Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4414
Revised: October 27, 2013
Accepted: January 8, 2014
Published online: April 21, 2014
Processing time: 224 Days and 13 Hours
AIM: To assess whether differential expression of caspase-3 in paired metastatic lymph nodes (LNs) is prognostic of survival in patients with resectable esophageal squamous cell carcinoma (ESCC).
METHODS: Capases-3 expression was evaluated immunohistochemically in 122 pairs of primary ESCCs and regional metastatic LNs assembled on tissue microarrays. The impact of caspase-3 expression on survival outcomes was analyzed by the Kaplan-Meier method and Cox proportional hazards regression model.
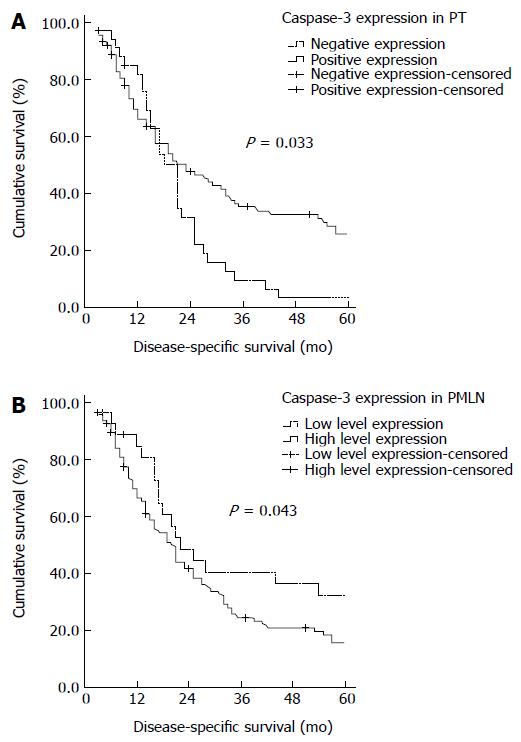
RESULTS: The level of caspase-3 expression was significantly higher in LN metastases than in primary tumors (P < 0.001). Caspase-3 expression in the primary tumors was associated with longer median survival (23 mo vs 21 mo, P = 0.033), whereas higher expression in paired metastatic LNs was associated with shorter median survival (20 mo vs 22 mo, P = 0.043). Multivariate analysis showed that both were independent prognostic factors.
CONCLUSION: Caspase-3 expression in metastatic LNs may be a potential independent predictor of poorer overall survival in patients with resected ESCC and LN metastasis. Protein expression in metastatic tumors may be a biomarker prognostic of survival.
Core tip: Reduced caspase-3 expression in primary esophageal squamous cell carcinoma (ESCC) is associated with poorer outcomes, but the prognostic value of caspase-3 in metastatic lymph nodes (MLNs) is unclear. Analysis of 122 patients with primary ESCCs and paired MLNs showed that higher caspase-3 expression in MLNs from ESCC was associated with poorer prognosis. To the best of our knowledge, this study is the first to report the potential prognostic value of caspase-3 expression in MLNs of ESCC. Additional studies are needed to assess the prognostic importance of this biomarker.
- Citation: Wang XS, Luo KJ, Bella AE, Bu SS, Wen J, Zhang SS, Hu Y. Caspase-3 expression in metastatic lymph nodes of esophageal squamous cell carcinoma is prognostic of survival. World J Gastroenterol 2014; 20(15): 4414-4420
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4414.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4414
Esophageal carcinoma is the eighth most common malignancy worldwide[1]. In China, esophageal squamous cell carcinoma (ESCC) accounts for most esophageal malignant tumors, where it bears more than half of the global burden[2]. Despite incorporation of new therapeutic approaches, it remains an aggressive disease with a dismal prognosis[3]. Biomarkers predictive of patient prognosis may help design more effective and targeted therapies for ESCC.
Caspase-3 (also known as CPP32, YAMA, and apopain) is a cysteine protease related to interleukin-1beta-converting enzyme (ICE) and the human homologue of Ced-3, a protein in Caenorhabditis elegans required for programmed apoptosis[4]. The normal apoptotic process can be initiated by a cascade of specific death-inducing signals with the activation of caspase-3 acknowledged as a penultimate step generally. The dysregulation of apoptotic pathways in many malignances can extend cell life span and may support anchorage-independent survival during metastasis[5-7]. Immunohistochemical studies have shown that caspase-3 is expressed in 55.4% to 79.7% of primary ESCCs[8-11], with reduced expression of caspase-3 associated with enhanced malignant potential and decreased survival.
Genomic instability is a hallmark of cancer caused by constant selection pressure. Specific populations of tumor cells may be more prone to metastasis than others, which is likely to result in an enrichment of the former and maintenance of their genetic aberrations in metastases. Alternatively, tumor cells may acquire new genetic modifications after spreading to metastatic sites[12]. Substantial genetic differences may therefore exist between primary tumors (PTs) and their metastases. Although the association between caspase-3 expression and clinical outcomes has been analyzed in PTs, it is unclear whether caspase-3 expression in lymphatic metastases is prognostic of patient outcomes. Therefore, the purposes of our study were to assess possible changes in caspase-3 expression between PTs and paired metastatic lymph nodes (PMLNs) and analyze whether capase-3 expression in the latter is associated with clinical outcomes.
Between June 1997 and December 2004, 1120 consecutive patients with ESCC underwent esophagectomy in the Department of Thoracic Surgery at Sun Yat-sen University Cancer Center. Patients were included with the following eligibility criteria: (1) histological proof of thoracic ESCC; (2) pathological evaluation of lymph node metastasis; (3) no neoadjuvant therapy; and (4) complete surgical resection (R0). Patients were excluded with the following criteria: history of other cancer or death during the perioperative period. The study protocol was approved by the Institutional Review Board of the Cancer Center of Sun Yat-sen University. All patients provided written informed consent before surgery and all had undergone transthoracic esophagectomy (the Sweet or Mckeown procedure) with standard or total dissection of thoracic and abdominal lymph nodes.
Of the 1120 patients who had undergone esophagectomy during the study period, 288 were deemed eligible for this study. We obtained 288 PT and 3720 regional lymph node samples from these patients. All samples were collected in the operating room and were routinely fixed immediately after collection in 10% neutral buffered formalin for approximately 24 h at room temperature. After fixation, the samples were dehydrated, incubated in xylene, infiltrated with paraffin, and finally embedded in paraffin (Oxford Labware, St Louis, MO). Each tissue sample was identified on hematoxylin-and-eosin stained slides, and the corresponding paraffin-embedded tissue blocks were obtained. Two trained pathologists, blindly to clinical data, selected those lymph node samples according to the following eligibility criteria: histologic proof of squamous cell carcinoma of the metastatic lymph node, and the diameter of metastatic lesion more than 3 mm. Forty-two patients had multiple lymph nodes satisfying these criteria. We randomly selected one lymph node from each. Finally, 164 pairs of surgically resected ESCC PTs and corresponding metastatic lymph nodes were selected.
Tissue microarrays (TMA) were constructed using a tissue microarrayer (Beecher Instruments, Sun Prairie, WI). During sample selection, the pathologists marked areas containing viable tumor on the paraffin wax tissue blocks. For each case, three 1-mm tissue cores from marked areas of the same tissue block were selected (three cores per case)[13] and transferred to a TMA. Hematoxylin- and eosin-stained sections from each TMA block were checked by the pathologists to ensure that adequate targeted tissues were included.
Immunohistochemical (IHC) staining was performed on 4 μm sections obtained from tissue microarray blocks. Rabbit polyclonal caspase-3 antibody (CPP32 Ab-4, Lab Vision Corporation, Cheshire, Unite Kingdom) was applied at a 1:100 dilution and incubated overnight at 4 °C. Immunoperoxidase staining was carried out using EliVison™ plus Kit (Maxim Bioscience, Fuzhou, China) according to the manufacturer’s instructions. Briefly, the slides were incubated with polymer enhancer for 15 min, washed three times in PBS, and incubated with secondary antibody for 30 min at room temperature. As a negative control, the slides were incubated with nonimmune rabbit immunoglobulin G. After staining, the pathologists checked the sections again to ensure that interpretable results were obtained from each pair of PT and matched metastatic lymph node samples. Interpretable results could not be obtained from 42 pairs of specimens due to secondary technical issues, including sectioning artifacts and obscuring debris.
Two trained pathologists, blindly to clinical data, evaluated all results independently at first and then reviewed together. The intensity (I) and proportion (Prop) of caspase-3 cytoplasmic staining in tumor cells were recorded individually for each core, with I defined as: none = 0, weak = 1, moderate = 2, strong = 3, Prop as the percentage of positive cells with at least 200 nuclei counted and the total score for each core calculated as I×Prop[14]. For statistical analysis, the minimum score was set at 1.00 with scores ranging from 1 to 300. The final score for each tumor was defined as the average of the score of three cores in the same case. Based on previous studies[9,11], we defined positive expression as a final score ≥ 80 and high level expression as a final score ≥ 160.
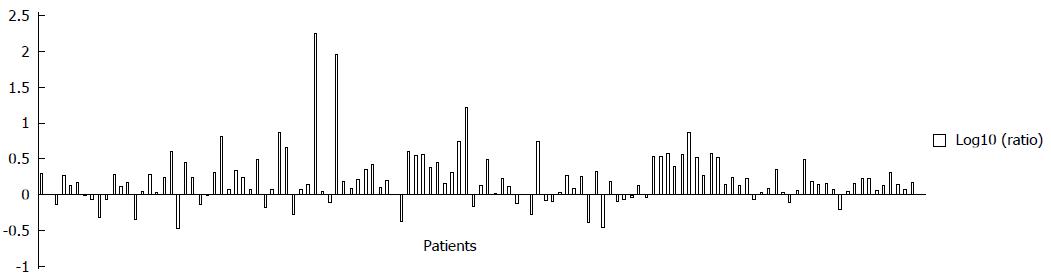
All statistical analyses were performed using SPSS 13.0 for Windows software (SPSS Inc, Chicago, IL). Differences in caspase-3 expression between group of PTs and PMLNs were analyzed by McNemar’s test, with intra-patient paired comparisons analyzed by Wilcoxon signed-rank paired tests. The correlation between caspase-3 expression and clinicopathologic parameters was analyzed by Mann-Whitney U-test or χ2 test where applicable. For graphical representations, the log10 of the ratio of PMLNs to PTs was calculated, with log10 (ratios) of < 0 and > 0 indicating higher and lower levels of expression, respectively, in PMLNs. Disease-specific survival (DSS) was calculated from the time of surgery to the time of death from ESCC. If the patient was lost to follow-up or died of a cause other than ESCC, data were censored at the time of last follow-up or death. Survival curves were constructed by the Kaplan-Meier method and analyzed by log-rank test. Multivariate analysis using Cox proportional hazards regression model with a forward stepwise procedure was performed to determine factors independently predictive of survival. A significant difference was defined as a two-tailed P value less than 0.05.
Following selection, 122 patients with paired PTs and MLNs were included. They consisted of 102 men and 20 women, ranging in age from 34 to 78 years (mean of 58.2 years). Other clinical and pathological characteristics are shown in Table 1. Eighteen patients received postoperative chemotherapy [cis-diaminedichloroplatinum (CDDP) plus fluorouracil]. Follow-up data were obtained from all patients, with a median survival of 19 mo (range, 3-123 mo).
| Characteristic | n (%) |
| Age at diagnosis (yr) | |
| ≤ 58 | 61 (50) |
| > 58 | 61 (50) |
| Gender | |
| Male | 102 (83.6) |
| Female | 20 (16.4) |
| Surgical approach1 | |
| Sweet | 58 (47.5) |
| McKeown | 64 (52.5) |
| Tumor location | |
| Upper | 13 (10.6) |
| Middle | 69 (56.6) |
| Lower | 40 (32.8) |
| Tumor grade | |
| G1 | 28 (22.9) |
| G2 | 59 (48.4) |
| G3 | 35 (28.7) |
| pT category | |
| T2 | 12 (9.8) |
| T3 | 103 (84.4) |
| T4a2 | 7 (5.7) |
| pN category | |
| N1 | 64 (52.5) |
| N2 | 45 (36.9) |
| N3 | 13 (10.6) |
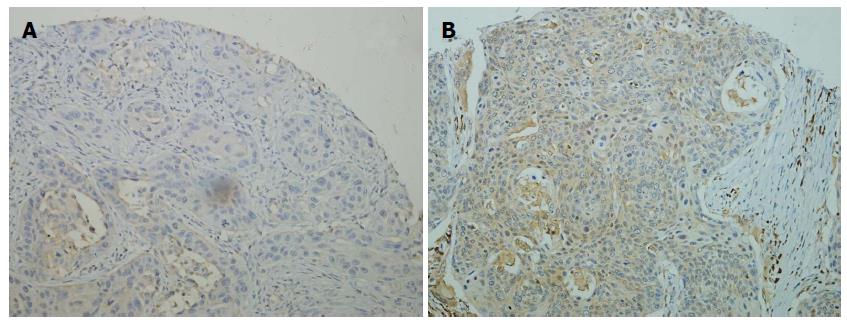
Representative tissue samples of IHC are depicted in Figure 1. Most ESCC specimens showed cytoplasmic expression, with nuclear staining being rare. Eighty-eight of 122 PTs (72.1%) and 116 of 122 PMLNs (95.1%) showed positive expression of caspase-3 (P < 0.001, McNemar’s test). High level expression was observed in 54 of 122 PTs (44.3%) and 94 of 122 PMLNs (77.0%). This discordance was statistically significant (P < 0.001, McNemar’s test). As shown in Figure 2, the log10 ratio of caspase-3 expression in PLMNs to PTs was > 0 in 94 of the 122 patients (77.0%), indicating that, in individual patients the level of caspase-3 expression was higher in PMLNs than in PTs from the same patient (P < 0.001, Wilcoxon signed-rank test).
Based on the 7th edition AJCC staging system[15] and previously assessed prognostic factors, patients’ clinicopathologic features were dichotomized for statistical analysis (Table 2). The levels of caspase-3 expression in PT and PMLN differed significantly in patients with pT2 (P < 0.001, Mann-Whitney U-test) and pT3/4 (P = 0.016, Mann-Whitney U-test) tumors. There was no association between caspase-3 expression and other clinicopathologic features.
| Characteristic | Number of patients | Caspase-3 expression in PT | Caspase-3 expression in PMLN | ||
| Mean rank | P value1 | Mean rank | P value1 | ||
| Gender | |||||
| Male | 102 | 60.22 | 0.365 | 63.18 | 0.237 |
| Female | 20 | 68.05 | 52.95 | ||
| Age (yr) | |||||
| ≤ 58 | 61 | 62.23 | 0.820 | 63.1 | 0.617 |
| > 58 | 61 | 60.77 | 59.9 | ||
| Tumor location | |||||
| Upper/middle | 82 | 62.27 | 0.731 | 64.76 | 0.145 |
| Lower | 40 | 59.92 | 54.82 | ||
| Tumor grade | |||||
| G1 | 28 | 66.11 | 0.432 | 55.32 | 0.292 |
| G2/G3 | 94 | 60.13 | 63.34 | ||
| pT category | |||||
| T2 | 12 | 103.7 | < 0.001 | 84.92 | 0.016 |
| T3/T4a | 110 | 56.9 | 58.95 | ||
| LN metastases2 | |||||
| N1 | 64 | 62.36 | 0.778 | 58.23 | 0.283 |
| N2/N3 | 58 | 60.55 | 65.11 | ||
Kaplan-Meier analysis (Table 3) showed that, median DSS was significantly longer in patients with PTs positive than negative for caspase-3 expression (23 mo vs 21 mo; P = 0.033, log-rank test; Figure 3A). In contrast, median DSS was significantly shorter in patients with PMLNs showing high level than low level caspase-3 expression (20 mo vs 22 mo; P = 0.043, log-rank test; Figure 3B).
Cox multivariate analysis of demographic and clinicopathologic characteristics, including gender, age, tumor location, differentiation grade, surgery approach, adjuvant therapy, pT category, pN category, caspase-3 expressions in PT, and high level caspase-3 expression in PMLN, showed that only age, lymph node involvement, caspase-3 expression in PT, and high level caspase-3 expression in PMLN were independent factors for long-term survival (Table 4).
| Prognostic factor | P value | RR | 95%CI |
| Univariate survival analysis | |||
| Gender (male vs female) | 0.91 | 1.031 | 0.611-1.740 |
| Age ( ≤ 58 vs > 58) | 0.012 | 1.661 | 1.118-2.469 |
| Location(lower vs upper/middle) | 0.293 | 0.877 | 0.687-1.120 |
| Differentiation (grade 1 vs grade 2/3) | 0.051 | 1.623 | 0.998-2.641 |
| Surgical approach (Sweet vs McKeown) | 0.637 | 0.91 | 0.615-1.347 |
| Adjuvant therapy (No vs Yes) | 0.501 | 0.907 | 0.683-1.205 |
| pT category (T2 vs T3/T4a) | 0.61 | 1.186 | 0.616-2.285 |
| pN category (N1 vs N2/N3) | < 0.001 | 2.19 | 1.463-3.277 |
| Caspase-3 expression in PT | 0.037 | 0.627 | 0.404-0.973 |
| (negative vs positive) | |||
| Caspase-3 expression in PMLN | 0.03 | 4.909 | 1.164-20.708 |
| (low vs high level expression) | |||
| Multivariate survival analysis | |||
| Age ( ≤ 58 vs > 58) | < 0.001 | 2.122 | 1.404-3.207 |
| pN category (N1 vs N2/N3) | < 0.001 | 2.422 | 1.590-3.690 |
| Caspase-3 expression in PT | 0.021 | 0.584 | 0.370-0.921 |
| (negative vs positive) | |||
| Caspase-3 expression in PMLN | 0.01 | 1.991 | 1.180-3.359 |
| (low vs high level) |
Numerous studies have been performed to identify useful markers associated with cancer progression and clinical outcomes. Most of these studies are performed on PTs, whereas others have not mentioned the origin of tumor tissue. The parallel progression model hypothesizes that tumor cells depart the primary lesion before the acquisition of fully malignant phenotypes undergoing somatic progression and metastatic growth at distant sites[16,17]. Thus, early dissemination and divergent progression of PT and disseminated tumor cells, the latter toward metastasis, suggest that assays of PTs may not be predictive of clinical outcomes. We therefore evaluated the patterns of caspase-3 expression in paired tissue samples from PTs and metastatic lymph nodes obtained from patients with ESCC in a single institution. This enabled an investigation of possible differences in caspase-3 expression in metastatic lymph nodes.
High level expression of caspase-3 was observed in a significantly higher fraction of metastatic lymph nodes than in PTs. In general, there was a trend toward increased expression of caspase-3 in metastases, a finding confirmed by comparisons of individual patients, which showed that caspase-3 expression was upregulated in metastatic lymph nodes compared with PTs. Neoplasms are biologically heterogeneous and contain subpopulations of cells with different malignant properties. By IHC method, the patterns of biomarker expression were previously analyzed in samples of PTs and corresponding metastatic lesions. Conflicting results on some biomarkers, such as epidermal growth factor receptor (EGFR), have been observed. For example, differential expression of EGFR was observed in 33% of primary lung cancers and metastases, with EGFR down-regulated in the latter[14,18]. In contrast, high degrees of concordant EGFR expression were observed in breast and colon cancers and their respective metastases[19,20]. According to limited previous studies on ESCC, the patterns of some biomarkers, including E-cadherin, VEGF, MMP-9, and CD44V6, have been shown to differ between ESCCs and their metastases[21-23]. The present findings were novel, showing that the level of caspase-3 protein expression was higher in metastatic lymph nodes of ESCC than in PTs.
After correlation analysis of protein expression and clinical outcomes, we found that there was no association between caspase-3 expression and any clinicopathological factors, although high caspase-3 expression in PT was associated with a favorable prognosis. Moreover, multivariate analysis of clinical factors and biomarkers indicated that caspase-3 expression in PT was an independent prognostic factor of improved survival, consistent with previous immunohistochemical[9,11] and flow cytometry[24] findings in ESCC. Caspase-3 is the main effecting caspase in apoptotic process. The active form of caspase-3 is generated by proteolytic cleavage of procaspase-3 into two subunits with molecular masses of 17 kDa and 12 kDa, respectively. The antibody we used to detect caspase-3 protein in paraffin sections recognizes the inactive form of caspase-3, i.e. procaspase-3 or full length human caspase-3 protein. A previous study using an antibody against pro-caspase-3 showed a concordance between caspase-3 expression in ESCC and apoptotic index, as assessed by TUNEL technique[8]. Another study, in which caspase-3 activation and procaspase-3 expression were determined using specific antibodies, reported that the absence of caspase-3 activation was significantly correlated with loss of procaspase-3 expression[25]. Thus, immunohistochemical analysis of caspase-3 protein expression could be considered as a feasible method for detecting apoptosis in our cohort. The high level of caspase-3 expression in primary ESCC could therefore reflect increased apoptosis and may suppress the progression of ESCC.
Interestingly, we found that caspase-3 expression in metastases of ESCC was associated with an unfavorable outcome. DSS was significantly poorer in patients with higher than lower level caspase-3 expression in PMLN. Further, Cox multivariate analysis showed that caspase-3 expression in PMLN was independently prognostic of poor prognosis. Apoptosis in esophageal lesions has been reported to increase gradually during the progression of esophageal carcinogenesis[26]. Caspase-3 expression in metastatic lesions does not accord with increased apoptosis, implying that this protein has a function other than the execution of cell death programs. Recently, Fujita et al[27] and Janzen et al[28] conducted a series of elegant studies to explore the unexpected role of caspase-3 which mediates the differentiation of embryonic stem cells (ESCs) and hematopoietic stem cells (HSCs), respectively. These findings suggested that caspase-3 protein is involved in regulating stem cell development and differentiation. Tissue development and maintenance that affect tumor metastasis are dependent on a complex interplay of stem cell self-renewal, differentiation, and apoptosis[29]. Further studies assessing the biological mechanisms by which caspases affect cancer stem cells may enhance our understanding of metastasis in ESCC.
In conclusion, our study revealed a novel phenomenon that the level of caspase-3 protein expression differed substantially between primary ESCCs and metastatic lymph nodes. In contrast to PTs, in which caspase-3 expression was associated with better survival, caspase-3 expression in metastatic lymph nodes was independently prognostic of poorer survival in patients resected for ESCC and lymph node metastasis. Based on our findings, it finally underlined that protein expression in metastatic tumors could be considered for prognostic prediction and further studies on caspase-3 will provide a new visual angle to make a better understanding of this familiar biomarker in ESCC.
The authors thank Ms Miao-Qing Lin for patient follow-up and Maxim Ltd. for technical assistance.
Esophageal squamous cell carcinoma (ESCC) remains an aggressive disease with poor prognosis despite incorporation of new therapeutic approaches. Recent research focused on biomarkers, including caspase-3, may help to find out more effective therapies for patients with ESCC. Previous studies suggest that reduced caspase-3 expression in primary tumors (PTs) is associated with poorer outcomes, but the prognostic value of caspase-3 expression in metastatic lymph nodes (MLNs) is unclear. Based on the question of tumor heterogeneity, additional studies are needed to assess the prognostic importance of this biomarker in MLNs.
Primary tumors and regional metastatic lymph nodes assembled on tissue microarrays in pairs were evaluated by immunohistochemical analysis for caspase-3. The impact of caspase-3 expression on survival outcome was analyzed by the Kaplan-Meier method and Cox proportional hazards regression model.
In analyzing 122 patients with primary ESCC tumors and paired MLNs, we found that reduced caspase-3 expression in ESCC from different lesions was associated with different prognoses. To the best of our knowledge, this study is the first to report the potential prognostic value of caspase-3 expression in MLNs. It suggested that ongoing studies should be needed to make a better understanding of this familiar biomarker.
Our results suggested that the level of caspase-3 expression was significantly increased in lymphatic metastases compared with primary tumors. Positive caspase-3 expression in PTs was associated with a favorable prognosis, but high level expression in paired MLNs was associated with a poor outcome. Multivariate analysis demonstrated that they were both independent prognostic factors.
Tissue microarrays consist of paraffin blocks in which up to 1000 separate cores from different tissue blocks are assembled in a new paraffin block. They can enable the high throughput analysis of a large number of tissue samples.
This is an interesting study analyzing relations between caspase-3 expression in MLNs and clinical outcomes of ESCC. The main results in this study show that the level of caspase-3 expression in MLNs is higher than in PTs, implying the existence of tumor heterogeneity, and caspase-3 expression in MLNs is also a biomarker independently predictive of survival. Further study is needed to indentify the different role of caspase-3 during the progression of ESCC.
P- Reviewers: Chen LF, Chi SG, Gonzalez-Aseguinolaza G, Mishra PK, Scarpa M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 3. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] |
| 4. | Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 3157] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 5. | Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2335] [Cited by in RCA: 2438] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 6. | Glinsky GV, Glinsky VV. Apoptosis amd metastasis: a superior resistance of metastatic cancer cells to programmed cell death. Cancer Lett. 1996;101:43-51. [PubMed] |
| 7. | Takaoka A, Adachi M, Okuda H, Sato S, Yawata A, Hinoda Y, Takayama S, Reed JC, Imai K. Anti-cell death activity promotes pulmonary metastasis of melanoma cells. Oncogene. 1997;14:2971-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kurabayashi A, Furihata M, Matsumoto M, Ohtsuki Y, Sasaguri S, Ogoshi S. Expression of Bax and apoptosis-related proteins in human esophageal squamous cell carcinoma including dysplasia. Mod Pathol. 2001;14:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Hsia JY, Chen CY, Chen JT, Hsu CP, Shai SE, Yang SS, Chuang CY, Wang PY, Miaw J. Prognostic significance of caspase-3 expression in primary resected esophageal squamous cell carcinoma. Eur J Surg Oncol. 2003;29:44-48. [PubMed] |
| 10. | Bellini MF, Cury PM, Silva AE. Expression of ki-67 antigen and caspase-3 protein in benign lesions and esophageal carcinoma. Anticancer Res. 2010;30:2845-2849. [PubMed] |
| 11. | Jiang H, Gong M, Cui Y, Ma K, Chang D, Wang TY. Upregulation of caspase-3 expression in esophageal cancer correlates with favorable prognosis: an immunohistochemical study from a high incidence area in northern China. Dis Esophagus. 2010;23:487-492. [PubMed] |
| 12. | Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1020] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 2974] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 14. | Gomez-Roca C, Raynaud CM, Penault-Llorca F, Mercier O, Commo F, Morat L, Sabatier L, Dartevelle P, Taranchon E, Besse B. Differential expression of biomarkers in primary non-small cell lung cancer and metastatic sites. J Thorac Oncol. 2009;4:1212-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 639] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 16. | Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 618] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | Weiss L, Holmes JC, Ward PM. Do metastases arise from pre-existing subpopulations of cancer cells? Br J Cancer. 1983;47:81-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Italiano A, Vandenbos FB, Otto J, Mouroux J, Fontaine D, Marcy PY, Cardot N, Thyss A, Pedeutour F. Comparison of the epidermal growth factor receptor gene and protein in primary non-small-cell-lung cancer and metastatic sites: implications for treatment with EGFR-inhibitors. Ann Oncol. 2006;17:981-985. [PubMed] |
| 19. | Tsutsui S, Ohno S, Murakami S, Kataoka A, Kinoshita J, Hachitanda Y. EGFR, c-erbB2 and p53 protein in the primary lesions and paired metastatic regional lymph nodes in breast cancer. Eur J Surg Oncol. 2002;28:383-387. [PubMed] |
| 20. | Italiano A, Saint-Paul MC, Caroli-Bosc FX, François E, Bourgeon A, Benchimol D, Gugenheim J, Michiels JF. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors correlates with EGFR expression in related metastatic sites: biological and clinical implications. Ann Oncol. 2005;16:1503-1507. [PubMed] |
| 21. | Luo KJ, Hu Y, Wen J, Fu JH. CyclinD1, p53, E-cadherin, and VEGF discordant expression in paired regional metastatic lymph nodes of esophageal squamous cell carcinoma: a tissue array analysis. J Surg Oncol. 2011;104:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sato F, Shimada Y, Watanabe G, Uchida S, Makino T, Imamura M. Expression of vascular endothelial growth factor, matrix metalloproteinase-9 and E-cadherin in the process of lymph node metastasis in oesophageal cancer. Br J Cancer. 1999;80:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Takayama N, Arima S, Haraoka S, Kotho T, Futami K, Iwashita A. Relationship between the expression of adhesion molecules in primary esophageal squamous cell carcinoma and metastatic lymph nodes. Anticancer Res. 2003;23:4435-4442. [PubMed] |
| 24. | Cao FM, Zhang XH, Yan X, Wang JL, Xing LX, Wang XL, Shen HT, Wang FR. [Expression and prognostic significance of survivin and caspase-3 in esophageal squamous-cell carcinoma and their relationship with HSPs expression]. Zhonghua Zhongliu Zazhi. 2005;27:416-419. [PubMed] |
| 25. | Oudejans JJ, Harijadi A, Cillessen SA, Busson P, Tan IB, Dukers DF, Vos W, Hariwiyanto B, Middeldorp J, Meijer CJ. Absence of caspase 3 activation in neoplastic cells of nasopharyngeal carcinoma biopsies predicts rapid fatal outcome. Mod Pathol. 2005;18:877-885. [PubMed] |
| 26. | Wang LD, Zhou Q, Yang WC, Yang CS. Apoptosis and cell proliferation in esophageal precancerous and cancerous lesions: study of a high-risk population in northern China. Anticancer Res. 1999;19:369-374. [PubMed] |
| 27. | Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, Thomson JA, Zwaka TP. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595-601. [PubMed] |
| 28. | Janzen V, Fleming HE, Riedt T, Karlsson G, Riese MJ, Lo Celso C, Reynolds G, Milne CD, Paige CJ, Karlsson S. Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell. 2008;2:584-594. [PubMed] |
| 29. | Abdul-Ghani M, Megeney LA. Rehabilitation of a contract killer: caspase-3 directs stem cell differentiation. Cell Stem Cell. 2008;2:515-516. [PubMed] |