Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4244
Revised: December 20, 2013
Accepted: February 20, 2014
Published online: April 21, 2014
Processing time: 201 Days and 20.5 Hours
Rectal cancer is a common cancer and a major cause of mortality in Western countries. Accurate staging is essential for determining the optimal treatment strategies and planning appropriate surgical procedures to control rectal cancer. Endorectal ultrasonography (EUS) is suitable for assessing the extent of tumor invasion, particularly in early-stage or superficial rectal cancer cases. In advanced cases with distant metastases, computed tomography (CT) is the primary approach used to evaluate the disease. Magnetic resonance imaging (MRI) is often used to assess preoperative staging and the circumferential resection margin involvement, which assists in evaluating a patient’s risk of recurrence and their optimal therapeutic strategy. Positron emission tomography (PET)-CT may be useful in detecting occult synchronous tumors or metastases at the time of initial presentation. Restaging after neoadjuvant chemoradiotherapy (CRT) remains a challenge with all modalities because it is difficult to reliably differentiate between the tumor mass and other radiation-induced changes in the images. EUS does not appear to have a useful role in post-therapeutic response assessments. Although CT is most commonly used to evaluate treatment responses, its utility for identifying and following-up metastatic lesions is limited. Preoperative high-resolution MRI in combination with diffusion-weighted imaging, and/or PET-CT could provide valuable prognostic information for rectal cancer patients with locally advanced disease receiving preoperative CRT. Based on these results, we conclude that a combination of multimodal imaging methods should be used to precisely assess the restaging of rectal cancer following CRT.
Core tip: In rectal cancer, accurate staging and circumferential resection margin assessment are essential for stratifying the risks of recurrence and determining the optimal therapeutic strategy for individual patients. In the preoperative setting, a combination of multimodal imaging methods, including endorectal ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI), can be used to precisely assess the preoperative staging of rectal cancer. However, restaging after neoadjuvant therapy remains a challenge with all of these modalities. Recently, high-resolution MRI with diffusion-weighted imaging and/or positron emission tomography-CT imaging methods have been developed to precisely assess the restaging of rectal cancer following neoadjuvant chemoradiation therapy.
- Citation: Heo SH, Kim JW, Shin SS, Jeong YY, Kang HK. Multimodal imaging evaluation in staging of rectal cancer. World J Gastroenterol 2014; 20(15): 4244-4255
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4244
Rectal cancer is a tumor that arises from the rectum within 15 cm of the anal verge[1]. In 2013, there were an estimated 40340 new cases of rectal cancer in the United States, and rectal cancer is a major cause of cancer-related deaths in the developed world[2]. The prognosis of rectal cancer patients depends on the disease stage at the time of diagnosis[3-5]; thus, accurate disease evaluation is necessary to properly treat rectal cancer.
Rectal cancer prognosis is largely determined by tumor-node-metastasis staging, which evaluates the depth of tumor invasion into the rectal wall as well as the presence of lymph node (LN) and other distant metastases (Table 1)[3,4]. Again, accurate staging is crucial to determine the optimal treatment strategy, including selecting the appropriate surgical procedure. For example, early-stage (T1 and some T2) tumors may be suitable for local excision or trans-anal endoscopic microsurgery, whereas more advanced lesions (T3-4 and/or N1-2) may merit neoadjuvant chemoradiation therapy (CRT) followed by total mesorectal excision [TME; the surgical removal of the rectum and mesorectum enveloped by the mesorectal fascia (MRF)][5,6]. In cases requiring TME, the status of the circumferential resection margin (CRM; defined by the spreading of the tumor to the MRF) is an important prognostic factor for predicting survival and local recurrence[1,5,7-9].
| Primary tumor (T) | |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades through the muscularis propria and into perirectal tissues |
| T4a | Tumor penetrates to the surface of the visceral peritoneum |
| T4b | Tumor directly invades or is adherent to other organs or structures |
| Regional lymph nodes (N) | |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastases in 1-3 regional lymph nodes |
| N1a | Metastasis in 1 regional lymph node |
| N1b | Metastases in 2-3 regional lymph nodes |
| N1c | Tumor deposit(s) in the subserosa, mesentery, pericolic, or perirectal tissues without regional nodal metastasis |
| N2 | Metastases in 4 or more regional lymph nodes |
| N2a | Metastases in 4-6 regional lymph nodes |
| N2b | Metastases in 7 or more regional lymph nodes |
| Distant metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confined to 1 organ or site (e.g., liver, lung, ovary, non-regional node, external iliac lymph node) |
| M1b | Metastases in > 1 organ/site or the peritoneum |
Because neoadjuvant CRT is the standard treatment for locally advanced rectal cancer, assessing tumor response using conventional imaging methods has been challenging[10-15]. The goal of neoadjuvant CRT is to preserve sphincter function while improving tumor resectability and reducing tumor mass prior to surgery[5]. Therefore, a more reliable imaging method would facilitate the design of more effective pre- and post-operative treatment strategies. Moreover, an effective assessment method would allow stratification of patients according to the risk of recurrence using preoperative and post-CRT assessments of the depth of tumor invasion and nodal status as well as of CRM involvement[16,17].
Currently, several imaging modalities, including endorectal ultrasound (EUS), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) or PET-CT, are used to assess preoperative staging and therapeutic response. This review will address the advantages and limitations for each of these imaging modalities in the context of staging and assessing therapeutic response in rectal cancer patients.
The preoperative staging of a rectal cancer tumor has been correlated with the patient’s long-term prognosis[3-5]. In particular, tumor staging facilitates the formulation of a structured multidisciplinary approach to manage the disease and assess a patient’s prognosis over the course of treatment. Preoperative staging of rectal cancer can be divided into either local or distant staging. Local staging primarily involves assessment of mural wall invasion and nodal status for metastasis as well as CRM involvement, while distant staging assesses the extent of metastatic disease. Currently, several modalities are used for the preoperative staging of rectal cancer (Table 2).
| Imaging modalities | Advantages | Disadvantages |
| Preoperative staging | ||
| EUS | Depth of tumor invasion in early rectal cancer | Limited field of view Perirectal invasion and LN metastasis |
| CT | Wide availability Fast scanning time | Local staging and CRM involvement in lower rectal cancer |
| MRI | High soft tissue resolution Local staging and CRM involvement | Distant metastasis |
| PET/CT | Distant metastasis | Poor spatial resolution Perirectal invasion and CRM involvement |
| Post-therapeutic restaging | ||
| EUS | None | Low accuracy of T staging and LN staging |
| CT | Relatively high accuracy of CRM status | Relatively low accuracy of T staging and LN staging |
| MRI | High specificity of therapeutic response assessment High accuracy of CRM involvement | Relatively poor sensitivity |
| PET/CT | Distant metastasis | No standard parameter and follow-up protocol |
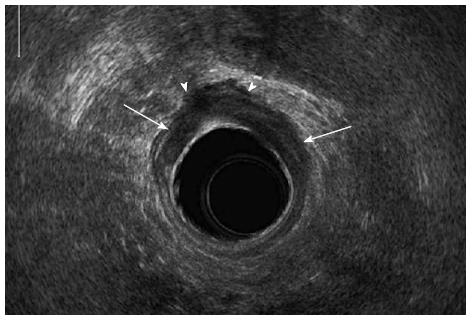
EUS is performed by introducing a water-filled balloon containing a high-frequency transducer and trans-anal probe into the rectum. This approach can delineate the five layers of the rectal wall as alternating hyper-echoic and hypo-echoic bands[18,19]. EUS is particularly effective for assessing the depth of tumor invasion into the rectal wall, with 69%-94% accuracy (Figure 1)[20-23]. EUS can accurately distinguish between early- and advanced-stage rectal lesions with sensitivity, specificity and accuracy rates of 96%, 85% and 94% respectively[6]. EUS is more specific in evaluating local tumor invasion when compared to MRI imaging (86% vs 69% specificity rates respectively), although both methods have similar high sensitivities for evaluating the depth of tumor penetration into the muscularis propria (94%)[24].
A recent meta-analysis of 5039 patients from 42 studies carried out between 1980 and 2008 supports the accuracy of EUS for determining T stages, with pooled sensitivity and specificity rates of approximately 81%-96% and 91%-98%, respectively[25]. However, the accuracy of EUS in assessing the depth of invasion into the rectal wall appears to vary with the tumor stage, with lower accuracy in T2 lesions, compared with that in early-(T1) and advanced-(T3-T4) stage lesions[25]. Furthermore, EUS cannot reliably distinguish between peritumoral inflammation or transmural tumor infiltration, which may lead to over-staging of T2 tumors as T3 tumors and subsequent overtreatment[21,26,27]. The staging of bulky, distal and/or stenotic lesions with EUS can also be challenging due to the limited field of view and the inability of probes (especially rigid probes) to traverse the lesion[19,23].
Lymph node (LN) staging with EUS remains difficult and is less accurate than T staging, with reported accuracy rates of 64%-83%[20,21,23,28]. Although EUS-detected morphological characteristics, including a round shape, peritumoral location, size, and hypo-echogenecity) could be associated with malignant LNs, these features are neither sensitive nor specific[10,19,29]. Moreover, EUS can evaluate only perirectal or mesorectal LNs, thereby limiting the screening capacity of this method. In contrast, other imaging modalities such as CT, MRI and PET/CT can visualize the iliac and mesenteric or retroperitoneal nodes, allowing for more comprehensive LN staging.
Recent developments in 3-dimensional EUS technology, with enhanced resolution and a multi-planar display, have resulted in improved T- and LN-staging accuracy rates compared to 2-dimensional EUS[30]. Nevertheless, an assessment of the MRF remains impossible and the accuracy of the procedure is largely dependent on the experience of the operator[19,21,30]. Thus, the major role of EUS in rectal cancer staging is for assessment of tumor invasion depth, particularly in early-stage rectal tumors, for which EUS can be used to evaluate whether tumors are suitable for treatment by trans-anal or local excision[6,17,25].
CT is commonly used as the initial staging modality for rectal cancer because of its wide availability and fast scanning times. In a single examination, CT can also assess the entire abdomen, pelvis and chest, allowing for both local staging and distant metastases evaluations[17,31]. Initial studies using conventional CT to assess locally advanced rectal cancers (i.e., ≥ T3) reported T staging accuracy rates of 79%-94%[32-34]. The technical advances in multi-detector CT (MDCT) have provided enhanced spatial resolution though thin-collimation scanning and multi-planar reformation, and have improved the accuracy rates to greater than 90%[35]. Nevertheless, MDCT has shown limited value for early-stage lesions confined to the rectal wall (e.g., differentiation between T1 and T2 tumors), for which EUS may be more effective. Furthermore, the lower resolution of MDCT is unable to reliably distinguish the layers of the rectal wall and to differentiate between desmoplastic or peritumoral inflammatory reactions and tumor infiltration into the perirectal fat[24,36]. These limitations lead to a tendency to over-stage T1 or T2 tumors as T3 tumors when using MDCT.
LN staging is predominantly based on the size criterion and, to a lesser extent, on morphology[17]. One study suggests that a LN with axis above 4.5 mm in diameter is usually considered malignant, but such size criteria are not generally considered to be accurate[37]. The lack of a clear cut-off diameter to determine whether a LN is metastatic has led to wide variability in LN-staging sensitivity and accuracy using CT, with respective rates of 25%-86% and 35%-84%[17,37]. Moreover, even with the enhanced resolution of MDCT, accurate assessment of the nodal status remains challenging because microscopic metastases in normal-sized LNs cannot be depicted on CT.
Regarding assessments of CRM involvement, in a multicenter study of 250 patients[38], MDCT showed overall sensitivity and specificity rates of 76% and 96%, respectively for mid to upper rectal cancer patients. This result suggests that CT may be an alternative to MRI to predict CRM involvement in such patients. In lower rectal cancer, however, CT is less accurate and inconsistently predicts CRM involvement[39,40]. Thus, in a preoperative setting, CT is best suited for evaluations of distant metastases, as this modality is limited with regard to local staging and CRM status determinations.
Since 1986, MRI has been used to delineate rectal tumors and locally stage rectal cancers. Initial MRI studies using a body coil have reported overall accuracy rates ranging from 59% to 88%, similar to the accuracy range for CT[41-43]. Recently, rectal MRI has been performed with either an endorectal coil or a phased-array surface coil. Several studies with phased-array MRI showed accuracy rates of 65%-86%, and studies with an endorectal coil reported slightly improved T staging rates (71%-91%)[43,44]. Although the endorectal coil permits a more detailed differentiation of the rectal wall, its routine use in clinical practice is controversial due to some limitations of the technology. Specifically, it is difficult to clearly differentiate between the mucosa and submucosa, to access high and/or stenotic rectal lesions, and to completely evaluate the mesorectum and surrounding structures due to the limited field of view[10,17,45]. However, in a comparative study on endorectal and phased-array coils, both techniques yielded similar diagnostic accuracy rates (80%) for the depth of tumor invasion[46].
In addition, recent studies have shown that the use of 3.0-T MRI improves the overall T staging accuracy for rectal cancer with accuracy rates of 86%-95%[47-49]. This represents a significant improvement over 1.5-T MRI, which has T staging accuracy rates of 67%-86%[50-52]. Moreover, improvements in scanner gradients and dedicated external coils have allowed rectal MRI to be successfully performed with phased-array surface coils; allowing high-resolution imaging with thin-sections and an enhanced field of view, and providing a more comfortable patient experience[43].
The use of rectal MRI techniques for clinical decisions regarding bowel preparation, the use of antispasmodic agents, and the use of rectal gel can vary according to the preference of the radiologists[53]. Although the use of gadolinium contrast enhancement rarely improves rectal cancer staging via MRI, many centers still acquire images both before and after gadolinium administration[54,55].
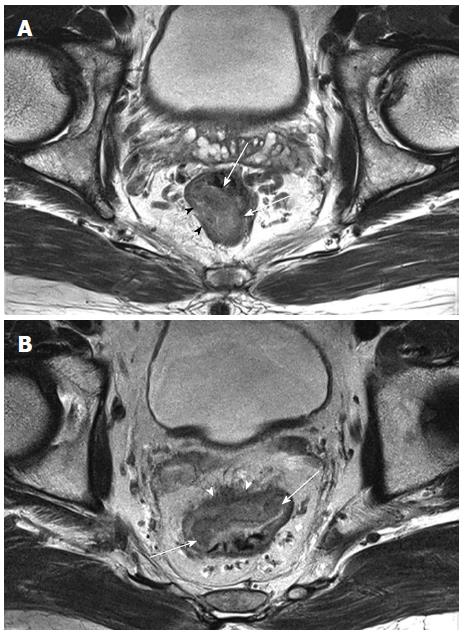
To identify and stage rectal cancers with MRI, high-resolution T2-weighted image with thin-sections is the primary approach used to differentiate between the tumor, mucosal and submucosal layers, muscular layer, perirectal fat, and MRF[56]. However, because the submucosal layer is not always efficiently visualized on MRI[16], the ability to differentiate between T1 and T2 lesions may be limited. Using MRI, a T3 lesion is defined as a tumor that extends through the hypo-intense muscle layer into the hyper-intense perirectal fat with a bulging or nodular appearance, whereas a T2 tumor is confined to the hypo-intense muscle layer (Figure 2)[56]. In prospective comparative studies with histopathology, MRI-based staging strongly correlated with the depth of tumor invasion (82%)[56] and agreed with the extramural depth of invasion (95%)[57]. However, staging failures commonly occur when differentiating T2 from borderline T3 tumors, as it is difficult to distinguish direct tumor infiltration (T3) from speculated structures in the perirectal fat due to fibrosis or peritumoral inflammation (T2)[58]. In contrast, MRI is particularly accurate when identifying T3 and T4 tumors, with a sensitivity of 80%-86% and specificity of 71%-76% for T3 tumors[59].
Regarding LN assessments with MRI, a size cut-off of 5 mm in diameter is the most commonly used criterion. However, 15%-42% of patients with rectal cancer have metastatic perirectal LNs that are smaller than 5 mm[60,61], indicating that this criterion is not accurate enough for reliable assessment. Using a combination of other criteria, including an irregular border and/or a mixed signal intensity, MRI for nodal staging shows an accuracy of 85%, with a moderate sensitivity of 75% and high specificity of 98%[61]. However, a recent meta-analysis of 1249 patients from 23 data sets showed that the ability to assess LN involvement was consistently poor with a pooled sensitivity of 77% and specificity of 71%[62]. Similarly, in another meta-analysis study, MRI showed poor accuracy when compared with EUS and CT[24]. With improvements to MRI scanners and techniques, the nodal staging accuracy has increased to 91%, with a high sensitivity of 89%[47]. High-resolution MRI with thin-sections has enabled evaluations of the detailed characteristics of LNs. Nevertheless, it remains challenging to detect micro-metastases in small or normal-sized LNs using MRI.
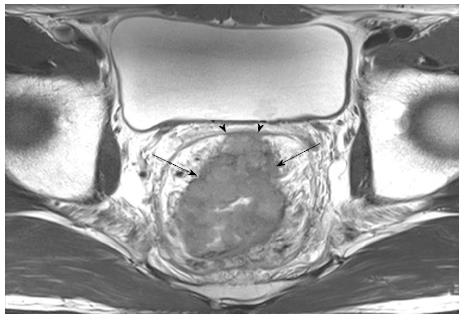
MRI is a reliable imaging modality for CRM status assessments, making the technique an important element of surgical planning and prognosis. Most commonly, MRI is used to measure the distance of a tumor from the MRF, as the MRF is observed as a thin hypo-intense line that envelops the perirectal fat and rectum. In a prospective study by the Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY) study group, which defines CRM involvement as a tumor within 1 mm of the MRF, MRI showed 92% specificity for preoperative predictions of a clear CRM (Figure 3)[63]. This 1 mm threshold is the most commonly used criterion to assess CRM involvement, although Beets-Tan et al[58] reported that a 5 mm cut-off on MRI could also predict CRM involvement with a high degree of certainty. Published data have shown that MRI has a high accuracy rate of 86% for predicting CRM involvement, with good intra- and inter-observer agreement[17,58,64]. Thus, MRI is a powerful method for preoperatively assessing both local staging and CRM involvement with high accuracy and reproducibility, effectively identifying patients who require preoperative CRT in order to minimize incomplete tumor resections and local recurrences[63].
PET enables the measurement and visualization of metabolic changes within cancer cells. Fluorodeoxyglucose (FDG) is the most common PET tracer used, as its uptake is increased in tumors due to increased metabolic activity in the tumor cells, which facilitates efficient radiolabelling. Studies have shown that PET-CT can reliably detect colorectal cancer but not its depth of tumor invasion[65,66]. The poor spatial resolution and lack of detailed anatomy provided by FDG-PET make the determination of the degree of local tumor spread, LN involvement, CRM status, or relationship to the sphincter complex difficult.
The utility of PET-CT scanning for initial staging of rectal cancer remains unclear[14]. While PET-CT is capable of detecting occult synchronous tumors or metastases at the time of initial presentation, the detection rate is too low to justify the costs and radiation exposure for its routine use. In addition, PET is limited to the identification of non-mucinous tumors because FDG uptake is hampered by the presence of mucin[67]. Currently, PET-CT is reserved for the staging of patients prior to surgical removal of recurrent lesions or distant metastases and to identify recurrent lesions in patients with an unexplained rise in serum carcinoembryonic antigen. PET-CT may be particularly useful for the staging of distant metastatic spread, particularly if radical surgery is being considered but no local staging information is available using other imaging methods[14].
One recent study demonstrated that preoperative PET altered the treatment strategy in 17% of patients, including 13% for whom surgery was cancelled and 4% for whom the radiotherapy field was changed[68]. One explanation for this finding is that PET-CT frequently yields additional staging information in patients with low rectal cancer. Thus, PET-CT could be used to improve the accuracy of pretreatment imaging, thereby allowing for more appropriate stage-specific therapy[69].
Preoperative CRT has been widely adopted for the management of patients with locally advanced rectal cancers because it can facilitate tumor downsizing and down-staging, leading to increased rates of sphincter-sparing surgeries and pathologic complete response[5]. If tumor responses to neoadjuvant CRT could be assesses before surgery, patients could receive appropriately tailored treatments. For example, patients who respond well the CRT could be offered less radical treatments, whereas those who fail to respond could be identified as candidates for more radical surgeries or second-line therapies[14]. Traditionally, tumor response assessments are achieved by measuring the percentage of the tumor size reduction according to the Response Evaluation Criteria in Solid Tumors criteria, as the change in tumor size is generally thought to correlate with treatment efficacy[70]. However, this assessment approach is insensitive to early treatment changes, and it can be difficult to distinguish between viable tumors and CRT-associated non-tumoral masses (e.g., inflammation, necrosis, or fibrosis)[11]. Given these limitations, the response of locally advanced rectal cancers should be addressed not only by tumor size, but also by additional prognostic factors, including the extramural depth of invasion, nodal status, and CRM involvement, as well as the relationship with pelvic structures (Table 2). Thus, it is important to determine which imaging modalities can provide accurate and reliable information regarding these criteria to accurately assess tumor responses.
EUS does not appear to play a role in post-therapeutic response assessments because it cannot reliably differentiate between post-radiation edema, inflammation, fibrosis, and viable tumors[19]. Due to this limitation, the accuracy of EUS for rectal cancer restaging after radiation therapy is markedly low (47%)[71]. In locally advanced rectal cancers, the T staging accuracy rates for EUS, when performed at 4-6 wk after the completion of CRT, were 29% in responsive patients and 82% in nonresponsive patients, with a high misinterpretation rate (71%) in responsive patients[72]. In the same study, the overall accuracy of EUS for LN involvement after CRT was 57%[72]. Thus, EUS is not recommended for restaging after radiotherapy or CRT.
CT is most commonly used to evaluate treatment responses in solid tumors. However, in CRT-treated rectal cancer, the utility of CT is limited for identifying and following-up metastatic lesions. In a recent study to correlate tumor responses measured by CT in accordance with the histopathology, the overall accuracy rates of T and N restaging were 65% and 67%[73] respectively, which represents an improvement over a previous study (reporting T restaging of 37% and N restaging of 62%)[13]. However, the tumor over-staging and under-staging rates after CRT were 23% and 12% respectively, because rectal wall thickening caused by CRT-induced fibrosis can be misidentified as residual tumors on CT[73]. In CRM status assessments after CRT, CT accurately predicted a negative CRM in 71% of cases[13]. Nevertheless, it remains unclear whether CT would have a clinical impact on the therapeutic outcome of local staging in patients with locally advanced, CRT-treated rectal cancer because of its poor resolution, further studies are necessary to evaluate the efficacy of this approach[14].
With the recent advances aimed at reducing radiation exposure, perfusion CT is an emerging and noninvasive technique that permits measurements of tumor vascularity and perfusion changes, allowing measurements of CRT response. In rectal cancers, perfusion CT has been shown to differentiate rectal cancer from the normal rectal wall[74,75]. In addition, perfusion CT could be useful for predicting tumor responses and clinical outcomes of CRT, as it can differentiate between tumors with normal vs low blood flow, as low blood flow before CRT is associated with a poor response[74,75].
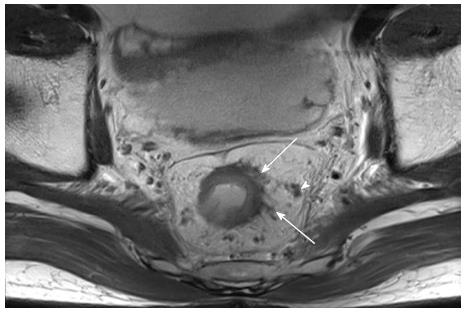
As in preoperative evaluation, T2-weighted MRI plays a major role in rectal cancer restaging after CRT. The concordance between the post-CRT MRI and the histological T stage was only fair to moderate (κ = 0.40), suggesting rather low accuracy[76]. However, when a morphological response assessment (e.g., responder vs non-responder) was used, the overall accuracy rate improved to 79%[77]. High-resolution MRI with thin-sections yielded an improved T staging accuracy rate after CRT, with good concordance (κ = 0.64)[78]. Despite these improvements, a recent meta-analysis showed that MRI-mediated rectal cancer restaging after preoperative CRT remains challenging, with poor sensitivity (50.4%) due to the difficulty of differentiating tumor changes from the residual tumor (Figure 4) using MRI[79]. In cases of mucinous tumors, which are hyper-intense on T2-weighted images, stage prediction errors can also arise, making it difficult to distinguish between a remnant tumor and a mucin pool[76,80]. However, the pooled specificity rate was as high as 91.2%, which suggests that positive MRI results can accurately identify CRT-responsive patients; however, negative MRI results may not similarly identify non-responsive patients[79].
To determine the LN status after CRT, the same pre-CRT morphological criteria (i.e., size, irregular borders, and signal homogeneity) are applied. This assessment method has a reported overall accuracy rate of 64%-68%[76,81,82]. Moreover, recent studies of MRI assessment of post-CRT LN status reported high accuracy rates of 87%-88%[77,78]. However, changes in morphological appearances after CRT, including high-signal interference from mucin pools or spiculated LN margins, can make MRI assessments prone to over-staging (Figure 4)[12,77,78].
According to a prospective study by the MERCURY group, the accuracy rate and negative predictive value for identifying CRM involvement in 97 CRT-treated patients were 77% and 98% respectively, whereas the same values were 91% and 93% in 311 patients who underwent primary surgery[63]. A recent meta-analysis study reported a similar result, with a mean sensitivity of 76.3% and mean specificity of 85.9%[79]. However, it should be noted that CRM involvement errors frequently occur in MRI assessments because of diffuse hypo-intense infiltration into the perirectal fat or MRF after CRT[63,83]. Nevertheless, MRI assessment is considered effective for restaging tumors after CRT, particularly with respect to potential CRM involvement.
Diffusion-weighted imaging (DWI) is a newly emerging, functional MRI technique that can supplement conventional MRI assessment and enable the noninvasive characterization of biological tissues based on the properties of water diffusion. Thus, DWI can provide microstructural information, including changes in cellularity and integrity of cellular membranes, which often precede conventional morphological alterations. DWI has been used to detect and characterize tumors and distinguish between necrotic and viable solid portions within tumors by quantifying apparent diffusion coefficient (ADC) values[84,85]. The ADC value decreases with increasing cell density due the restricted diffusion of water molecules, which tends to increase in necrotic regions[84,86].
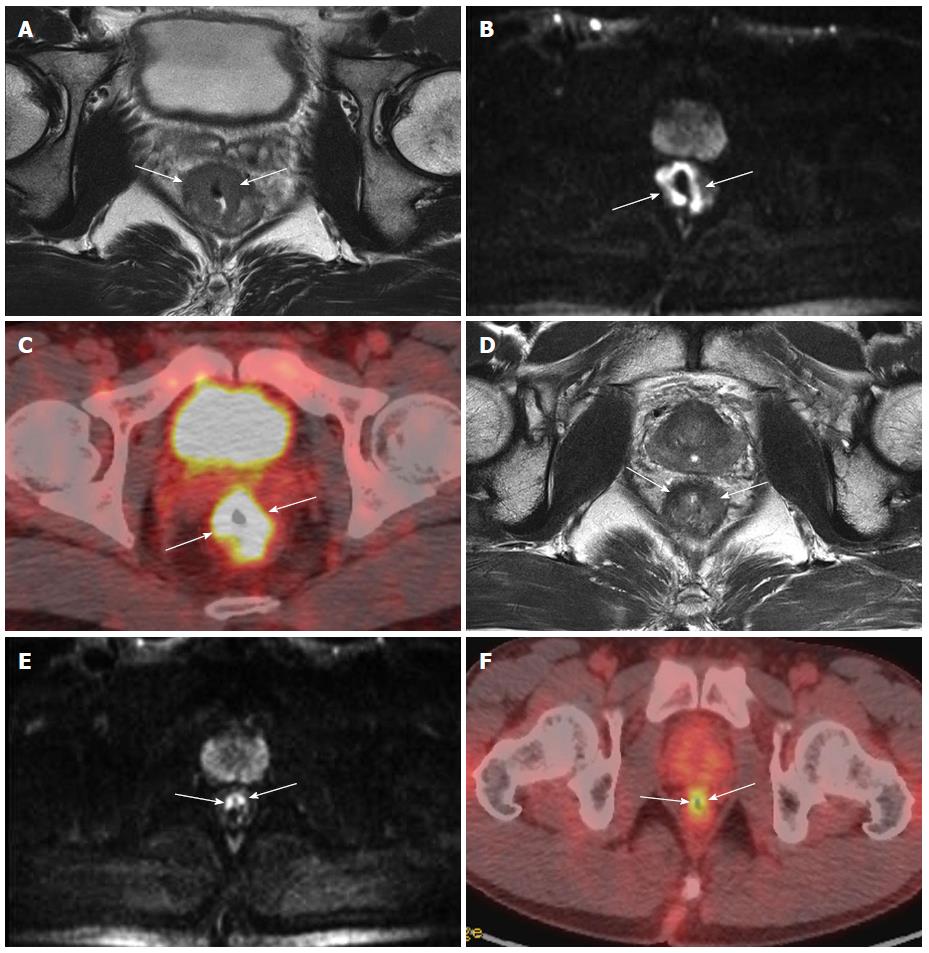
The ADC values of tumors with good or complete responses were significantly higher than those of tumors with poor responses, and the presence of higher pre-CRT ADC values reflected necrotic CRT-resistant tumors[86,87], suggesting that this approach is useful for predicting CRT response ahead of treatment. In contrast, the post-CRT ADC values of rectal cancers were lower than those of the pre-CRT tumors; a difference that was attributed to fibrosis or scar tissue formation and was not correlated with tumor response. These results indicate that the addition of DWI to standard MRI may facilitate the detection of viable tumors and identify CRT-responsive patients (Figure 5)[88-90]. Moreover, in a recent meta-analysis, restaging with DWI showed good pooled sensitivity (83.6%) and specificity rates (84.8%)[79]. Thus, DWI can provide a useful biomarker to assess and monitor treatment responses to CRT[86,91].
PET-CT is a promising modality for identifying recurrent rectal cancer and distant metastasis. A significant reduction of standardized uptake values on post-CRT PET of responders compared with non-responders has been noted by a number of studies (Figure 5), suggesting that this approach can effectively assess CRT response[15,92,93]. However, the efficacy of PET-CT for the prediction of tumor response remains controversial, especially for assessing pathological complete response to CRT in rectal cancer due to several limitations of the technology. One limitation is the variation in definitions and parameters used for defining a good vs a poor response. Another limitation is the uncertainty regarding the timing of PET imaging after the completion of radiotherapy. The optimal time to carry out PET assessment after the completion of CRT remains unclear, with initial studies suggesting an interval of only 6 wk being too soon to confirm metabolic response[93]. Therefore, at present, the role of PET-CT in post-CRT restaging is not well established. It is possible that PET-CT may provide additional information in assessing response; however, PET-CT needs to be formally compared with existing methods to properly evaluate its efficacy.
Multimodal imaging assessments in rectal cancer facilitate the design of treatment strategies and predict patient prognosis. Continued improvement of imaging techniques will provide superior image resolution, three-dimensional viewing, and decreased image acquisition times, and may provide new functional qualities. The use of EUS is likely to increase in future staging investigations, which will be complementary to the management of rectal cancer. DWI has been applied as a useful biomarker, which could be used to assess and monitor treatment responses to CRT, although protocol standardization and experienced radiologists are required to maximize the efficacy of this approach. Although the role of PET-CT needs to be formally compared with existing methods, PET-CT could provide additional valuable information for the assessment of post-CRT response. Restaging using a combination of imaging methods including high-resolution MRI, DWI and PET-CT will provide valuable prognostic information before definitive surgery. Despite these improvements, determination of the LN metastasis remains challenging in rectal cancer staging. LN research is further needed.
In preoperative settings, EUS is suitable for assessing the depth of tumor invasion, particularly in superficial and early-stage rectal tumors. CT imaging is a common method for evaluation of distant metastases. MRI is a useful and reliable modality for assessing preoperative staging and CRM involvement, allowing physicians to stratify the risks of recurrence and determine the optimal therapeutic planning for individual patients. PET or PET-CT assessment is recommended when there is clinical, biochemical or radiological suspicion of systemic disease.
Restaging after neoadjuvant therapy, particularly CRT, is challenging with all modalities because it is difficult to reliably differentiate between radiation-induced changes and the tumor itself. However, recent data suggest that a combination of high-resolution MRI with DWI, and PET-CT could provide valuable prognostic information before surgery for patients who were treated with preoperative CRT for locally advanced rectal cancer.
P- Reviewers: Bujanda L, Cai SJ, Riss S, Triantafyllou K S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Salerno G, Sinnatamby C, Branagan G, Daniels IR, Heald RJ, Moran BJ. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis. 2006;8 Suppl 3:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 3. | AJCC cancer staging manual; Edge SB, American Joint Committee on Cancer. Colon and Rectum. 7th ed. New York: Springer 2010; 143-164. |
| 4. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fuchs CS. Rectal cancer. J Natl Compr Canc Netw. 2012;10:1528-1564. [PubMed] |
| 6. | Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23:1384-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 7. | Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707-711. [PubMed] |
| 8. | Hall NR, Finan PJ, al-Jaberi T, Tsang CS, Brown SR, Dixon MF, Quirke P. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent. Predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41:979-983. [PubMed] |
| 9. | Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP, Lacasta A, Bujanda L. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res. 2007;13:6877s-6884s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Bodén K, Eriksson-Alm Y, Berg E, Fujii H, Kubo A, Blomqvist L. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Barbaro B, Vitale R, Leccisotti L, Vecchio FM, Santoro L, Valentini V, Coco C, Pacelli F, Crucitti A, Persiani R. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics. 2010;30:699-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Pomerri F, Pucciarelli S, Maretto I, Zandonà M, Del Bianco P, Amadio L, Rugge M, Nitti D, Muzzio PC. Prospective assessment of imaging after preoperative chemoradiotherapy for rectal cancer. Surgery. 2011;149:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Evans J, Patel U, Brown G. Rectal cancer: primary staging and assessment after chemoradiotherapy. Semin Radiat Oncol. 2011;21:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Tong J, Sun X, Liu J, Wang Y, Huang G. 18F-FDG-PET evaluation of treatment response to neo-adjuvant therapy in patients with locally advanced rectal cancer: a meta-analysis. Int J Cancer. 2012;131:2604-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Beets-Tan RG, Beets GL. Local staging of rectal cancer: a review of imaging. J Magn Reson Imaging. 2011;33:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Greene FL, Hindman NM, Jones B, Katz DS. ACR Appropriateness Criteria pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012;9:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Beynon J, Foy DM, Temple LN, Channer JL, Virjee J, Mortensen NJ. The endosonic appearances of normal colon and rectum. Dis Colon Rectum. 1986;29:810-813. [PubMed] |
| 19. | Krajewski KM, Kane RA. Ultrasound staging of rectal cancer. Semin Ultrasound CT MR. 2008;29:427-432. [PubMed] |
| 20. | Herzog U, von Flüe M, Tondelli P, Schuppisser JP. How accurate is endorectal ultrasound in the preoperative staging of rectal cancer? Dis Colon Rectum. 1993;36:127-134. [PubMed] |
| 21. | Garcia-Aguilar J, Pollack J, Lee SH, Hernandez de Anda E, Mellgren A, Wong WD, Finne CO, Rothenberger DA, Madoff RD. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10-15. [PubMed] |
| 22. | Kulig J, Richter P, Gurda-Duda A, Gach T, Klek S. The role and value of endorectal ultrasonography in diagnosing T1 rectal tumors. Ultrasound Med Biol. 2006;32:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Lin S, Luo G, Gao X, Shan H, Li Y, Zhang R, Li J, He L, Wang G, Xu G. Application of endoscopic sonography in preoperative staging of rectal cancer: six-year experience. J Ultrasound Med. 2011;30:1051-1057. [PubMed] |
| 24. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 721] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 25. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Hulsmans FJ, Tio TL, Fockens P, Bosma A, Tytgat GN. Assessment of tumor infiltration depth in rectal cancer with transrectal sonography: caution is necessary. Radiology. 1994;190:715-720. [PubMed] |
| 27. | Maier AG, Barton PP, Neuhold NR, Herbst F, Teleky BK, Lechner GL. Peritumoral tissue reaction at transrectal US as a possible cause of overstaging in rectal cancer: histopathologic correlation. Radiology. 1997;203:785-789. [PubMed] |
| 28. | Beynon J, Mortensen NJ, Foy DM, Channer JL, Rigby H, Virjee J. Preoperative assessment of mesorectal lymph node involvement in rectal cancer. Br J Surg. 1989;76:276-279. [PubMed] |
| 29. | Hulsmans FH, Bosma A, Mulder PJ, Reeders JW, Tytgat GN. Perirectal lymph nodes in rectal cancer: in vitro correlation of sonographic parameters and histopathologic findings. Radiology. 1992;184:553-560. [PubMed] |
| 30. | Kim JC, Kim HC, Yu CS, Han KR, Kim JR, Lee KH, Jang SJ, Lee SS, Ha HK. Efficacy of 3-dimensional endorectal ultrasonography compared with conventional ultrasonography and computed tomography in preoperative rectal cancer staging. Am J Surg. 2006;192:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Thoeni RF, Moss AA, Schnyder P, Margulis AR. Detection and staging of primary rectal and rectosigmoid cancer by computed tomography. Radiology. 1981;141:135-138. [PubMed] |
| 33. | Zaunbauer W, Haertel M, Fuchs WA. Computed tomography in carcinoma of the rectum. Gastrointest Radiol. 1981;6:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | van Waes PF, Koehler PR, Feldberg MA. Management of rectal carcinoma: impact of computed tomography. AJR Am J Roentgenol. 1983;140:1137-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Kulinna C, Eibel R, Matzek W, Bonel H, Aust D, Strauss T, Reiser M, Scheidler J. Staging of rectal cancer: diagnostic potential of multiplanar reconstructions with MDCT. AJR Am J Roentgenol. 2004;183:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Sinha R, Verma R, Rajesh A, Richards CJ. Diagnostic value of multidetector row CT in rectal cancer staging: comparison of multiplanar and axial images with histopathology. Clin Radiol. 2006;61:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 37. | Perez RO, Pereira DD, Proscurshim I, Gama-Rodrigues J, Rawet V, São Julião GP, Kiss D, Cecconello I, Habr-Gama A. Lymph node size in rectal cancer following neoadjuvant chemoradiation--can we rely on radiologic nodal staging after chemoradiation? Dis Colon Rectum. 2009;52:1278-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Wolberink SV, Beets-Tan RG, de Haas-Kock DF, van de Jagt EJ, Span MM, Wiggers T. Multislice CT as a primary screening tool for the prediction of an involved mesorectal fascia and distant metastases in primary rectal cancer: a multicenter study. Dis Colon Rectum. 2009;52:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Vliegen R, Dresen R, Beets G, Daniels-Gooszen A, Kessels A, van Engelshoven J, Beets-Tan R. The accuracy of Multi-detector row CT for the assessment of tumor invasion of the mesorectal fascia in primary rectal cancer. Abdom Imaging. 2008;33:604-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Maizlin ZV, Brown JA, So G, Brown C, Phang TP, Walker ML, Kirby JM, Vora P, Tiwari P. Can CT replace MRI in preoperative assessment of the circumferential resection margin in rectal cancer? Dis Colon Rectum. 2010;53:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Hodgman CG, MacCarty RL, Wolff BG, May GR, Berquist TH, Sheedy PF, Beart RW, Spencer RJ. Preoperative staging of rectal carcinoma by computed tomography and 0.15T magnetic resonance imaging. Preliminary report. Dis Colon Rectum. 1986;29:446-450. [PubMed] |
| 42. | Butch RJ, Stark DD, Wittenberg J, Tepper JE, Saini S, Simeone JF, Mueller PR, Ferrucci JT. Staging rectal cancer by MR and CT. AJR Am J Roentgenol. 1986;146:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Gualdi GF, Casciani E, Guadalaxara A, d’Orta C, Polettini E, Pappalardo G. Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum. 2000;43:338-345. [PubMed] |
| 45. | Iafrate F, Laghi A, Paolantonio P, Rengo M, Mercantini P, Ferri M, Ziparo V, Passariello R. Preoperative staging of rectal cancer with MR Imaging: correlation with surgical and histopathologic findings. Radiographics. 2006;26:701-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Matsuoka H, Nakamura A, Masaki T, Sugiyama M, Takahara T, Hachiya J, Atomi Y. Comparison between endorectal coil and pelvic phased-array coil magnetic resonance imaging in patients with anorectal tumor. Am J Surg. 2003;185:328-332. [PubMed] |
| 47. | Winter L, Bruhn H, Langrehr J, Neuhaus P, Felix R, Hänninen LE. Magnetic resonance imaging in suspected rectal cancer: determining tumor localization, stage, and sphincter-saving resectability at 3-Tesla-sustained high resolution. Acta Radiol. 2007;48:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Fütterer JJ, Yakar D, Strijk SP, Barentsz JO. Preoperative 3T MR imaging of rectal cancer: local staging accuracy using a two-dimensional and three-dimensional T2-weighted turbo spin echo sequence. Eur J Radiol. 2008;65:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Sani F, Foresti M, Parmiggiani A, D’Andrea V, Manenti A, Amorotti C, Scotti R, Gallo E, Torricelli P. 3-T MRI with phased-array surface coil in the local staging of rectal cancer. Radiol Med. 2011;116:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Blomqvist L, Holm T, Rubio C, Hindmarsh T. Rectal tumours--MR imaging with endorectal and/or phased-array coils, and histopathological staging on giant sections. A comparative study. Acta Radiol. 1997;38:437-444. [PubMed] |
| 51. | Gagliardi G, Bayar S, Smith R, Salem RR. Preoperative staging of rectal cancer using magnetic resonance imaging with external phase-arrayed coils. Arch Surg. 2002;137:447-451. [PubMed] |
| 52. | Poon FW, McDonald A, Anderson JH, Duthie F, Rodger C, McCurrach G, McKee RF, Horgan PG, Foulis AK, Chong D. Accuracy of thin section magnetic resonance using phased-array pelvic coil in predicting the T-staging of rectal cancer. Eur J Radiol. 2005;53:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Dewhurst CE, Mortele KJ. Magnetic resonance imaging of rectal cancer. Radiol Clin North Am. 2013;51:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Vliegen RF, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, Beets-Tan RG. Rectal cancer: MR imaging in local staging--is gadolinium-based contrast material helpful? Radiology. 2005;234:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Jao SY, Yang BY, Weng HH, Yeh CH, Lee LW. Evaluation of gadolinium-enhanced T1-weighted magnetic resonance imaging in the preoperative assessment of local staging in rectal cancer. Colorectal Dis. 2010;12:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, Bourne MW, Williams GT. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215-222. [PubMed] |
| 57. | MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 58. | Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, von Meyenfeldt MF, Baeten CG, van Engelshoven JM. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497-504. [PubMed] |
| 59. | Gowdra Halappa V, Corona Villalobos CP, Bonekamp S, Gearhart SL, Efron J, Herman J, Kamel IR. Rectal imaging: part 1, High-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol. 2012;199:W35-W42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Dworák O. Number and size of lymph nodes and node metastases in rectal carcinomas. Surg Endosc. 1989;3:96-99. [PubMed] |
| 61. | Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 607] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 62. | Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 63. | MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 659] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 64. | Purkayastha S, Tekkis PP, Athanasiou T, Tilney HS, Darzi AW, Heriot AG. Diagnostic precision of magnetic resonance imaging for preoperative prediction of the circumferential margin involvement in patients with rectal cancer. Colorectal Dis. 2007;9:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Falk PM, Gupta NC, Thorson AG, Frick MP, Boman BM, Christensen MA, Blatchford GJ. Positron emission tomography for preoperative staging of colorectal carcinoma. Dis Colon Rectum. 1994;37:153-156. [PubMed] |
| 66. | Calvo FA, Domper M, Matute R, Martínez-Lázaro R, Arranz JA, Desco M, Alvarez E, Carreras JL. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58:528-535. [PubMed] |
| 67. | Shin SS, Jeong YY, Min JJ, Kim HR, Chung TW, Kang HK. Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT. Abdom Imaging. 2008;33:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Heriot AG, Hicks RJ, Drummond EG, Keck J, Mackay J, Chen F, Kalff V. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum. 2004;47:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 71. | Napoleon B, Pujol B, Berger F, Valette PJ, Gerard JP, Souquet JC. Accuracy of endosonography in the staging of rectal cancer treated by radiotherapy. Br J Surg. 1991;78:785-788. [PubMed] |
| 72. | Rau B, Hünerbein M, Barth C, Wust P, Haensch W, Riess H, Felix R, Schlag PM. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13:980-984. [PubMed] |
| 73. | Lee CT, Chow NH, Liu YS, Lin SC, Lin PC, Wu YH, Lee JC, Tsai HM. Computed tomography with histological correlation for evaluating tumor regression of rectal carcinoma after preoperative chemoradiation therapy. Hepatogastroenterology. 2012;59:2484-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, Mueller PR, Lee TY. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005;234:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 75. | Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 76. | Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol. 2007;188:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, Rizzo G, Coco C, Crucitti A, Ratto C. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 78. | Koh DM, Chau I, Tait D, Wotherspoon A, Cunningham D, Brown G. Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2008;71:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 80. | Kim DJ, Kim JH, Lim JS, Yu JS, Chung JJ, Kim MJ, Kim KW. Restaging of Rectal Cancer with MR Imaging after Concurrent Chemotherapy and Radiation Therapy. Radiographics. 2010;30:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM, Chung YL, Fang WT. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48:23-28. [PubMed] |
| 82. | Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | Vliegen RF, Beets GL, Lammering G, Dresen RC, Rutten HJ, Kessels AG, Oei TK, de Bruïne AP, van Engelshoven JM, Beets-Tan RG. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology. 2008;246:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 84. | Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1418] [Cited by in RCA: 1462] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 85. | Heo SH, Shin SS, Kim JW, Lim HS, Jeong YY, Kang WD, Kim SM, Kang HK. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2013;14:616-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32:2-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 87. | Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, Kim HR, Kang HK. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging. 2012;35:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 88. | Song I, Kim SH, Lee SJ, Choi JY, Kim MJ, Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol. 2012;85:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18:2224-2231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 90. | Sassen S, de Booij M, Sosef M, Berendsen R, Lammering G, Clarijs R, Bakker M, Beets-Tan R, Warmerdam F, Vliegen R. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol. 2013;23:3440-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Harry VN, Semple SI, Parkin DE, Gilbert FJ. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010;11:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 92. | Kim JW, Kim HC, Park JW, Park SC, Sohn DK, Choi HS, Kim DY, Chang HJ, Baek JY, Kim SY. Predictive value of (18)FDG PET-CT for tumour response in patients with locally advanced rectal cancer treated by preoperative chemoradiotherapy. Int J Colorectal Dis. 2013;28:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Guillem JG, Ruby JA, Leibold T, Akhurst TJ, Yeung HW, Gollub MJ, Ginsberg MS, Shia J, Suriawinata AA, Riedel ER. Neither FDG-PET Nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg. 2013;258:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |