Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4059
Revised: December 23, 2013
Accepted: January 3, 2014
Published online: April 14, 2014
Processing time: 197 Days and 13.4 Hours
AIM: To evaluate the long-term results of radiofrequency ablation (RFA) compared to left lateral sectionectomy (LLS) in patients with Child-Pugh class A disease for the treatment of single and small hepatocellular carcinoma (HCC) in the left lateral segments.
METHODS: We retrospectively reviewed the data of 133 patients with single HCC (≤ 3 cm) in their left lateral segments who underwent curative LLS (n = 66) or RFA (n = 67) between 2006 and 2010.
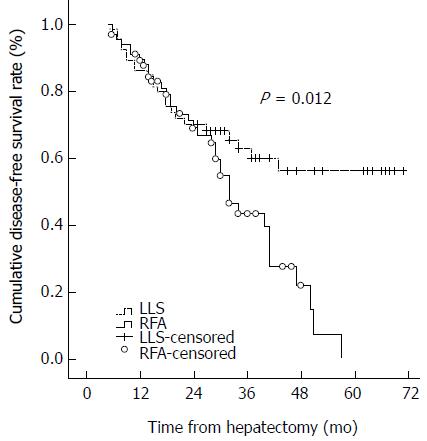
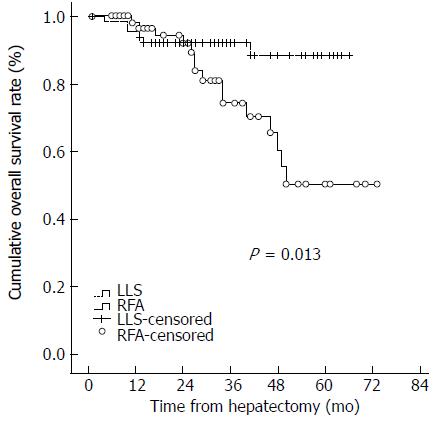
RESULTS: The median follow-up period was 33.5 mo in the LLS group and 29 mo in the RFA group (P = 0.060). Most patients had hepatitis B virus-related HCC. The hospital stay was longer in the LLS group than in the RFA group (8 d vs 2 d, P < 0.001). The 1-, 2-, and 3-year disease-free survival and overall survival rates were 80.0%, 68.2%, and 60.0%, and 95.4%, 92.3%, and 92.3%, respectively, for the LLS group; and 80.8%, 59.9%, and 39.6%, and 98.2%, 92.0%, and 74.4%, respectively, for the RFA group. The disease-free survival curve and overall survival curve were higher in the LLS group than in the RFA group (P = 0.012 and P = 0.013, respectively). Increased PIVKA-II levels and small tumor size were associated with HCC recurrence in multivariate analysis.
CONCLUSION: Liver resection is suitable for single HCC ≤ 3 cm in the left lateral segments.
Core tip: Many papers have reported the relative outcomes between liver resection and radiofrequency ablation, but here we selected patients with small and single hepatocellular carcinoma (HCC) in the left lateral segments. The present study showed that the disease-free survival curve and the overall survival curve were higher in the left lateral sectionectomy (LLS) group than in the radiofrequency ablation (RFA) group for those patients. However, the hospital stay was longer for the LLS group than for the RFA group. We conclude that liver resection is suitable for single HCC ≤ 3 cm in the left lateral segments.
- Citation: Kim JM, Kang TW, Kwon CHD, Joh JW, Ko JS, Park JB, Rhim H, Lee JH, Kim SJ, Paik SW. Single hepatocellular carcinoma ≤ 3 cm in left lateral segment: Liver resection or radiofrequency ablation? World J Gastroenterol 2014; 20(14): 4059-4065
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4059.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4059
Screening programs for patients with hepatitis B virus (HBV) have led to increasingly earlier diagnoses of hepatocellular carcinoma (HCC)[1]. Recent progress in imaging modalities has also facilitated increased diagnosis of small HCC in endemic areas, such as South Korea.
Following the Milan criteria (single HCC ≤ 5 cm or up to 3 nodules < 3 cm), the best treatment for HCC is liver transplantation, but this procedure is limited by the scarcity of donors[2]. Surgical resection is thus considered the first-choice treatment for patients with early stage HCC, and offers a 5-year-survival rate of over 50%[3]. Percutaneous ablation is usually reserved for patients who are not candidates for surgery owing to impaired liver function or co-morbidity, or for those who refuse surgery[4].
The American Association for the Study of Liver Diseases (AASLD) recommends percutaneous radiofrequency ablation (RFA) for three or fewer 3 cm or smaller early-stage HCCs, or 2 cm or smaller very-early-stage HCCs with complications such as portal hypertension[5]. Currently, RFA competes with liver resection and liver transplantation as the primary treatment for small HCC. RFA has attracted the greatest interest due to its advantages over liver resection, including less destruction of normal liver tissue, lower cost, no necessity for blood transfusion, lower complication rate, and shorter hospital stay[6,7]. However, there is still debate with regard to whether percutaneous RFA or liver resection is the most suitable therapy for small HCC or certain tumor locations. Several randomized controlled trials and many non-randomized controlled trials have been published in an attempt to address this question.
The purpose of this study was to retrospectively evaluate the long-term results of percutaneous RFA compared with left lateral sectionectomy (LLS) in patients with Child-Pugh class A liver cirrhosis for the treatment of single and small HCC in the left lateral segment.
We retrospectively reviewed the data of 133 patients with HCC in their left lateral segments (S2 and/or S3) who underwent curative LLS or percutaneous RFA at Samsung Medical Center between January 2006 and June 2010. All patients had a single tumor of 3 cm or less in diameter without extrahepatic metastasis detected during pre-treatment imaging such as 3-phase computed tomography (CT) and/or dynamic magnetic resonance imaging (MRI). Enrolled patients had Child-Pugh class A liver cirrhosis or non-cirrhotic livers and no previous history of surgical resection or locoregional therapy for HCC. The diagnosis of HCC was based on pathologic confirmation, elevated serum α-fetoprotein (AFP) (≥ 400 ng/mL) with radiologic findings, or at least two coincidental radiologic findings compatible with HCC in high-risk patients[8]. Patients younger than 18 years or with tumor size more than 3 cm, tumor in segments other than the left lateral segments (S2 or S3), other pathological or radiological malignancy in liver, or those lost to follow-up after hepatectomy or RFA were excluded from this study. The demographic and preoperative laboratory data of all patients were retrieved from electronic medical records (EMR) and were retrospectively reviewed. None of the patients in either group received postoperative adjuvant therapy before recurrence was detected.
Patients with small HCC in their left lateral segments were screened by planning ultrasonography to determine the feasibility of percutaneous RFA[9]. If the tumor was located at risk locations for RFA, such as superficially and adjacent to the hepatic vein, portal vein, and/or heart, liver resection was preferentially recommended. All RFA procedures were performed percutaneously under real-time ultrasound guidance with conscious sedation. Procedures were performed on an inpatient basis by one of six radiologists, each of whom had at least 7 years of experience performing this procedure by the end of the study period. We used either internally cooled, multi-tined expandable, or perfusion electrode systems according to temporal availability or operator preference. When we used internally cooled electrodes, we started at 50 W and continuously increased the power during the initial 2 min to minimize the popping phenomenon. All patients were treated with 2% lidocaine hydrochloride at the puncture site and intravenous drip infusion of 50 mg pethidine hydrochloride mixed with 50 mL of 5% dextrose water. Patient cardiovascular and respiratory systems were continuously monitored during the procedure. Our therapeutic strategy for RF ablation was to obtain at least 0.5 cm of the normal liver surrounding the tumor as a tumor-free margin insofar possible[10].
Before surgery, each patient underwent conventional liver function tests and indocyanine green retention rate measurements at 15 min (ICG-R15). Preoperative tests of liver function included serum bilirubin, transaminases, alkaline phosphatase, albumin, and prothrombin time. The levels of AFP and protein induced by vitamin K absence/antagonism-II (PIVKA-II) were also measured in all patients. Selection criteria for the liver resection procedure in the left lateral segments depended on bulging tumor in a superficial site and/or location close to vessel and heart. Child-Pugh class C, severe comorbidity, and distant metastasis were considered contraindications for hepatectomy. Standard operative techniques for hepatectomy were used[11].
Patients were followed every 2-3 mo postoperatively. Follow-up included physical examination, serum AFP, PIVKA-II, liver function tests, and chest X-ray. Helical dynamic triple phase CT was performed every 3 mo for the detection of local tumor progression, new intra-hepatic recurrence, and extrahepatic metastasis or when recurrence was suspected. MRI and/or positron emission tomography (PET) scan were performed when CT was not definitive. Diagnoses of HCC recurrence were based on CT and/or MRI. Needle biopsies of recurrent tumors were not performed.
Continuous variables were presented as median and range and were compared by the Mann-Whitney U test. Categorical variables were compared by Fisher’s exact test, as appropriate. Disease-free survival rates and overall survival rates were calculated by the Kaplan-Meier method. Differences between the curves were assessed using the log-rank test. Variables that showed statistical significance in univariate analyses were included in multivariate analyses using Cox proportional hazard models. A value of P < 0.05 was considered statistically significant. All data were analyzed using SPSS statistical software (Ver 21.0; SPSS Inc., Chicago, IL, United States).
A total of 133 patients with HCC ≤ 3 cm in their lateral segments (S2 and S3) were reviewed. Sixty-six patients were initially treated with surgical resection, such as LLS, while 67 patients were initially treated with percutaneous RFA. The baseline characteristics of all patients are outlined in Table 1. The median follow-up period was 33.5 mo (range, 1-66 mo) for LLS and 29 mo (range, 1-73 mo) for percutaneous RFA (P = 0.060). Most patients had HBV-related HCC, and the proportion of HCV-related HCC was higher in the percutaneous RFA group than in the LLS group (25.4% vs 6.2%). The age, serum AST levels, and ICG-R15 were higher in the RFA group than in the LLS group, but white blood cell counts, serum hemoglobin levels, platelet counts, serum albumin levels, PIVKA-II levels, and tumor size were higher in the LLS group. General liver function was better in the LLS than in the RFA group despite the Child-Pugh class A status of patients. The median hospitalization of the LLS group was 8 d (range, 3-68 d), as opposed to 2 d (range, 2-26 d) for the RFA group. The hospital stay was longer in the LLS group than in the RFA group (P < 0.001).
| Characteristics | LLS (n = 66) | RFA (n = 67) | P value |
| Gender-male | 48 (72.7) | 52 (77.6) | 0.514 |
| Age (yr) | 55 (27-76) | 59 (39-85) | 0.002 |
| BMI | 23.5 (17.8-33.4) | 23.6 (18.5-32.0) | 0.374 |
| Etiology | 0.014 | ||
| HBV | 51 (78.5) | 44 (65.7) | |
| HCV | 4 (6.2) | 17 (25.4) | |
| Alcoholic | 2 (3.1) | 2 (3.0) | |
| NBNC | 4 (6.2) | 4 (6.0) | |
| Others | 4 (6.2) | 0 (0) | |
| WBC (/μL) | 5345 (2600-8950) | 4000 (2000-11000) | 0.007 |
| Hemoglobin (g/dL) | 14.2 (10.8-17.7) | 14.0 (8.0-17.0) | 0.001 |
| Platelet (/μL) | 149500 (51000-276000) | 103000 (50000-257000) | 0.000 |
| INR | 1.1 (0.9-1.3) | 1.00 (1.0-2.0) | 0.000 |
| Albumin (g/dL) | 4.3 (3.5-4.9) | 4.0 (3.0-5.0) | 0.000 |
| Total bilirubin (mg/dL) | 0.7 (0.2-1.7) | 1.0 (0.2-2.0) | 0.538 |
| AST (IU/L) | 33 (16-95) | 38 (12-124) | 0.007 |
| ALT (IU/L) | 30 (10-162) | 35 (8-138) | 0.477 |
| ALP (IU/L) | 78 (35-176) | 83 (45-189) | 0.392 |
| Creatinine (mg/dL) | 0.91 (0.50-1.27) | 0.88 (0.46-2.64) | 0.507 |
| ICG-R15 | 10.5% (2.3%-24.9%) | 16.8% (3.3%-45.2%) | 0.000 |
| AFP (ng/mL) | 28.5 (1-7102) | 20.0 (2-5652) | 0.323 |
| PIVKA-II (mAU/mL) | 25 (3-500) | 18 (9-500) | 0.011 |
| Tumor size (cm) | 2.1 (0.8-3.0) | 1.8 (1.0-2.9) | 0.035 |
At last assessment, 23 patients in the LLS group and 35 in the RFA group had developed tumor recurrence. The 1-, 2-, and 3-year disease-free survival and overall survival rates were 80.0%, 68.2%, and 60.0%, and 95.4%, 92.3%, and 92.3%, respectively, for the LLS group; and 80.8%, 59.9%, and 39.6%, and 98.2%, 92.0%, and 74.4%, respectively, for the RFA group. The disease-free survival curve and overall survival curve were higher in the LLS group than the RFA group (P = 0.012 and P = 0.013, respectively) (Figures 1 and 2). Eleven patients in the RFA group developed local tumor progression. The 1-, 2-, and 3-year local tumor progression rates in the RFA group were 90.9%, 85.1%, and 82.3%, respectively. For HCC less than or equal to 2 cm, the 1-, 2-, and 3-year disease-free survival and overall survival rates were 75.8%, 69.7%, and 50.3%, and 97.0%, 88.2%, and 88.2%, respectively, in the LLS group; and 78.6%, 60.5%, and 35.3%, and 97.4%, 94.3%, and 80.9%, respectively, in the RFA group (P = 0.183 and P = 0.074, respectively). There were no statistically significant differences in disease-free survival and overall survival between the RFA group and the LLS group in patients with HCC ≤ 2 cm.
In the RFA group, 35 patients had intrahepatic recurrence and 10 patients showed concurrent intrahepatic and systemic recurrence. None developed only extrahepatic recurrence. Of these 35 patients, 12 were treated with a second percutaneous RFA and 15 with transarterial chemoembolization (TACE). Six patients were treated with simultaneous RFA and TACE. Two patients were treated with surgical resection because HCC recurred in segment 6 in one patient and segment 8 in the other patient with good liver function. Of the 23 patients with recurrence in the LLS group, 17 patients had intrahepatic recurrence and two had systemic recurrence, while four patients had concurrent intrahepatic and systemic recurrence. Of the recurrent patients in the LLS group, eight were treated with TACE, four with RFA, and five with simultaneous TACE and RFA, while four patients received no treatment, and two patients were treated with a second liver resection. The 3-year overall survival rate was 93.3% in the LLS group and 74.4% in the RFA group (P = 0.018). The overall survival curve was higher for the LLS group than for the RFA group (Figure 2, P = 0.013).
Among all the variables, treatment allocation (such as RFA), platelet counts, serum albumin, ICG-R15, PIVKA-II levels, and tumor size were found to be significant risk factors of disease-free survival by univariate analysis (Table 2). Multivariate Cox regression hazard regression analyses showed that PIVKA-II levels (OR = 1.005; 95%CI: 1.001-1.009, P = 0.010) and tumor size (OR = 0.915; 95%CI: 0.853-0.981, P = 0.012) were significant prognostic factors for disease-free survival.
| Risk factors | OR | 95%CI | P value |
| Group-RFA | 1.934 | 1.141-3.277 | 0.014 |
| Gender-female | 0.536 | 0.264-1.090 | 0.085 |
| Age | 1.015 | 0.991-1.040 | 0.217 |
| BMI | 1.066 | 0.972-1.169 | 0.177 |
| WBC | 0.924 | 0.781-1.093 | 0.354 |
| Hemoglobin | 0.900 | 0.773-1.047 | 0.171 |
| Platelet | 0.993 | 0.988-0.998 | 0.008 |
| INR | 0.730 | 0.019-27.673 | 0.865 |
| Albumin | 0.407 | 0.255-0.650 | 0.000 |
| Total bilirubin | 0.970 | 0.539-1.746 | 0.920 |
| AST | 1.008 | 0.996-1.019 | 0.196 |
| ALT | 1.002 | 0.992-1.011 | 0.736 |
| ALP | 1.001 | 0.993-1.009 | 0.816 |
| Creatinine | 1.511 | 0.490-4.655 | 0.473 |
| ICG-R15 | 1.048 | 1.015-1.082 | 0.004 |
| AFP | 1.000 | 1.000-1.000 | 0.980 |
| PIVKA-II | 1.004 | 1.001-1.007 | 0.017 |
| Tumor size | 0.952 | 0.907-1.000 | 0.050 |
Many studies have reported that surgical resection reduces the risk of recurrence of HCC, but failed to demonstrate any difference in the overall survival following resection versus RFA in patients with small HCC[4,12-14]. Our study showed that liver resection was associated with a significantly lower risk of both death and recurrence than was RFA in patients with small HCC in the left lateral segments. This difference is particularly evident in the long term. The curves of disease-free survival and overall survival in the LLS group were higher than in the RFA group, despite the RFA group showing low liver function via such metrics as high ICG-R15, low platelet count, and low serum albumin levels. However, all RFA patients were treatable with liver resection. This study reconfirmed that liver resection is associated with a reduced recurrence rate in HCC located in the left lateral segments and revealed that resection yielded longer overall survival than did RFA.
Treating hepatocellular carcinoma in patients with chronic liver disease has always presented a challenge because of the clinical complexity of managing these patients and the potential risks associated with postoperative complications. The risk factors for tumor recurrence after treatment include tumor size, insufficient safety margin, multi-nodular tumor, and tumor location[15]. Liver resection in patients with resectable HCC who have normal liver function and are in good general condition is still considered the gold standard therapy for delivering curability[12,16]. However, patients with central HCC are not usually good candidates for surgical resection because of the risk of additional injury to normal liver tissue and blood loss, which may induce further complications and negatively impact treatment outcome. RFA, however, preserves the liver parenchyma, and has a low risk of blood loss. In recent years, it has been possible to reduce perioperative mortality to less than 0.5% depending on the extent of resection and hepatic reserve[11]. The improved outcome is primarily due to advances in surgical and radiologic techniques, perioperative care and more cautious patient selection[17]. Surgical resection of tumors located in the left lateral segments is considered a safe procedure because it is easily practicable from a technical standpoint, as well as due to ease of accessibility. Recently, laparoscopic LLS has been established as a safe and feasible standard treatment option for malignant liver tumors at some specialized centers[18]. In the present study, some patients were treated with laparoscopic LLS. However, the follow-up period of those patients was too short, and we did not compare the laparoscopic LLS group with the RFA group. We will continue to collect data on laparoscopic LLS.
The RFA procedure can be performed under conscious sedation and most patients only require a short hospitalization after the procedure. There is general consensus that complete response to percutaneous RFA therapy in patients with tumors of less than or equal to 3 cm is associated with improved outcome[13,14,19]. Whether RFA or surgical resection is the better treatment option for small HCC has been debated since RFA was recommended as a treatment option in the 2005 practice guidelines issued by the AASLD[20]. Two recent meta-analyses reached significantly different conclusions, mainly because the majority of the data were obtained from non-randomized controlled trials and the overall level of clinical evidence was low[14,19]. The conclusions reported from two randomized-controlled trials were also contradictory[16,21]. Another recent randomized controlled study showed that percutaneous RFA may provide therapeutic effects similar to those of liver resection in patients with small HCC[13]. However, outcomes of RFA and resection have not been compared for left lateral segments. In this study, we therefore limited our objectives to patients with HCC ≤ 3 cm in left lateral segments.
Compared to surgical resection, percutaneous RFA is more likely to be incomplete for the treatment of small HCCs located at specific sites of the liver, such as those with bulging tumor, as well as the adjacent regions of the heart and diaphragm, and major vessels. Open or laparoscopic surgery may be the better choice in these patients. HCC mainly disseminates through the portal and hepatic veins. The tumor dissemination can invade the tributaries of the portal branches and shed tumor emboli in the neighboring branches of the same liver segment[22]. Liver resection has the advantage of complete excision of tumor tissue and hepatic parenchyma around the tumor, which might contain undetectable intrahepatic metastases and microvascular invasion[23]. Therefore, liver resection with safe tumor-free margins has better results than RFA with respect to tumor recurrence.
In this study, local recurrence was found to be more frequent after RFA than LLS, as eleven patients in the RFA group developed local tumor progression, whereas none developed it in the LLS group. This may be a result of the safety margin of RFA being narrower than that of LLS. LLS removes the entire left lateral segment containing the primary tumor and venous tumor thrombus[24,25], and the clearance of tumors and any potential sites of microscopic disease will be more complete in these patients. Local recurrences after RFA may be attributed to insufficient ablation of the primary tumor and/or the presence of tumor venous invasion in the adjacent regions of the liver. However, our study showed that the LLS group may have poor prognostic factors, such as microvascular invasion because patients with vessel-adjacent tumors were treated by surgical resection.
This study suggests that disease-free and overall survival rates following liver resection were superior to those following RFA. We therefore consider RFA to be significantly worse than LLS in the long-term. Percutaneous RFA was demonstrated to have an advantage over liver resection in terms of shorter hospitalization length. We suspected that some factors were correlated with early tumor recurrence after treatment, independent of the treatment strategy, and such factors of early recurrence were identified in this study.
In our study, patients who chose RFA as the first treatment modality were significantly older than those who underwent liver resection. Older patients may choose RFA because they more commonly have comorbidities that make liver resection unfeasible. In addition, RFA is less invasive and has lower rates of complications and lower costs, and higher repeatability when recurrence occurs[7]. The choice of RFA by older patients is consistent with data from a large, nationwide cohort study from Japan[26].
Our study had several limitations. First, it was a retrospective study. Thus, the present study was inherently flawed by a selection bias evident in the differences in tumor, etiology, and liver functions. Second, we did not assess the histopathologic diagnosis of HCC in the RFA group. Patients with poorly differentiated HCC have a poorer outcome than patients with well to moderately differentiated HCC after percutaneous RFA[27], and our study showed that a small tumor size was associated with risk factors for tumor recurrence. It is possible that HCC in the RFA group was associated with benign liver diseases, such as nodular liver cirrhosis or inflammatory pseudotumors, which may have influenced the overall survival and recurrence rates found in this study. Third, data on liver function during the follow-up was absent, which precluded assessment of the relationship between liver function and the choice of treatment at recurrence. For HCC, the influence of the first treatment is considered to be smaller than for other primary malignant diseases, because liver function significantly affects recurrence rate. Fourth, the absence of recurrence was not verified by pathologic examination, which suggests that the reported local recurrence rates for RFA may have been underestimated.
We created groups with three uniform criteria: tumor size ≤ 3 cm, Child-Pugh class A, and tumor located in left lateral segments, with the aim of producing a focused study and contributing to the current discussion on the management of HCC. We believe that despite the inherent drawbacks of our study design, our results are useful given the current lack of reliable data derived from well-designed randomized controlled trials.
In conclusion, liver resection is suitable in single HCC ≤ 3 cm in the left lateral segments. A future prospective multi-center study of the local recurrence rates of small HCC stratified according to tumor location is needed to provide clinically useful data on this issue.
Liver resection is considered the first-choice treatment for patients with early stage hepatocellular carcinoma (HCC), but recently radiofrequency ablation in patients with small HCC achieved the similar outcomes of surgical liver resection. Many studies have reported the efficacy between liver resection and radiofrequency ablation.
Nobody recommend the liver resection or radiofrequency ablation in small HCC patients. In addition, all studies do not consider the location of tumor and the extent of surgical resection.
This study has a high value because this was the first study that evaluated patients with HCC located in left lateral segments. Present study showed that the disease-free survival curve and overall survival curve were higher in the left lateral sectionectomy group than in the radiofrequency ablation group.
Present study suggests that liver resection is suitable for single HCC ≤ 3 cm in the left lateral segments.
The authors compared the outcome of liver resection (left lateral sectionectomy, left lateral sectionectomy) vs radiofrequency ablation for single HCC ≤ 3 cm in left lateral segments. The paper is relevant to this journal, and in general well written.
P- Reviewers: Haemmerich D, Miyoshi E, Vigano L S- Editor: Cui XM L- Editor: A E- Editor: Liu XM
| 1. | Yang JD, Kim WR. Surveillance for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Vivarelli M, Bellusci R, Cucchetti A, Cavrini G, De Ruvo N, Aden AA, La Barba G, Brillanti S, Cavallari A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation. 2002;74:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 4. | Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Majno PE, Mentha G, Mazzaferro V. Partial hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: confirming the trial that will never be, and some comments on the indications for liver resection. Hepatology. 2010;51:1116-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 8. | Park JW. [Practice guideline for diagnosis and treatment of hepatocellular carcinoma]. Korean J Hepatol. 2004;10:88-98. [PubMed] |
| 9. | Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol. 2010;75:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Lee HY, Rhim H, Lee MW, Kim YS, Choi D, Park MJ, Kim YK, Kim SH, Lim HK. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol. 2013;23:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Kim JM, Kwon CH, Joh JW, Ko JS, Park JB, Lee JH, Kim SJ, Paik SW, Park CK. C-reactive protein may be a prognostic factor in hepatocellular carcinoma with malignant portal vein invasion. World J Surg Oncol. 2013;11:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Xu G, Qi FZ, Zhang JH, Cheng GF, Cai Y, Miao Y. Meta-analysis of surgical resection and radiofrequency ablation for early hepatocellular carcinoma. World J Surg Oncol. 2012;10:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 598] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, El-Malah A. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1104] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 17. | Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 367] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 18. | Rao A, Rao G, Ahmed I. Laparoscopic left lateral liver resection should be a standard operation. Surg Endosc. 2011;25:1603-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 21. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 22. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 24. | Wakai T, Shirai Y, Suda T, Yokoyama N, Sakata J, Cruz PV, Kawai H, Matsuda Y, Watanabe M, Aoyagi Y. Long-term outcomes of hepatectomy vs percutaneous ablation for treatment of hepatocellular carcinoma & lt; or =4 cm. World J Gastroenterol. 2006;12:546-552. [PubMed] |
| 25. | Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 540] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 27. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |