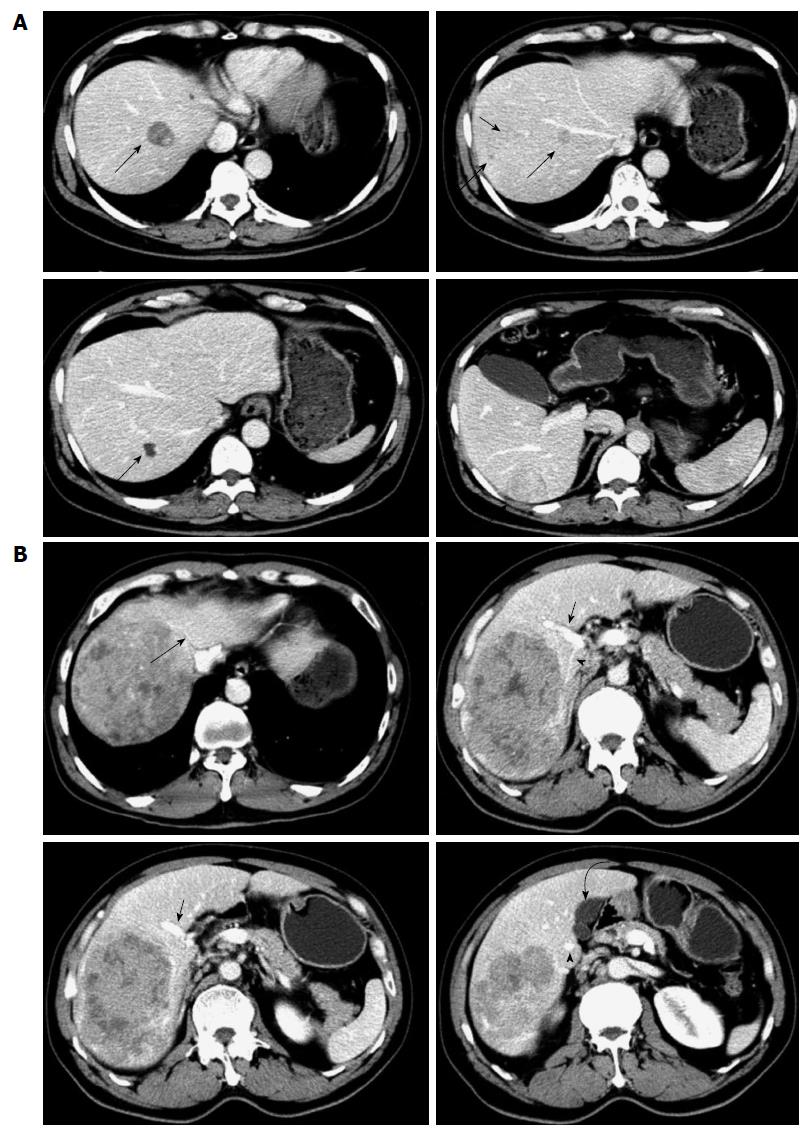
Six patients undergoing anatomic liver resection of segments 6, 7 and 8 from December 2011 to November 2013 in our department were included in this study. All these patients were male with a mean age of 55 years (range: 43-61 years), and had a history of hepatitis B virus infection. All the tumors were located in the right liver with multifocal tumors in two patients and huge tumors in four (Table 1). Preoperative imaging showed that segment 5 was free of tumor in all patients (Figure 1). Serum α-fetoprotein (AFP) level was elevated in all patients. Conventional follow-up was done, ranging from 6 to 23 mo.
| No. | Sex | Age (yr) | Diagnosis | ICG-R15 | Child-Pugh liver function grade | %FLRV for right hemihepatectomy | %FLRV for 6-8 segmentectomy | Survival period (d) |
| 1 | Male | 61 | Multifocal tumors | 6.8% | A | 29.8 | 44.7 | 690, DFS |
| 2 | Male | 43 | Huge tumor, 12.2 cm × 9.3 cm | 5.5% | A | 34.5 | 46.2 | 383, dead |
| 3 | Male | 59 | Huge tumor, 11.5 cm × 10.7 cm | 13.8% | B | 32.6 | 46.9 | 270, intrahepatic recurrence, alive |
| 4 | Male | 54 | Huge tumor, 13.2 cm × 10.6 cm | 8.3% | A | 31.8 | 48.6 | 256, lung metastasis, alive |
| 5 | Male | 53 | Multifocal tumors | 2.6% | A | 37.5 | 50.3 | 239, DFS |
| 6 | Male | 60 | Huge tumor, 13.5 cm × 11.6 cm | 7.9% | A | 33.4 | 46.5 | 181, DFS |
Liver function, hepatic functional reserve and hepatic imaging, including ultrasound B and computed tomography (CT), were carried out preoperatively. Liver function was classified by Child-Pugh score and hepatic functional reserve was evaluated by the indocyanine green retention at 15 min (ICG-R15) test. Contrast-enhanced CT scans were generated with a helical scanner. Manual 3D reconstructions of the liver were made using reconstructed 5-mm-thick axial slices from 2-3-mm original slices. The total liver, left liver and segment 5, as well as the tumors were manually outlined using portal and hepatic veins as landmarks for segmental division[9]. The volumes of the total liver, left liver, segment 5 and tumor were calculated. The percentage of future liver remnant volume (%FLRV) was expressed using the formula: %FLRV = (remnant liver volume × 100)/(total liver volume - tumor volume)[10]. If right hemihepatectomy was performed, %FLRV would be < 40% in all of the patients. The risk of postoperative liver failure would be high due to insufficient remnant functional liver because all these cases had liver cirrhosis. However, if resection of segments 6, 7 and 8 was performed, %FLRV would increase by an average of 13.9% (Table 1). The risk of postoperative liver failure would be low due to sufficient remnant functional liver. Therefore, we designed anatomic resection of liver segments 6, 7 and 8, with retention of segment 5, to guarantee the maximum preservation of remnant functional liver tissue.
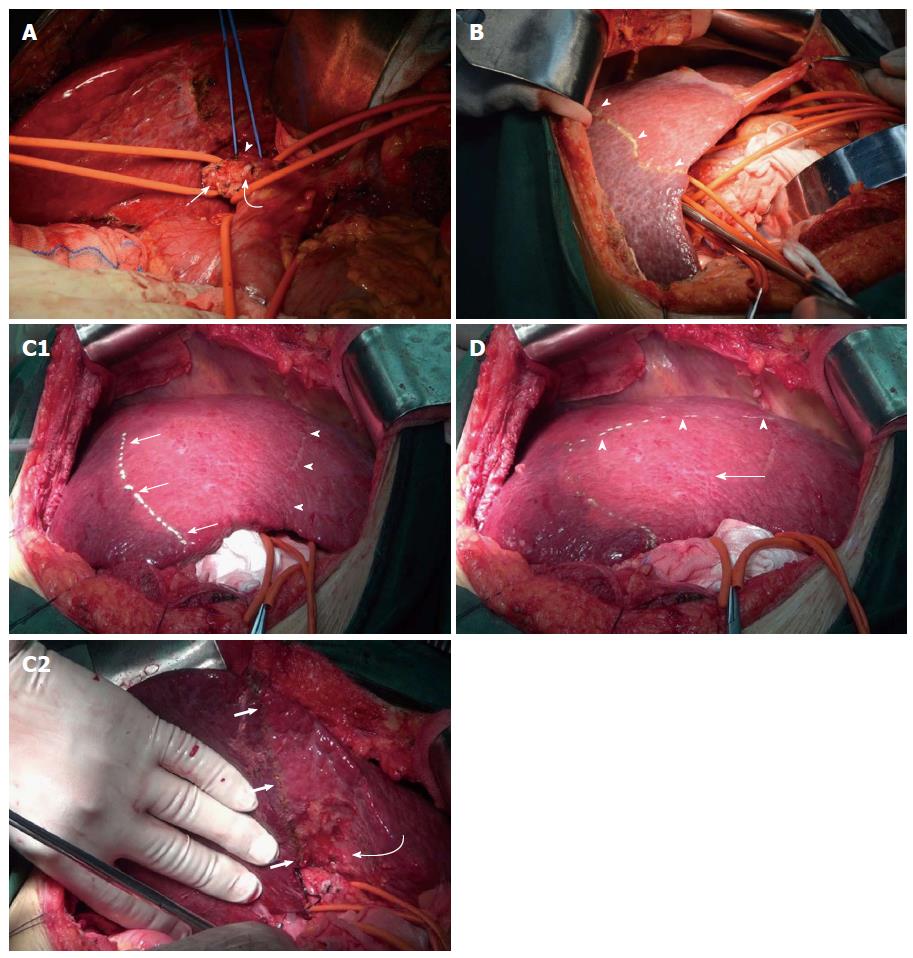
Selective occlusion of hepatic inflow was the key to complete anatomic liver resection of segments 6, 7 and 8. Segment 5 was determined by two steps of Glissonean pedicle occlusion. After cholecystectomy, the porta hepatis was dissected based on Glissonean pedicle anatomy[11-13]. The right hemihepatic Glissonean pedicle and the segment 6 and 7 Glissonean pedicle were sequentially divided (Figure 2A). After occlusion of the right hemihepatic Glissonean pedicle, the right liver developed obvious ischemia. So, the demarcation between the right and left liver could be easily determined (Figure 2B). After demarcation, the right hemihepatic Glissonean pedicle was left unoccluded. Then the segment 6 and 7 Glissonean pedicle was dissected and ligated, and segments 6 and 7 showed obvious ischemia (Figure 2C). The interface between segments 6 and 5 could be easily demarcated. Intraoperative ultrasound B was used to form the demarcation between segments 8 and 5, with a transverse marked line upon the diaphragmatic surface of the liver. This line was located 1-2 cm below the tumor in segment 8. Finally, segment 5 was determined and a “┏┛” shaped broken resection line was marked upon the diaphragmatic surface of the liver (Figure 2D). Short hepatic veins were dissected, isolated and ligated. Parenchymal transection was performed along the broken resection line using an ultrasonic scalpel and cavitron ultrasonic surgical aspirator. Selective right hemihepatic inflow occlusion was used to reduce blood loss during parenchymal transection between segments 6 and 5 and between segments 8 and 5. If needed, total hepatic Glissonean pedicle occlusion was used during parenchymal transection between segment 8 and the left liver.
Postoperative follow-up was done with conventional liver function tests and measurement of serum AFP level. Abdominal ultrasonography and CT were performed at intervals of 30 d in the first 3 mo postoperatively, and at intervals of 2-3 mo thereafter. Chest X-ray, whole-body bone scan, or other imaging studies were undertaken in patients who had suspected metastasis. All the patients received three sessions of postoperative transcatheter arterial chemoembolization (TACE). If affordable, sorafenib, a multi-targeted tyrosine kinase receptor inhibitor was used for metastatic or recurrent cases.
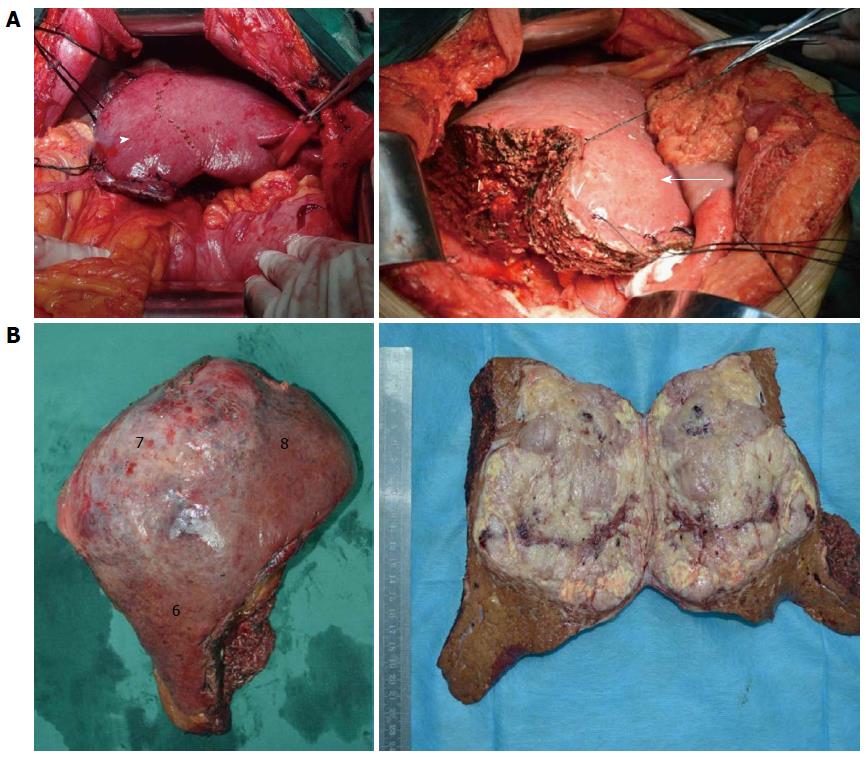
All hepatectomies were uneventfully completed with an average operation time of 326 min (range: 260-470 min) and average blood loss of 758 mL (range: 400-1800 mL). After hepatectomy, the inflow and outflow of segment 5 were maintained (Figure 3A). Gross specimens showed that tumors were completely resected and postoperative pathology verified a diagnosis of HCC (Figure 3B). There was no perioperative mortality, postoperative abdominal bleeding, or bile leakage. Serum AFP decreased to the normal range within 2 mo postoperatively.
Case 2 survived well with normal AFP level and liver function for about 12 mo postoperatively. However, he was hospitalized 381 d postoperatively due to fever and 15 d progressive elevation of jaundice at home without any treatment. CT and ultrasound B showed no tumor recurrence and metastasis inside or outside the liver. Serum total bilirubin was 193.4 μmol/L (normal range: 1.7-20 μmol/L) at admission with direct bilirubin being 129.1 μmol/L (normal range: 0-6 μmol/L). Two days after admission, this patient died from obstructive suppurative cholangitis.
Case 3 had Child-Pugh score 7 and 13.8% ICG, which indicated severe postoperative liver dysfunction. His serum total bilirubin increased constantly and peaked at 142.5 μmol/L on postoperative day 8. Ascites and hepatoencephalopathy occurred consecutively due to liver dysfunction. These symptoms resolved gradually as liver dysfunction improved. However, intrahepatic recurrence was found by CT and digital substraction angiography 4 mo postoperatively. Although AFP level increased to 219.9 ng/mL at that time, it decreased to the normal range after two sessions of TACE. To date, this tumor-bearing patient has had > 6 mo survival.
Lung metastasis was found by CT 5 mo postoperatively in Case 4. Sorafenib was administered to this patient. To date, this patient has had > 4 mo tumor-bearing survival with AFP in the normal range.
No tumor recurrence was found in the other patients and the parameters including liver function and AFP level were in the normal range. Postoperative outcome and quality of life are summarized in Table 1.
Inadequate future liver remnant may not meet the hepatic metabolic demands after major hepatectomy[14,15]. Therefore, patients with huge or multifocal tumors in the right liver and a small volume of left liver cannot undergo right hemihepatectomy because of possible postoperative liver failure. These patients are excluded from radical operations. In the present study, all %FLRV would have been < 40% if right hemihepatectomy was performed. The risk of postoperative liver failure would have been high because of liver cirrhosis and insufficient remnant functional liver. However, if resection of segments 6, 7 and 8 was performed, %FLRV would increase by an average of 13.9%. The risk of postoperative liver failure would be low because of sufficient remnant functional liver. Therefore, all patients underwent anatomic liver resection of segments 6, 7 and 8, with retention of segment 5. The patients underwent radical surgery with tumor-free margins and maximum preservation of remnant functional liver tissue[16-19]. This technique could improve the resection rate in patients with huge or multifocal tumors in the right liver. No remnant tumor was found on imaging shortly after the operation and AFP decreased to the normal range within 2 mo postoperatively. The tumors were completely resected by anatomic liver resection of segments 6, 7 and 8. These six patients have survived > 6 mo with satisfactory quality of life postoperatively. We demonstrated that this operation had a good therapeutic efficacy.
It is worth mentioning that the hepatectomies were performed with a “┏┛” shaped broken resection line. This naturally increased the resection length, area, operation time and blood loss. However, the blood loss in our study (mean: 758 mL) was no more than that previously reported for huge HCC (e.g., 1015[20] and 780 mL[21]).
There were no severe complications such as perioperative mortality, postoperative abdominal bleeding, and bile leakage. We demonstrated that liver resection of segments 6, 7 and 8 is safe and effective for the treatment of HCC. Anatomic liver resection of segments 6, 7 and 8 could become a conventional operation for patients whose tumors do not involve segment 5, to enable maximum preservation of remaining normal liver tissue. It may be an alternative modality for maximal hepatectomy in the treatment of HCC.
Finally, selective occlusion of right hemihepatic inflow was applied during parenchymal transection between segments 6 and 5 and between segments 8 and 5. If needed, total hepatic inflow occlusion was used during parenchymal transection between segment 8 and the left liver. Selective right hemihepatic Glissonean pedicle occlusion enables blood inflow to the left liver and avoids splanchnic stasis during hepatectomy[22-24]. Thus, liver ischemia-reperfusion injury and hemodynamic instability were reduced during the operation. It particularly benefits patients with cirrhosis[25-27].
Hepatic resection is the only curative treatment for patients with huge and multifocal tumors. However, patients with huge or multifocal tumors in the right liver and a small volume of left liver cannot undergo right hemihepatectomy because of possible postoperative liver failure, thus making the overall resection rate for hepatocellular carcinoma (HCC) low. To increase the number of resectable patients, we devised anatomic liver resection of segments 6, 7 and 8 in patients with right liver tumors.
Anatomic liver resection of segments 6, 7 and 8 has not been reported previously. By the techniques of selective occlusion of hepatic inflow and anatomic liver resection, complicated hepatectomy can be performed. Anatomic liver resection of segments 6, 7 and 8 could be a conventional technique for patients whose tumors do not involves segment 5, to enable maximum preservation of remaining normal liver tissue, thus improving the overall resection rate for HCC.
Compared to right hemihepatectomy, the percentage of future liver remnant volume (%FLRV) was increased by an average of 13.9% when resection of segments 6, 7 and 8 was performed. Segmentectomy was completed uneventfully in this group. Segment 5 was determined by two steps of hepatic inflow occlusion. A “┏┛” shaped broken resection line was marked upon the diaphragmatic surface of the liver. Selective right hemihepatic inflow occlusion was used to reduce blood loss during parenchymal transection between segments 6 and 5 and between segments 8 and 5. If needed, total hepatic Glissonean pedicle occlusion was used during parenchymal transection between segment 8 and the left liver. Thus, liver ischemia-reperfusion injury and hemodynamic instability were maximally reduced during the operation. It particularly benefits patients with cirrhosis.
Patients with huge or multifocal tumors in the right liver and with a small volume of left liver cannot undergo right hemihepatectomy because of the possibility of postoperative liver failure. To improve the overall resection rate for HCC, anatomic resection of segments 6, 7 and 8 can be performed in these cases. This operation is radical and benefits these patients because it avoids postoperative liver failure.
%FLRV is an important measure for patients undergoing major liver resection. Selective occlusion of hepatic inflow was the key procedure for anatomic liver resection.
Partial hepatectomy is a powerful tool for HCC patients. Selective resection of right anterior/posterior segments often requires an experienced surgeon. The procedures may involve a unique technique. Surgical technique of parenchyma-sparing liver resection for right liver HCC (huge or multiple) developed on liver cirrhosis. This is a description of an atypical technique of anatomic liver resection.
P- Reviewers: Hori T, Regimbeau JM, Wang DS S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 2. | Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, Wei SH, Curley SA, Laurent A, Poon RT. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17:66-77; discussion p77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Dittmar Y, Altendorf-Hofmann A, Schüle S, Ardelt M, Dirsch O, Runnebaum IB, Settmacher U. Liver resection in selected patients with metastatic breast cancer: a single-centre analysis and review of literature. J Cancer Res Clin Oncol. 2013;139:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Risk factors of poor prognosis and portal vein tumor thrombosis after curative resection of solitary hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Akai H, Kiryu S, Matsuda I, Satou J, Takao H, Tajima T, Watanabe Y, Imamura H, Kokudo N, Akahane M. Detection of hepatocellular carcinoma by Gd-EOB-DTPA-enhanced liver MRI: comparison with triple phase 64 detector row helical CT. Eur J Radiol. 2011;80:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Ishizawa T, Mise Y, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Kokudo N. Surgical technique: new advances for expanding indications and increasing safety in liver resection for HCC: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Shirabe K, Motomura T, Takeishi K, Morita K, Kayashima H, Taketomi A, Ikegami T, Soejima Y, Yoshizumi T, Maehara Y. Human early liver regeneration after hepatectomy in patients with hepatocellular carcinoma: special reference to age. Scand J Surg. 2013;102:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | de Graaf W, van Lienden KP, Dinant S, Roelofs JJ, Busch OR, Gouma DJ, Bennink RJ, van Gulik TM. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Okabe H, Beppu T, Chikamoto A, Hayashi H, Yoshida M, Masuda T, Imai K, Mima K, Nakagawa S, Kuroki H. Remnant liver volume-based predictors of postoperative liver dysfunction after hepatectomy: analysis of 625 consecutive patients from a single institution. Int J Clin Oncol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg. 1998;5:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 209] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Karamarković A, Doklestić K, Milić N, Djukić V, Bumbasirević V, Sijački A, Gregorić P, Bajec D. Glissonean pedicle approach in major liver resections. Hepatogastroenterology. 2012;59:1896-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Katagiri S, Ariizumi S, Kotera Y, Takahashi Y, Yamamoto M. Right hepatectomy using Glissonean pedicle transection method with anterior approach (with video). J Hepatobiliary Pancreat Sci. 2012;19:25-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Chun YS, Ribero D, Abdalla EK, Madoff DC, Mortenson MM, Wei SH, Vauthey JN. Comparison of two methods of future liver remnant volume measurement. J Gastrointest Surg. 2008;12:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Di Domenico S, Santori G, Balbis E, Traverso N, Gentile R, Bocca B, Gelli M, Andorno E, Cottalasso D, Valente U. Biochemical and morphologic effects after extended liver resection in rats: preliminary results. Transplant Proc. 2010;42:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Chen J, Huang K, Wu J, Zhu H, Shi Y, Wang Y, Zhao G. Survival after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2011;56:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Nanashima A, Sumida Y, Abo T, Nagasaki T, Tobinaga S, Fukuoka H, Takeshita H, Hidaka S, Tanaka K, Sawai T. Comparison of survival between anatomic and non-anatomic liver resection in patients with hepatocellular carcinoma: significance of surgical margin in non-anatomic resection. Acta Chir Belg. 2008;108:532-537. [PubMed] |
| 20. | Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, DeMatteo RP. Outcome of partial hepatectomy for large (& gt; 10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Chen X, Wu Z, Qiu F. Hepatectomy for huge primary liver cancer: report of 171 patients. Zhonghua Waike Zazhi. 2000;38:6-9. [PubMed] |
| 22. | Giordano M, Lopez-Ben S, Codina-Barreras A, Pardina B, Falgueras L, Torres-Bahi S, Albiol M, Castro E, Figueras J. Extra-Glissonian approach in liver resection. HPB (Oxford). 2010;12:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Dello SA, Reisinger KW, van Dam RM, Bemelmans MH, van Kuppevelt TH, van den Broek MA, Olde Damink SW, Poeze M, Buurman WA, Dejong CH. Total intermittent Pringle maneuver during liver resection can induce intestinal epithelial cell damage and endotoxemia. PLoS One. 2012;7:e30539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Yanaga K, Matsumata T, Nishizaki T, Shimada M, Sugimachi K. Alternate hemihepatic vascular control technique for hepatic resection. Am J Surg. 1993;165:365-366. [PubMed] |
| 25. | Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, Schmidt J, Weitz J, Büchler MW. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. Br J Surg. 2008;95:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Wang HQ, Yang JY, Yan LN. Hemihepatic versus total hepatic inflow occlusion during hepatectomy: a systematic review and meta-analysis. World J Gastroenterol. 2011;17:3158-3164. [PubMed] |
| 27. | Arkadopoulos N, Kyriazi MA, Theodoraki K, Vassiliou P, Perelas A, Vassiliou I, Smyrniotis V. Central hepatectomy under sequential hemihepatic control. Langenbecks Arch Surg. 2012;397:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |