Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4141
Revised: January 17, 2014
Accepted: March 12, 2014
Published online: April 21, 2014
Processing time: 169 Days and 22.6 Hours
Hepatocellular carcinoma (HCC) is a major health problem with a high incidence and mortality all over the world. Natural history of HCC is severe and extremely variable, and prognostic factors influencing outcomes are incompletely defined. Over time, many staging and scoring systems have been proposed for the classification and prognosis of patients with HCC. Currently, the non-ideal predictive performance of existing prognostic systems is secondary to their inherent limitations, as well as to a non-universal reproducibility and transportability of the results in different populations. New serological and histological markers are still under evaluation with promising results, but they require further evaluation and external validation. The aim of this review is to highlight the main tools for assessing the prognosis of HCC and the main concerns, pitfalls and warnings regarding its staging systems currently in use.
Core tip: Hepatocellular carcinoma is a major health problem, with a heterogeneous natural history that makes it difficult to identify accurate prognostic factors. The aim of this review is to highlight the main tools for assessing the prognosis of HCC and the main concerns, pitfalls and warnings regarding its staging systems currently in use.
- Citation: Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: A review of literature. World J Gastroenterol 2014; 20(15): 4141-4150
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4141
Hepatocellular carcinoma (HCC) has an increasing incidence worldwide, and it is the leading cause of death in patients with cirrhosis[1]. It is the fifth most common cancer and the third most common cause of cancer-related death[2].
Despite intensive surveillance programs, considerable recent therapeutic advances and use of potentially radical treatments, prognosis and life expectancy remain poor in this setting[3]. Curative treatments are applicable for early stage tumors only, and include resection, liver transplantation and percutaneous ablation, while transarterial chemoembolization (TACE) and sorafenib are regarded as non-curative treatments able to improve survival in intermediate and advanced stages, respectively[4].
According to the definition by Sackett et al[5], natural history is the course of a disease from its biological onset to its recovery or permanent disability or death.
The natural history of HCC is extremely severe, as confirmed by mortality rates overlapping the incidence of the tumor[6]. In addition, it is extremely heterogeneous, due to the complex interplay between the biological characteristics of the tumor and the frequent presence of an underlying chronic liver disease, as showed by a recent meta-analysis which analyzed the survival rates of the placebo and untreated arms of several randomized controlled trials (RCTs) on HCC patients[7].
According to this study, the survival rates were 17.5% at 1 year and 7.3% at 2 years, with a significant heterogeneity among all studies (P < 0.0001) both for 1-year and 2-year survival. By meta-regression analysis, impaired performance status (PS), B and C Child-Pugh classes, and presence of portal vein thrombosis (PVT) were independently associated with a poor survival.
The natural history of early HCC can not be evaluated by RCTs for ethical reasons, although a milestone paper published in 1989 showed that overall survival (OS) of asymptomatic patients with HCC and cirrhosis was 96% and 50% at 1- and 2-year, respectively[8].
A recent study analyzed a cohort of 320 patients affected by HCC and not suitable for curative or palliative treatments, confirming the heterogeneous behaviour of untreated HCC[9]. The overall median survival was 6.8 mo, and the 1-year survival was 32%. The 1-year survival according to barcelona clinic liver cancer (BCLC) classes was 100%, 79%, 12% and 0%, for BCLC A, B, C and D, respectively, with a significant difference in survival among each BCLC class.
Staging systems assess and describe the extent of tumor burden in the originally primary organ and its spread throughout the body.
They have a key role in the management of all cancers, allowing an accurate prognostic stratification of the tumor and the choice of the best therapeutic approach according to the stage. Furthermore, they are useful for grouping patients homogeneously in clinical trials and scientific research, and to make comparable patients of different clinical studies.
An ideal staging system should be simple, easy and quick to be determined as soon as possible after diagnosis; it should provide information on prognosis and guide therapeutic decisions. In contrast to classical staging systems, which consider only the inherent characteristics of the tumor, prognostic scores include also all the variables that influence the patient’s prognosis, over the tumor extension. Each staging system and prognostic score must be reproducible and externally validated, in order to be recommended and used on a large scale. Internal validation is an estimate of the internal reproducibility and answers the question asking if “The score can be properly applied to the patient population from which it was derived”.
Using survival time as an outcome measure, important criteria for assessing the internal validation of a prognostic system are homogeneity, discrimination and monotonicity[10]. Homogeneity is the characteristic whereby the difference in survival time is small among patients classified into the same staging group, while discriminatory ability is the feature by which there are much greater differences in the survival times among patients classified into different groups. Finally, monotonicity is defined as the property of a staging system by which the mean survival time for a group classified as favourable by that system is always longer than the mean survival times experienced in less favourable groups (monotonicity of gradients). Besides, external validation is an estimate of transportability of the results, and answers the question asking if “It is possible to apply the results of a prognostic study to any single patient”. It is assessed by validation studies that are performed on populations other than, but related to the one from which the prognostic score was originally derived.
Generally, the extension of the tumour burden in the original primary organ and its spread throughout the body, is per se exhaustive for the staging of most solid tumors. Nevertheless, unlike other tumors, HCC usually occurs on a background of a liver disease, making the level of management complexity unique among all malignancies.
It is well known that the functional impairment of the underlying liver disease has a significant impact on prognosis, irrespective of the tumour stage[4]. For this reason, systems that include the anatomical characteristics of the tumor only, such as the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) staging system that stratifies patients using a Tumor-Node-Metastasis (TNM) classification, do not have per se a good predictive capability[11,12] (Table 1). Thus the TNM, along with all other systems that enclose it, represents a group of models useful for assessment of tumor extension only, but inadequate as prediction models.
| Okuda staging system[13] | |||||||
| Score | |||||||
| 0 | 1 | ||||||
| Tumor size | ≤ 50% of the liver | > 50% of the liver | |||||
| Albumin (g/dL) | ≥ 3 | < 3 | |||||
| Bilirubin (mg/dL) | < 3 | ≥ 3 | |||||
| Ascites | Absent | Present | |||||
| CLIP score[14] | |||||||
| Score | |||||||
| 0 | 1 | 2 | |||||
| Tumour morphology | Uninodular and extension ≤ 50% | Multinodular and extension ≤ 50% | Massive or extension > 50% | ||||
| Child–Pugh score | A | B | C | ||||
| Alpha-fetoprotein (ng ⁄ mL) | < 400 | ≥ 400 | - | ||||
| Portal vein thrombosis | Absent | Present | - | ||||
| GRETCH score[21] | |||||||
| Score | |||||||
| 0 | 1 | 2 | 3 | ||||
| Karnofsky index | ≥ 80% | < 80% | |||||
| Bilirubin (μmol ⁄ L) | < 50 | ≥ 50 | |||||
| Alkaline phosphatase | < 2 X ULN | ≥ 2 X ULN | |||||
| Alpha-fetoprotein (μg⁄L) | < 35 | ≥ 35 | |||||
| Portal vein thrombosis | Absent | Present | |||||
| BCLC[16] | |||||||
| Stage | |||||||
| 0 (very early) | A (early) | B (intermediate) | C (advanced) | D (end stage) | |||
| ECOG Performance Status | 0 | 0 | 0 | 1-2 | 3-4 | ||
| Liver function | Child-Pugh A-B | Child-Pugh A-B | Child-Pugh A-B | Child-Pugh A-B | Child-Pugh C | ||
| Tumor stage | Single | Single or 3 nodules < 3 cm | Multinodular | Vascular invasion or extrahepatic spread | Any | ||
| CUPI[23] | |||||||
| VARIABLE | Weight | ||||||
| TNM stage | I and II | -3 | |||||
| III | -1 | ||||||
| IV | 0 | ||||||
| Total Bilirubin (μmol ⁄ L) | < 34 | 0 | |||||
| 34-51 | 3 | ||||||
| ≥ 52 | 4 | ||||||
| Ascites | 3 | ||||||
| Alpha-fetoprotein > 500 ng/mL | 2 | ||||||
| Alkaline phosphatase > 200 IU/L | 3 | ||||||
| Asymptomatic disease on presentation | -4 | ||||||
| JIS[24] | |||||||
| Score | |||||||
| 0 | 1 | 2 | 3 | ||||
| Child-Pugh score | A | B | C | ||||
| TNM stage by LCSGJ | I | II | III | IV | |||
| Tokyo[22] | |||||||
| Score | |||||||
| 0 | 1 | 2 | |||||
| Albumin (g/dL) | > 3.5 | 2.8-3.5 | < 2.8 | ||||
| Bilirubin (mg/dL) | < 1 | 1-2 | > 2 | ||||
| Tumor size (cm) | < 2 | 2-5 | > 5 | ||||
| Numbers of nodules | ≤ 3 | - | > 3 | ||||
| AJCC/UICC TNM staging system 7th ed[12] | |||||||
| Group | Description | Stage grouping | |||||
| T1 | Single tumor without vascular invasion | STAGE I | T1 | N0 | M0 | ||
| T2 | Single tumor with vascular invasion or multiple tumors, none > 5 cm | STAGE II | T2 | N0 | M0 | ||
| T3a | Multiple tumors, any > 5 cm | STAGE IIIA | T3a | N0 | M0 | ||
| T3b | Single tumor or multiple tumors of any size involving a major branch of portal or hepatic vein(s) | STAGE IIIB | T3b | N0 | M0 | ||
| T4 | Tumors with direct invasion of adjacent organs other than the gallbladder, or perforation of visceral peritoneum | STAGE IIIC | T4 | N0 | M0 | ||
| N1 | Regional lymph node metastasis | STAGE IVA | Any T | N1 | M0 | ||
| M1 | Distant metastasis | STAGE IVB | Any T | Any N | M1 | ||
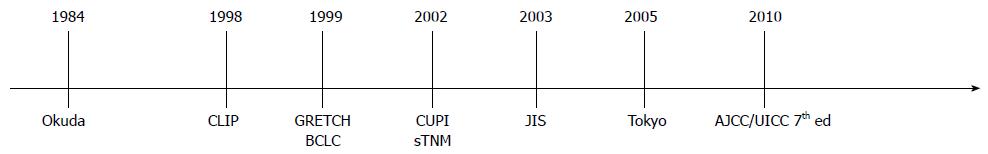
Therefore, many staging and scoring systems for the classification and prognosis of patients with HCC have been proposed over time (Figure 1).
The staging system proposed by Okuda et al[13] in 1984 (Table 1) is the first attempt to successfully combine the anatomical features of the tumor to the degree of the underlining liver disease. A distinction is made by three stages, considering the volume of the tumor (occupancy extended to ≤ or > 50% of the liver) together with the main indices of liver function (albumin, bilirubin, presence of ascites). This system has been widely adopted and used throughout the world for over two decades. However, the development of more advanced diagnostic techniques over the years has permitted an increasing detection of small tumors, with an occupancy of the entire liver well below 50%, which are poorly discriminable within the two dimensional groups proposed by Okuda. In addition other prognostic variables not included in that model were identified, leading to the development of more accurate staging systems (Table 2).
| Variables | Prognostic scores | ||||||
| Okuda[13] | CLIP[14] | GRETCH[21] | BCLC[16] | CUPI[23] | JIS[24] | Tokyo[22] | |
| Child-Pugh score | X | X | X | ||||
| Ascites | X | X | |||||
| Albumin | X | X | |||||
| Total Bilirubin | X | X | X | X | |||
| Alkaline phosphatase | X | X | |||||
| Alpha-fetoprotein | X | X | X | ||||
| Tumor size | X | X | X | X | |||
| Numbers of nodules | X | X | X | ||||
| TNM stage | X | X | |||||
| Portal vein thrombosis | X | X | X | ||||
| Metastasis | X | ||||||
| Portal hypertension | X | ||||||
| Presence of symptoms and/or General Status | X | X | X | ||||
Cancer of the Liver Italian Program (CLIP) is a simple scoring system designed by an Italian group with the aim of overcoming the main limitations of the TNM and Okuda.
It has been derived from a retrospective cohort study of 435 patients[14] and then externally validated comparing its discriminatory ability and predictive power with the one of the Okuda staging system by a randomized trial that prospectively enrolled 196 patients with cirrhosis and HCC[15]. The CLIP includes the liver function according to Child-Pugh score, the morphology of the tumor (uninodular, multinodular, massive), its extension in the liver, the levels of Alpha-fetoprotein and the eventual presence of PVT. The combination of the different variables places all patients into 6 categories (Table 1). Although it was built with a correct methodology and externally validated, this score presents some limits, including the absence of general well-being assessment of the patient, and the inability to identify the early stages, which are susceptible to percutaneous or surgical therapies.
The BCLC staging classification for HCC is currently the only staging system that includes an integrated assessment of liver disease, tumor extension, and presence of constitutional symptoms, providing in the meantime an indication of the first-line treatment. It classify stages of disease into five subgroups, from 0 to D, each associated with a specific therapy and prognosis (Table 1)[16]. Stage 0 is defined as very early stage disease, and is featured by a single nodule ≤ 2 cm without tumor invasion into surrounding tissues, in asymptomatic patients with preserved liver function. Stage A, or early disease, is classified as a solitary HCC of any size, or in maximum 3 nodules, each of them ≤ 3 cm, in asymptomatic patients with Child-Pugh A or B. Stage 0 and A can be effectively treated with curative therapies, such as surgical resection, liver transplantation, or by percutaneous ablation methods, including percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA). These treatments allow to reach a complete response[17], with potential long-term curative effect and a 5-year survival better than 40%-70%. It must be emphasized that tumor size (< 5 cm) has been recently removed as a contraindication for radical therapy in single nodule HCC. However, in this particular case, the choice of the surgeon is finally decisive in defining a tumor as resectable. Stage B, or intermediate disease, consists of multinodular tumor, without macrovascular invasion or extrahepatic spread (ES), in asymptomatic patients with well-preserved liver function, and PS lower than 2. This subset of patients may be treated with TACE, which has proven a significant increase in survival compared with best supportive care (median survival, 20 mo vs 16 mo)[18].
Patients with mild related symptoms and/or macrovascular invasion or ES are classified as stage C (advanced stage). According to two pivotal RCTs[19,20], the standard of care in this group is Sorafenib, an inhibitor of Raf kinase and vascular endothelial growth factor receptor. Patients with cancer symptoms related to advanced liver failure, tumor growth with vascular involvement, ES, or physical impairment (PS > 2), are classified as stage D (end stage disease). They can not benefit from any specific cancer therapy and could only receive the best available supportive care. Although it is not an ideal staging system and has several gaps, the BCLC has been endorsed by EASL and AASLD as the standard staging system for patients with HCC, and it is currently the most used in Western countries.
The GRETCH (Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire) score was derived by a prospective cohort of 761 patients with hepatocellular carcinoma from 24 medical centers in France, Belgium and Canada enrolled during a period of 30 mo (Table 1)[21]. Five prognostic factors were selected: Karnofsky index, serum bilirubin, serum alkaline phosphatase, serum alpha-fetoprotein > 35 pg/L, and ultrasonographic evidence of portal obstruction. Three risk groups with different 1-year survival rates were derived, and then independently validated in the test sample. However, it is not a well validated or a widely used staging system.
The lower survival of Asian patients with HCC seems to be due not only to the different ethnic origin, but also to a different etiologic distribution and to a different and more severe natural history of liver disease. On this background, many prognostic scores have been built on Asian cohorts. The Tokyo score[22] was developed from a cohort of 403 consecutive Japanese patients with HCC treated by percutaneous ablation, and it is composed by four factors including serum albumin, bilirubin, size and number of tumors (Table 1). In the testing sample, the predictive power of this score resulted equal to CLIP and better than BCLC staging. Unfortunately, the score has been derived on a cohort of patients with early HCC, therefore its predictive ability can be transferred only to patients susceptible to radical therapies, whereas it poorly fits to patients with advanced HCC. The CUPI (Chinese University Prognostic Index for hepatocellular carcinoma)[23] was designed in 2002 by the analysis of a cohort of 926 Chinese patients with HCC, adding five prognostic factors (total bilirubin, presence of ascites, alkaline phosphatase, alpha fetoprotein, and asymptomatic disease on presentation) to the TNM, in order to set up 3 classes of risk with highly significant differences in survival (Table 1). This score was obtained from a mono-centric and mono-ethnic cohort of patients, and most of the patients had a liver disease secondary to HBV infection. Therefore, the transportability of data of this score could be limited to this specific subset of patients. Finally, the JIS (Japan Integrated Staging Score)[24] is a score system that combines two existing classifications, named TNM and Child-Pugh (Table 1). It is widely used in Japan but it lacks external validation in Western countries.
To date, a growing attention is focused on the lookout for new prognostic markers able to increase the power of predictor models for HCC.
Several years ago, the role of Estrogen Receptor (ER), defined as wild-type (wtER) or variant (vER)[25], has been described for the biologic characterization of the tumor. A study by Villa et al[26] showed that the presence of wtER is directly related with a good prognosis, and the survival is five-fold better in patients with HCC presenting with wtER compared with the ones presenting with vER. Similarly to what happens in breast cancer, the presence of variant forms of ER seems to correlate with lack of hormonal control on the tumor growth, elevated proliferation rate and tumor aggressiveness. Thus, although ER characterization requires an invasive procedure - liver biopsy - it can be useful for an accurate prognosis and as a reliable assessment of sensitivity to treatment also for clinical decision making. More recently, some studies have focused on the impact of new serological markers in predicting prognosis of patients with HCC. For example, low serum vascular endothelial growth factor (VEGF) levels seems to be associated with a longer survival at each stage according to CLIP or BCLC, and the inclusion of baseline plasma VEGF levels increases the precision of the CLIP scoring system for predicting HCC prognosis (“V-CLIP” staging)[27,28]. Likewise, high Insulin-like growth factor-1 (IGF-1) plasma levels seems to reflect time-to-recurrence, as well as overall survival[29], and the addition of plasma IGF-1 levels to CLIP (“I-CLIP” staging) significantly improves prognostic stratification of patients with advanced HCC[30]. Finally, overexpression of the Forkhead box M1 (FOXM1) gene is associated with a poor outcome after OLT[31], and expression of the AKR1B10 (aldo-keto reductase enzyme) gene reflects a less aggressive tumour behaviour[32]. All these new markers have shown promising results, but require further evaluation and external validation.
To date several staging systems for HCC have been proposed, but currently none of these has been universally accepted, as pointed out by AASLD guidelines, which emphasize how there is not a worldwide consensus on the use of any given model for stadiation of HCC[33].
Several studies comparing the predictive power of different models have shown conflicting results, both in the general population and in the different subgroups of treatment (Table 3).
| Ref. | Country | Year | Case number | Patient population | The best |
| Levy et al[58] | Canada | 2002 | 257 | All | CLIP |
| Kudo et al[59] | Japan | 2004 | 4525 | All | JIS |
| Cillo et al[41] | Italy | 2004 | 187 | All | BCLC |
| Grieco et al[39] | Italy | 2005 | 268 | Early to intermediate | BCLC |
| Marrero et al[34] | United States | 2005 | 244 | All | BCLC |
| Nanashima et al[60] | Japan | 2005 | 210 | Surgery | CLIP |
| Huang et al[61] | Taiwan | 2005 | 599 | Surgery | TNM |
| Toyoda et al[62] | Japan | 2005 | 1508 | All | JIS |
| Pascual et al[63] | Spain | 2006 | 115 | All | BCLC |
| Georgiades et al[42] | United States | 2006 | 172 | TACE | Child-Pugh |
| Cillo et al[64] | Italy | 2006 | 195 | All | BCLC |
| Nanashima et al[65] | Japan | 2006 | 230 | Surgery | Modified JIS |
| Kondo et al[66] | Japan | 2007 | 235 | Surgery | JIS |
| Seong et al[67] | South Korea | 2007 | 305 | Radiotherapy | TNM |
| Chen et al[40] | Taiwan | 2007 | 382 | Surgery | CLIP |
| Huo et al[68] | Taiwan | 2007 | 430 | All | CLIP |
| Cammà et al[37] | Italy | 2008 | 406 | All | CLIP |
| Guglielmi et al[69] | Italy | 2008 | 112 | RFA | BCLC |
| Collette et al[70] | French | 2008 | 538 | Advanced | CLIP |
| Lu et al[71] | China | 2008 | 234 | Surgery | TNM |
| Chung et al[72] | Japan | 2008 | 290 | All | JIS |
| Lin et al[38] | Taiwan | 2009 | 3668 | All | CLIP |
| Hsu et al[36] | Taiwan | 2010 | 1713 | All | CLIP |
| Op den Winkel et al[73] | German | 2012 | 405 | Non-surgical | CLIP |
| Kim et al[35] | South Korea | 2012 | 1717 | All | BCLC |
According to the analysis performed by Marrero et al[34] on a cohort of 244 United States patients of any stage, the BCLC showed the best independent predictive power for survival when compared with the other 6 prognostic systems (TNM, CLIP, CUPI, JIS, GRETCH and Okuda). Similar results were found by an Asian study performed on 1717 treatment-naïve HCC patients, showing as BCLC was the best prognostic model if compared with other 5 systems (CLIP, CUPI, JIS, GRETCH and Tokyo score)[35]. Conversely, a study by Hsu et al[36] investigating the prognostic ability of the 5 staging systems (BCLC, CLIP, JIS, TNM and Tokyo score), showed the CLIP was the best long-term prognostic model in a cohort of patient with early to advanced stage HCC. Similarly, a recent study comparing the performance of BCLC, CLIP and GRETCH in a cohort of 406 consecutive patients with cirrhosis and HCC[37], showed the CLIP had the best discriminative capacity in the entire HCC cohort and in the advanced untreatable cases, while BCLC proved to be the best in predicting survival in treated patients. Finally, a subsequent study on a larger cohort of 3868 treated patients confirmed a modest discriminatory ability of CLIP for early HCC[38].
In addition, several studies have been also performed over time in order to weigh the performance of staging and prognostic systems in specific subsets of patients receiving different class of treatments (Table 3).
An Italian retrospective study compared the performance of Okuda, CLIP and BCLC in a cohort of 268 patients treated with non-surgical therapy[39]. Both CLIP and BCLC scores were more effective than the Okuda score in stratifying patients into different risk groups of patients with early-intermediate HCC, even if BCLC showed a better prediction of prognosis in patients with very early stage HCC. Furthermore, a subsequent study compared Okuda, TNM, CLIP, BCLC, CUPI, JIS and MELD in the prediction of survival among patients with HCC treated with major or minor hepatectomy[40]. Among all the seven staging systems, CLIP and JIS showed the best results. In particular, CLIP had a better discriminatory ability in the subset of patients treated with major hepatectomy, while JIS proved to be the most accurate in the minor hepatectomy group.
Conversely, another retrospective analysis of 187 HCC Italian patients mainly treated with radical therapies (resection and percutaneous ablation) showed that BCLC had the greatest prognostic power among five systems (BCLC, CLIP, GRETCH, CUPI and Okuda) both for the whole study group and for the 2 subgroups of surgical and non-surgical patients[41].
These results do not seem to be confirmed in the group of patients treated with “non-curative” therapies. In this regard, the prognostic accuracy of 12 liver staging systems (nominal and categoric Child-Pugh, Okuda, CLIP, BCLC, MELD, CUPI, JIS, TNM, GRETCH, Liver Cancer Study Group of Japan, and Tokyo score) has been assessed in a cohort of 172 consecutive patients with unresectable HCC treated with TACE[42]. According to the results of this study, nominal Child-Pugh, CUPI, and Tokyo score provided the best prognostic accuracy, and the nominal Child-Pugh was the most accurate among them in predicting survival of patients with unresectable HCC treated with TACE.
As already mentioned, currently there is not an ideal staging and prognostic system for HCC. Anyway, the BCLC seems to be the most comprehensive, since it integrates information about tumor extension, liver function and the presence of constitutional symptoms. It also provides prognostic information and guidance to the therapeutic choices, and it has been endorsed by EASL and AASLD as standard for patients with HCC.
Since survival outcomes can be inevitably confounded by treatment strategies that may be quite different from one center to another[43], It must be noted that the external validation which uses the natural history of untreated HCC cohorts might be the most useful way to compare the prognostic value of each staging system[44,45]. In this regard, the previously mentioned meta-analysis by Cabibbo et al[7] which analyzed the survival rates of the untreated and placebo arms of several RCTs on HCC patients, confirmed that many of the prognostic variables of the BCLC (PS, Child-Pugh B-C class, and presence of PVT) are also robust predictors of death in untreated patients. This provides further evidence that the BCLC has a good discriminative capacity as prognostic score, regardless of the treatment strategy applied.
Anyway, it does not represent a perfect model and still it has several unmet points. First, unlike CLIP[14], GRETCH[21] and CUPI[23], the BCLC was not derived from a cohort of HCC patients by a multivariate analysis, and therefore it is not a prognostic model able to predict the mortality of HCC patients, being internally and externally validated just as a staging system. Second, acting as classification model, it presents itself some inherent drawbacks. For example, the intermediate stage (BCLC B) includes an extremely heterogeneous population in terms of both liver function and tumor characteristics. In addition, according to the BCLC, any patient with a PS equal to 1 automatically falls in the advanced stage (BCLC C), even if this condition identifies a “subject capable of performing all the normal daily activities” according the original ECOG (Eastern Cooperative Oncology Group) definition.
In addition, acting as treatment algorithm, the main limitation of the BCLC is represented by its rigidity. First, some prognostic factors, such as the presence of clinically significant portal hypertension, are outlined as contraindications that preclude a therapy, whereas evidences suggest that hepatic resection can be performed successfully, in highly selected cases, even in patients with portal hypertension and multiple hepatic lesions[46,47]. Second, it should be noted that not all patients defined by each stage of BCLC are ultimately candidates for the suggested treatment modality. For instance, TACE can be performed at earlier stages in patients not eligible to RFA or PEI because of tumor location (proximity to the gallbladder, biliary tree, or blood vessel), or failure of previous curative treatments and/or presence of medical comorbidities. Moreover, BCLC algorithm does not provide indications concerning second-line therapies, re-treatment choices or combined treatments[48,49].
An important management problem is still represented by the indications for transplantation suggested by BCLC. For example, several lines of evidence show that transplant can get similar results in patients exceeding the Milan criteria, but conform to the “up-to-seven”[50] or the “San Francisco” criteria[51]. Furthermore, transplant is not indicated for end stage disease (BCLC D), which includes, among others, also patients with early tumor but with severe hepatic decompensation (Child-Pugh C). Despite the recommendations of the BCLC suggesting supportive care as the only available therapy, this subset of patients gets anyway the best benefit after transplantation[52,53]. As a result of its rigidity and unmet points, the BCLC is frequently difficult to apply, and its adherence in clinical practice is low[54]. Finally, to date, none of these staging systems have been analyzed or validated taking into account the prognosis of OLT, and therefore can not be recommended in the setting of liver transplantation[55].
Currently, the non-ideal predictive performance of existing prognostic systems is secondary to their inherent limitations, as well as to a non-universal reproducibility and transportability of the results in different populations. In addition, other key factors must be considered. First, most of prognostic models are derived by a multiple regression analysis using time-fixed Cox model in order to identify independent factors for mortality. It is already well known as this kind of models may be unreliable because of the potential interaction of time-varying predictors. In this context, compared with the time-fixed models, a time-dependent Cox model could have a better potential to estimate prognosis in HCC patients, as already demonstrated by a recent study[56]. Second, as already mentioned, the natural history of HCC is extremely heterogeneous. This is probably secondary to the existence of specific factors not accounted in the prognostic models that can have some impact on patient outcomes. In this regard, the evaluation of gene expression profiling may have an important role in the future to better understand the tumor biology and to improve the predictive power of the models.
In conclusion, due to a non-perfect homogeneity and discrimination (internal validity) and a not absolute transportability of prognostic models in different populations (external validity), currently they are still far away from getting a good confidence in predicting outcome in the individual patient[57]. For these reasons, prognostic models should be used with caution, and staging systems that include integrated therapeutic algorithms should be considered as a general guide only.
P- Reviewers: Takagi H, Troncoso MF, Yoon JH S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 3. | Cabibbo G, Craxì A. Hepatocellular cancer: optimal strategies for screening and surveillance. Dig Dis. 2009;27:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] |
| 5. | Sackett DL, Hayness RB, Guyatt G, Tugwell P. Clinical epidemiology: A basic science for clinical medicine. 2nd ed. London: Little, Brown and Company 1991; . |
| 6. | Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191-211, v. [PubMed] |
| 7. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [PubMed] |
| 8. | Cottone M, Virdone R, Fusco G, Orlando A, Turri M, Caltagirone M, Maringhini A, Sciarrino E, Demma I, Nicoli N. Asymptomatic hepatocellular carcinoma in Child’s A cirrhosis. A comparison of natural history and surgical treatment. Gastroenterology. 1989;96:1566-1571. [PubMed] |
| 9. | Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M, Brancatelli G, Cammà C, Craxì A, Di Marco V. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol. 2012;4:256-261. [PubMed] |
| 10. | Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529-534. [PubMed] |
| 11. | Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, editors . AJCC cancer staging manual. 6th ed. Chicago: Springer 2002; 435. |
| 12. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer 2010; . |
| 13. | Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4:3S-6S. [PubMed] |
| 14. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] |
| 15. | Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840-845. [PubMed] |
| 16. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] |
| 17. | Cabibbo G, Maida M, Genco C, Alessi N, Peralta M, Butera G, Galia M, Brancatelli G, Genova C, Raineri M. Survival of patients with hepatocellular carcinoma (HCC) treated by percutaneous radio-frequency ablation (RFA) is affected by complete radiological response. PLoS One. 2013;8:e70016. [PubMed] |
| 18. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] |
| 19. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] |
| 20. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] |
| 21. | Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133-141. [PubMed] |
| 22. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [PubMed] |
| 23. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [PubMed] |
| 24. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [PubMed] |
| 25. | Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A, Buttafoco P, Losi L, Manenti F. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995;55:498-500. [PubMed] |
| 26. | Villa E, Colantoni A, Cammà C, Grottola A, Buttafoco P, Gelmini R, Ferretti I, Manenti F. Estrogen receptor classification for hepatocellular carcinoma: comparison with clinical staging systems. J Clin Oncol. 2003;21:441-446. [PubMed] |
| 27. | Kaseb AO, Hassan MM, Lin E, Xiao L, Kumar V, Pathak P, Lozano R, Rashid A, Abbruzzese JL, Morris JS. V-CLIP: Integrating plasma vascular endothelial growth factor into a new scoring system to stratify patients with advanced hepatocellular carcinoma for clinical trials. Cancer. 2011;117:2478-2488. [PubMed] |
| 28. | Kaseb AO, Morris JS, Hassan MM, Siddiqui AM, Lin E, Xiao L, Abdalla EK, Vauthey JN, Aloia TA, Krishnan S. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892-3899. [PubMed] |
| 29. | Cho EJ, Lee JH, Yoo JJ, Choi WM, Lee MJ, Cho Y, Lee DH, Lee YB, Kwon JH, Yu SJ. Serum insulin-like growth factor-I level is an independent predictor of recurrence and survival in early hepatocellular carcinoma: a prospective cohort study. Clin Cancer Res. 2013;19:4218-4227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Kaseb AO, Abbruzzese JL, Vauthey JN, Aloia TA, Abdalla EK, Hassan MM, Lin E, Xiao L, El-Deeb AS, Rashid A. I-CLIP: improved stratification of advanced hepatocellular carcinoma patients by integrating plasma IGF-1 into CLIP score. Oncology. 2011;80:373-381. [PubMed] |
| 31. | Sun H, Teng M, Liu J, Jin D, Wu J, Yan D, Fan J, Qin X, Tang H, Peng Z. FOXM1 expression predicts the prognosis in hepatocellular carcinoma patients after orthotopic liver transplantation combined with the Milan criteria. Cancer Lett. 2011;306:214-222. [PubMed] |
| 32. | Schmitz KJ, Sotiropoulos GC, Baba HA, Schmid KW, Müller D, Paul A, Auer T, Gamerith G, Loeffler-Ragg J. AKR1B10 expression is associated with less aggressive hepatocellular carcinoma: a clinicopathological study of 168 cases. Liver Int. 2011;31:810-816. [PubMed] |
| 33. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] |
| 34. | Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707-716. [PubMed] |
| 35. | Kim BK, Kim SU, Park JY, Kim do Y, Ahn SH, Park MS, Kim EH, Seong J, Lee do Y, Han KH. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int. 2012;32:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Lee PC, Loong CC, Chiang JH, Huo TI, Lee SD. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62-75. [PubMed] |
| 38. | Lin CY, Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC. Is the Cancer of the Liver Italian Program system an adequate weighting for survival of hepatocellular carcinoma? Evaluation of intrascore prognostic value among 36 subgroups. Liver Int. 2009;29:74-81. [PubMed] |
| 39. | Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411-418. [PubMed] |
| 40. | Chen TW, Chu CM, Yu JC, Chen CJ, Chan DC, Liu YC, Hsieh CB. Comparison of clinical staging systems in predicting survival of hepatocellular carcinoma patients receiving major or minor hepatectomy. Eur J Surg Oncol. 2007;33:480-487. [PubMed] |
| 41. | Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, D’Amico F, Ciarleglio FA, Boccagni P, Brolese A. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124-131. [PubMed] |
| 42. | Georgiades CS, Liapi E, Frangakis C, Park JU, Kim HW, Hong K, Geschwind JF. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:1619-1624. [PubMed] |
| 43. | Huo TI, Huang YH. Staging for hepatocellular carcinoma: treatment strategy matters. Hepatology. 2005;41:678; author reply 678-679. [PubMed] |
| 45. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [PubMed] |
| 46. | Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G, Zanello M, Ravaioli M, Capussotti L, Pinna AD. Indication of the extent of hepatectomy for hepatocellular carcinoma on cirrhosis by a simple algorithm based on preoperative variables. Arch Surg. 2009;144:57-63; discussion 63. [PubMed] |
| 47. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [PubMed] |
| 48. | Cabibbo G, Latteri F, Antonucci M, Craxì A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2009;6:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Bolondi L, Cillo U, Colombo M, Craxì A, Farinati F, Giannini EG, Golfieri R, Levrero M, Pinna AD, Piscaglia F. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 50. | Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543-1554. [PubMed] |
| 51. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [PubMed] |
| 52. | Cillo U, Vitale A, Volk ML, Frigo AC, Grigoletto F, Brolese A, Zanus G, D’Amico F, Farinati F, Burra P. The survival benefit of liver transplantation in hepatocellular carcinoma patients. Dig Liver Dis. 2010;42:642-649. [PubMed] |
| 53. | Vitale A, Morales RR, Zanus G, Farinati F, Burra P, Angeli P, Frigo AC, Del Poggio P, Rapaccini G, Di Nolfo MA. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654-662. [PubMed] |
| 54. | Borzio M, Sacco R. Nonadherence to guidelines in the management of hepatocellular carcinoma: an Italian or universal phenomenon? Future Oncol. 2013;9:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 55. | Olthoff KM, Forner A, Hübscher S, Fung J. What is the best staging system for hepatocellular carcinoma in the setting of liver transplantation? Liver Transpl. 2011;17 Suppl 2:S26-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Cabibbo G, Genco C, Di Marco V, Barbara M, Enea M, Parisi P, Brancatelli G, Romano P, Craxì A, Cammà C. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment Pharmacol Ther. 2011;34:196-204. [PubMed] |
| 57. | Cammà C, Cabibbo G. Prognostic scores for hepatocellular carcinoma: none is the winner. Liver Int. 2009;29:478-480. [PubMed] |
| 58. | Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881-885. [PubMed] |
| 59. | Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396-1405. [PubMed] |
| 60. | Nanashima A, Omagari K, Tobinaga S, Shibata K, Sumida Y, Mine M, Morino S, Shibasaki S, Ide N, Shindou H. Comparative study of survival of patients with hepatocellular carcinoma predicted by different staging systems using multivariate analysis. Eur J Surg Oncol. 2005;31:882-890. [PubMed] |
| 61. | Huang YH, Chen CH, Chang TT, Chen SC, Wang SY, Lee HS, Lin PW, Huang GT, Sheu JC, Tsai HM. Evaluation of predictive value of CLIP, Okuda, TNM and JIS staging systems for hepatocellular carcinoma patients undergoing surgery. J Gastroenterol Hepatol. 2005;20:765-771. [PubMed] |
| 62. | Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Yamaguchi A, Isogai M, Kaneoka Y, Washizu J. Comparison of the usefulness of three staging systems for hepatocellular carcinoma (CLIP, BCLC, and JIS) in Japan. Am J Gastroenterol. 2005;100:1764-1771. [PubMed] |
| 63. | Pascual S, Zapater P, Such J, García-Herola A, Sempere L, Irurzun J, Palazón JM, Carnicer F, Pérez-Mateo M. Comparison of staging systems to predict survival in hepatocellular carcinoma. Liver Int. 2006;26:673-679. [PubMed] |
| 64. | Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723-731. [PubMed] |
| 65. | Nanashima A, Sumida Y, Abo T, Shindou H, Fukuoka H, Takeshita H, Hidaka S, Tanaka K, Sawai T, Yasutake T. Modified Japan Integrated Staging is currently the best available staging system for hepatocellular carcinoma patients who have undergone hepatectomy. J Gastroenterol. 2006;41:250-256. [PubMed] |
| 66. | Kondo K, Chijiiwa K, Nagano M, Hiyoshi M, Kai M, Maehara N, Ohuchida J, Nakao H, Ohkuwa Y. Comparison of seven prognostic staging systems in patients who undergo hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2007;54:1534-1538. [PubMed] |
| 67. | Seong J, Shim SJ, Lee IJ, Han KH, Chon CY, Ahn SH. Evaluation of the prognostic value of Okuda, Cancer of the Liver Italian Program, and Japan Integrated Staging systems for hepatocellular carcinoma patients undergoing radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1037-1042. [PubMed] |
| 68. | Huo TI, Lin HC, Hsia CY, Wu JC, Lee PC, Chi CW, Lee SD. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: a prospective sequential survey. Am J Gastroenterol. 2007;102:1920-1930. [PubMed] |
| 69. | Guglielmi A, Ruzzenente A, Pachera S, Valdegamberi A, Sandri M, D’Onofrio M, Iacono C. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol. 2008;103:597-604. [PubMed] |
| 70. | Collette S, Bonnetain F, Paoletti X, Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Bedenne L, Barbare JC. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Lu W, Dong J, Huang Z, Guo D, Liu Y, Shi S. Comparison of four current staging systems for Chinese patients with hepatocellular carcinoma undergoing curative resection: Okuda, CLIP, TNM and CUPI. J Gastroenterol Hepatol. 2008;23:1874-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, Minami Y, Ueshima K, Fukunaga T, Matsunaga T. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445-452. [PubMed] |
| 73. | op den Winkel M, Nagel D, Sappl J, op den Winkel P, Lamerz R, Zech CJ, Straub G, Nickel T, Rentsch M, Stieber P. Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging-systems in a large western HCC-cohort. PLoS One. 2012;7:e45066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |